Abstract
Purpose
Cisplatin is an effective chemotherapeutic agent used in the treatment of a wide variety of malignancies. Acute kidney injury (AKI) is the major toxicity associated with cisplatin and sometimes necessitates a reduction in dose or discontinuation of treatment. Atrial natriuretic peptide (ANP) is secreted by the heart and exerts a wide range of renoprotective effects, including anti-inflammatory activity. The objective of this study was to investigate the protective effects of ANP on cisplatin-induced AKI in mice.
Methods
Mice were randomly divided into three groups: control, cisplatin (20 mg/kg, intraperitoneal)/vehicle treatment, and cisplatin/ANP (1.5 μg/kg/min via osmotic-pump, subcutaneous) treatment. At 72 h after cisplatin injection, serum blood urea nitrogen and creatinine, