Abstract
Background
Efficacies of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) rechallenge have been demonstrated in EGFR-mutant non-small cell lung cancer (NSCLC). However, their efficacies were only moderate. Some preclinical studies suggested synergistic effects of bevacizumab to EGFR-TKI in TKI-resistant models.
Methods
We retrospectively evaluated clinical efficacy and safety of EGFR-TKI rechallenge with bevacizumab. Rebiopsy was performed on all studied cases to examine T790M-resistant mutation status.
Results
Between January 2010 and June 2014, a total of 24 EGFR-mutant NSCLC patients who had been previously treated with EGFR-TKIs (gefitinib, erlotinib, and/or afatinib) received EGFR-TKI rechallenge with bevacizumab. Twenty-two (92 %) patients underwent erlotinib and two (8 %) gefitinib as rechallenge EGFR-TKIs in combination with bevacizumab. Three patients achieved partial response, and 18 had stable disease, resulting in the response rate (RR) of 13 % and disease control rate (DCR) of 88 %, respectively. The median progression-free survival (PFS) was 4.1 [95 % confidence interval (CI) 2.3–4.9] months, and the median overall survival (OS) was 13.5 (95 % CI 9.7–27.4) months. The RR, DCR, median PFS, and median OS for T790M-positive versus T790M-negative were 0 versus 18 % (p = 0.530), 86 versus 88 % (p = 1.00), 3.3 versus 4.1 months (p = 0.048), and 15.1 versus 13.5 months (p = 0.996), respectively. Severe adverse events (≥grade 3): grade 3 of 1 (4 %) rash; grade 3 of 1 (4 %) paronychia; grade 3 of 1 (4 %) hypertension; and grade 3 of 1 (4 %) anemia, were observed.
Conclusions
EGFR-TKI rechallenge with bevacizumab demonstrated higher DCR and modestly longer PFS than historical data on EGFR-TKI rechallenge alone. Its activity was notably higher in T790M-negative population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In systemic chemotherapies for advanced non-small cell lung cancer (NSCLC), molecular-targeted therapies have been recently developed and have provided a remarkable benefit to patients harboring specific genetic alterations such as epidermal growth factor receptor (EGFR) gene mutations or anaplastic lymphoma kinase gene fusions [1]. Somatic mutations in EGFR have been identified in patients with radiographic responses to EGFR-tyrosine kinase inhibitors (TKIs) [2, 3]. At present, EGFR sensitive mutation is established as the most reliable predictive marker for the efficacy of EGFR-TKIs [4, 5]. Currently, the efficacy of up-front EGFR-TKIs has been demonstrated for patients harboring EGFR sensitive mutations in prospective randomized phase III trials compared with platinum doublet cytotoxic chemotherapies, exhibiting a median progression-free survival (PFS) of approximately 12 months [6–11]. Despite an initial dramatic response, most patients finally acquire resistance to EGFR-TKI.
Several acquired resistant mechanisms to EGFR-TKI have been identified [12–17], and the “gatekeeper” EGFR mutation, a threonine-to-methionine substitution at amino acid position 790 in exon 20 (T790M), is the most common mechanism, accounting for approximately half of acquired resistance. Some reports demonstrated emergence of T790M was a favorable prognostic maker after acquired resistance [18, 19]. Furthermore, upcoming third-generation EGFR-TKIs have shown remarkable effectiveness for patients with T790M after acquired resistance to classical EGFR-TKIs [20, 21]. T790M is thus an important biomarker, and rebiopsy to confirm T790M status will become more essential in clinical practice.
In present clinical practice after acquired resistance to EGFR-TKIs, several guidelines recommend platinum doublet chemotherapies for patients maintaining good performance status (PS) [22, 23]. Similar to EGFR wild-type patients, docetaxel and pemetrexed are administered as salvage treatments following platinum doublets. After failure of these agents, EGFR-TKI rechallenge is occasionally effective. Several reports have shown efficacies of EGFR-TKI rechallenge in EGFR-mutant NSCLC, but the efficacies were only moderate [response rate (RR), 0–22 % disease control rate (DCR), 29–67 %, and median PFS, 2.0–3.3 months] [24–29].
Bevacizumab is a recombinant, humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF), a key factor in tumor-associated angiogenesis. The survival benefit of bevacizumab with paclitaxel plus carboplatin has been established in the frontline setting for metastatic non-squamous NSCLC, as demonstrated in a randomized phase III trial [30]. Recently, several clinical trials have exhibited the efficacy of EGFR-TKIs with bevacizumab in chemo-naïve patients with EGFR-mutant NSCLC [31, 32]. Some preclinical studies also suggested synergistic effects of bevacizumab to EGFR-TKI in TKI-resistant models [33, 34]. EGFR-TKI with bevacizumab could be a potent therapeutic strategy for patients after acquired resistance to EGFR-TKIs, but to the best of our knowledge, there is almost no clinical evidence regarding this combination therapy. The aim of our study was to evaluate clinical efficacy and safety of EGFR-TKI rechallenge with bevacizumab after acquired resistance to TKI in EGFR-mutant NSCLC. Additionally, we compared efficacies between T790M-positive and T790M-negative populations to explore the effect of this prominent mutation on combination therapy with EGFR-TKI and anti-VEGF antibody.
Patients and methods
Patients
We screened all patients with EGFR-mutant NSCLC to identify cases after acquired resistance to EGFR-TKI who had received EGFR-TKI rechallenge in combination with bevacizumab at our institute. Patients’ results were analyzed using medical and radiographic records to take age, gender, Eastern Cooperative Oncology Group (ECOG) PS, histology, smoking history, primary EGFR mutation status, previous EGFR-TKI therapies, and clinical course details into account. We retrospectively evaluated the RR, DCR, PFS, overall survival (OS), and safety. Efficacies were also compared between T790M-positive and T790M-negative populations. This study was approved by the institutional review board of Institute of Biomedical Research and Innovation.
Treatment
EGFR-TKI (erlotinib or gefitinib) was orally prescribed daily. The initial doses of erlotinib and gefitinib were 250 and 150 mg/day, respectively. Therapeutic dose was adjusted by the discretion of physicians in charge. In cases with intolerable toxicities, erlotinib dose reduction was performed from 150–100 mg/day or 50 mg/day, and gefitinib administration was modified from daily to alternating days or every 3 days. Some patients underwent EGFR-TKIs at 2 weeks on/1 week off. In patients with leptomeningeal metastases, erlotinib was prescribed at 300 mg on alternating days. Bevacizumab was intravenously administered at 15 mg/kg triweekly. Tumor evaluations were performed every 4–8 weeks with computed tomography.
Rebiopsy and EGFR mutational analysis
Rebiopsy was performed on all studied cases to examine T790M status before receiving EGFR-TKI rechallenge with bevacizumab. Tumor specimens were obtained by various methods: ultrasound or computed tomography (CT)-guided needle biopsy; bronchoscopic transbronchial biopsy; cell blocks of malignant effusions; and/or surgery. We isolated tumor DNA from these histologically or cytologically confirmed cancer cell specimens, and EGFR mutations were analyzed using the peptide nucleic acid-locked nucleic acid PCR clamp method [35].
Statistical analysis
Tumor response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1). The DCR was defined as the rate of complete response (CR)/partial response (PR) + stable disease (SD) ≥ 6 weeks in our study. RR and DCR between T790M-positive and T790M-negative populations were compared using the Fisher’s exact test. The PFS was calculated from the date of therapy initiation to disease progression or death. The OS was calculated from the date of therapy initiation to death and censored at the date of last visit for patients whose deaths could not be confirmed. PFS and OS were analyzed using the Kaplan–Meier method to estimate the median points with 95 % confidence interval (CI). PFS and OS between T790M-positive and T790M-negative populations were compared using the log-rank test. Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (version 3.0). A p value <0.05 was considered significant. The statistical analyses were performed using JMP 7 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
Between January 2010 and June 2014, a total of 24 EGFR-mutant NSCLC patients who had been previously treated with EGFR-TKIs received EGFR-TKI rechallenge in combination with bevacizumab at our institute. Patient characteristics are shown in Table 1. Median age was 64 (range 50–81). Female (18 of 24, 75 %), good PS (0/1) (21 of 24, 88 %), and never smoker (17 of 24, 71 %) were dominant. Primary EGFR mutation status was 16 (67 %) exon 21 (L858R), 5 (21 %) exon 19 (deletion), 1 (4 %) exon 19 (deletion) + exon 21 (L858R), 1 (4 %) exon 18 (G719S), and 1 (4 %) exon 18 (G719S) + exon 21 (L861Q). Patients previously underwent gefitinib (9 of 24, 38 %), erlotinib (1 of 24, 4 %), gefitinib and erlotinib (11 of 24, 46 %), or gefitinib and afatinib (3 of 24, 12 %), before treatment of EGFR-TKI rechallenge with bevacizumab. Twelve (50 %) patients had EGFR-TKI rechallenge with bevacizumab successively without a TKI-free interval, and 12 (50 %) patients after 1–4 intervening cytotoxic regimens. Eighteen (75 %) bevacizumab-naïve patients received EGFR-TKI rechallenge with bevacizumab, and 6 (25 %) after previous bevacizumab-containing regimens.
Efficacy and safety
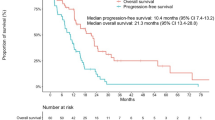
Twenty-two (92 %) patients underwent erlotinib and two gefitinib (8 %) as EGFR-TKI rechallenge therapies in combination with bevacizumab. Median course of bevacizumab administration was 6 (range 1–19). No (0 %) CR, 3 (13 %) PR, and 18 (75 %) SD were confirmed, resulting in the RR of 13 % and DCR of 88 %, respectively. The median PFS was 4.1 [95 % confidence interval (CI) 2.3–4.9] months (Fig. 1a), and the median OS was 13.5 (95 % CI 9.7–27.4) months (Fig. 1b).
Table 2 summarizes adverse events. Rash was the most frequent (15 of 24, 65 %) side effect of the therapy. Severe adverse events (≥grade 3): 1 (4 %) grade 3 rash; 1 (4 %) grade 3 paronychia; 1 (4 %) grade 3 hypertension; and 1 (4 %) grade 3 anemia, were observed. Neither grade 4 nor 5 adverse events were confirmed. There were no liver dysfunctions, interstitial lung disease, nor bevacizumab-related severe adverse events such as pulmonary hemorrhage, gastrointestinal bleeding, or thromboembolic events.
Rebiopsy results and comparison of efficacies between T790M-positive and T790M-negative populations
Table 3 shows primary and rebiopsy EGFR mutation status. T790M was confirmed by rebiopsy in 7 (29 %) of 24 patients. At the time of rebiopsy, sensitive EGFR mutations were not detected in 2 (8 %) cases. Two complex mutations consisting of exon 19 (deletion) + exon 21 (L858R) and exon 18 (G719X) + exon 21 (L861Q) changed to exon 21 (L858R) alone and exon 21 (L861Q) alone, respectively, after acquired resistance to EGFR-TKIs.
The RR, DCR, median PFS, and median OS in patients with T790M-positive (n = 7) versus T790M-negative (n = 17) were 0 versus 18 % (p = 0.530), 86 versus 88 % (p = 1.00), 3.3 (95 % CI 0.6–4.1) months versus 4.1 (95 % CI 2.1–8.0) months (p = 0.048) (Fig. 2a), and 15.1 (95 % CI 1.0-inestimable) months versus 13.5 (95 % CI 7.5–31.1) months (p = 0.996) (Fig. 2b), respectively.
Case report
The patient is a 79-year-old female diagnosed with adenocarcinoma of the lung harboring L858R. She had pleural disseminations and multiple brain metastases and was initially treated with gefitinib for 8 months, achieving PR. After progression, she underwent carboplatin plus pemetrexed with bevacizumab as the second-line chemotherapy. PR continued for 5 months, but brain metastases progressed. After whole brain radiation therapy, rebiopsy of primary tumor revealed T790M-negative status. We initiated erlotinib 150 mg/day daily + bevacizumab 15 mg/kg triweekly. Two months later, chest CT demonstrated a favorable response (Fig. 3a, b). Erlotinib was reduced to 100 mg/day due to grade 2 anorexia and grade 3 paronychia. After dose reduction, the treatment was well tolerated and the response continued for 16 months.
Discussion
EGFR-TKI rechallenge with bevacizumab demonstrated RR of 13 %, DCR of 88 %, and median PFS of 4.1 months in our study. These results exhibited higher DCR and modestly longer PFS than results from several recent studies of EGFR-TKI rechallenge alone [24–29]. As shown in the case report, a longitudinal clinical benefit was achieved in some cases. Interestingly, in the presented case, PFS was longer in third-line erlotinib with bevacizumab (16 months) than in first-line gefitinib (8 months). A favorable toxicity profile was also shown in the treatment of EGFR-TKI rechallenge with bevacizumab. Grade 3 adverse events were observed in only 4 (17 %) patients, and there were neither grade 4 nor grade 5 adverse events. Although our study included many heavily pretreated patients, active dose reductions and modifications might have improved the safety and maintained the efficacy. EGFR-TKI rechallenge with bevacizumab could be effective and safe even for heavily pretreated patients after acquired resistance to EGFR-TKI.
Activity of this combination therapy was notably higher in T790M-negative population. Dual blockade of EGFR and VEGF pathways may provide greater clinical benefit in T790M-negative cases. The reason for this result is unclear, but we present two hypotheses. First, VEGF is associated with T790M-negative acquired resistant mechanisms. Some preclinical studies reported that dual blockade of EGFR and VEGF pathways could delay tumor progression [33, 34]. Second, T790M-negative status is a predictive marker of EGFR-TKI rechallenge. A few investigators demonstrated T790M disappearance (T790M status from positive to negative) could be a predictive marker of EGFR-TKI rechallenge [17, 36]. TKI-free interval could reduce the proportion of T790M-negative cells enough that T790M is undetectable by PCR. Resistant tumors are likely to be a mixed population of TKI-sensitive (T790M-negative) and TKI-resistant (T790M-positive) cells, and upon withdrawal of the selective pressure from TKI, previously arrested TKI-sensitive cells can repopulate more quickly than TKI-resistant cells, and tumors may regain their sensitivity to TKI. This theory is based on the indolent nature of T790M-positive cells and the rapid growth potential of TKI-sensitive cells [37, 38]. Incidence of T790M in our study was 29 % (7 of 24), which is relatively lower than historical incidence of T790M (40–60 %) [19, 39]. T790M status might have changed from positive to negative in some cases after TKI-free interval. Notably, upcoming third-generation EGFR-TKIs have shown remarkable effectiveness for patients with T790M after acquired resistance to classical EGFR-TKIs [20, 21]. On the other hand, there have been almost no good therapeutic options for T790M-negative populations. Our proposed EGFR-TKI rechallenge with bevacizumab therapy could be a therapeutic option for patients without T790M.
Results of rebiopsy exhibited some interesting insights. At the time of rebiopsy, sensitive EGFR mutations “disappeared” in two (8 %) cases. Although cancer cells were definitely confirmed in our specimens, this phenomenon might have represented false negative results by inadequate process of mutation analysis. However, several current studies have actually insisted loss of activating mutation was a possible acquired resistant mechanism [40, 41]. Further investigations are warranted to confirm whether this phenomenon is a false negative result or a true acquired resistant mechanism. Two complex mutations consisting of exon 19 (deletion) + exon 21 (L858R) and exon 18 (G719X) + exon 21 (L861Q) changed to exon 21 (L858R) alone and exon 21 (L861Q) alone, respectively, after acquired resistance to EGFR-TKIs. We assume that these results imply an intratumor heterogeneity of EGFR mutations [42, 43]. Above all, rebiospy results occasionally raise intriguing questions and will become more essential in future clinical practice to confirm T790M status.
Our study has several limitations. First, it is retrospective and small sample size, including some biases, inevitably. Second, half of patients underwent EGFR-TKI with bevacizumab without TKI-free interval. This might have affected our results. Heon et al. demonstrated that 16 patients with a longer TKI-free interval (>6 months) were able to obtain greater benefit from erlotinib rechallenge than 8 patients with a shorter TKI-free interval (≤6 months) (median time to progression: 4.4 vs. 1.9 months, p = 0.026) [27]. We also previously showed that higher efficacy of TKI rechallenge with erlotinib after gefitinib failure can be achieved with proper patient selection criteria, including good PS, a benefit from prior gefitinib, and the insertion of cytotoxic chemotherapies between gefitinib and erlotinib therapies [28]. Unfortunately, our data did not reveal such a trend, which might be due to small sample size, but patients with longer TKI-free intervals (presumably interspersed with cytotoxic chemotherapies) are likely to obtain more benefit from EGFR-TKI with bevacizumab therapy. Finally, variable timings and locations of rebiopsy might have influenced T790M status. T790M status is spatiotemporally heterogenous due to selective pressure from EGFR-TKI [44]. T790M status appears to be frequently negative in cerebrospinal fluid and after a longer TKI-free interval. Our presented T790M incidence was 29 %, which is lower than historical T790M incidence (40–60 %) [19, 39].
In conclusion, EGFR-TKI rechallenge with bevacizumab demonstrated higher DCR and modestly longer PFS than historical data on EGFR-TKI rechallenge alone. It was well tolerated and feasible for heavily pretreated patients. The activity was notably higher in T790M-negative population. EGFR-TKI rechallenge with bevacizumab could be a potent therapeutic option after acquired resistance to EGFR-TKI, especially in those without T790M. Further studies are needed to evaluate this strategy. We are thus conducting a prospective phase II study of afatinib plus bevacizumab after acquired resistance to EGFR-TKI (UMIN000014710).
References
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561–566
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Fukuoka M, Wu YL, Thongprasert S et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334
Wu YL, Zhou C, Hu CP et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15:213–222
Pao W, Miller VA, Politi KA et al (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2:e73
Kobayashi S, Boggon TJ, Dayaram T et al (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352:786–792
Engelman JA, Zejnullahu K, Mitsudomi T et al (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039–1043
Yano S, Wang W, Li Q et al (2008) Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 68:9479–9487
Wang W, Li Q, Yamada T et al (2009) Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res 15:6630–6638
Sequist LV, Waltman BA, Dias-Santagata D et al (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3:75ra26
Oxnard GR, Arcila ME, Sima CS et al (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17:1616–1622
Hata A, Katakami N, Yoshioka H et al (2013) Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and -negative populations. Cancer 119:4325–4332
Jänne PA, Yang JC, Kim DW et al (2015) AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372:1689–1699
Sequist LV, Soria JC, Goldman JW et al (2015) Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 372:1700–1709
National Comprehensive Cancer Network:NCCN Clinical Practice Guidelines in Oncology—Non-Small Cell Lung Cancer, Version 6.2015. http://www.nccn.org
Besse B, Adjei A, Baas P et al (2014) 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 25:1475–1484
Asahina H, Oizumi S, Inoue A et al (2010) Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology 79:423–429
Koizumi T, Agatsuma T, Ikegami K et al (2012) Prospective study of gefitinib readministration after chemotherapy in patients with advanced non-small-cell lung cancer who previously responded to gefitinib. Clin Lung Cancer 13:458–463
Oh IJ, Ban HJ, Kim KS et al (2012) Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer 77:121–127
Heon S, Nishino M, Goldberg SB et al (2012) Response to EGFR tyrosine kinase inhibitor (TKI) retreatment after a drug-free interval in EGFR-mutant advanced non-small cell lung cancer (NSCLC) with acquired resistance. J Clin Oncol 30 (suppl; abstr 7525)
Hata A, Katakami N, Yoshioka H et al (2011) Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer 74:268–273
Kaira K, Naito T, Takahashi T et al (2010) Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer 68:99–104
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med 355:2542–2550
Seto T, Kato T, Nishio M et al (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15:1236–1244
Ichihara E, Hotta K, Nogami N et al (2015) Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 10:486–491
Naumov GN, Nilsson MB, Cascone T et al (2009) Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 15:3484–3494
Ichihara E, Ohashi K, Takigawa N et al (2009) Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res 69:5091–5098
Nagai Y, Miyazawa H, Huqun et al (2005) Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res 65:7276–7282
Hata A, Katakami N, Kaji R et al (2013) Does T790M disappear? Successful gefitinib rechallenge after T790M disappearance in a patient with EGFR-mutant non-small cell lung cancer. J Thorac Oncol 8:e27–e29
Chmielecki J, Foo J, Oxnard GR et al (2011) Optimization of dosing for EGFR-mutant non–small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 3:9059
Oxnard GR, Arcila ME, Chmielecki J et al (2011) New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res 17:5530–5537
Yu HA, Arcila ME, Rekhtman N et al (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19:2240–2247
Tabara K, Kanda R, Sonoda K et al (2012) Loss of activating EGFR mutant gene contributes to acquired resistance to EGFR tyrosine kinase inhibitors in lung cancer cells. PLoS One 7:e41017
Ji W, Choi CM, Rho JK et al (2013) Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer 13:606
Hata A, Yoshioka H, Fujita S et al (2010) Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol 5:1524–1528
Hata A, Fujita S, Kaji R et al (2011) Do complex mutations of the epidermal growth factor receptor gene reflect intratumoral heterogeneity? J Thorac Oncol 6:1144–1146
Hata A, Katakami N, Yoshioka H et al (2015) Spatiotemporal T790M heterogeneity in individual patients with non-small cell lung cancer (NSCLC) after acquired resistance to EGFR-tyrosine kinase inhibitor (TKI). J Thorac Oncol (in press)
Acknowledgments
We thank Mr. David Martin for writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest.
Rights and permissions
About this article
Cite this article
Otsuka, K., Hata, A., Takeshita, J. et al. EGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancer. Cancer Chemother Pharmacol 76, 835–841 (2015). https://doi.org/10.1007/s00280-015-2867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2867-8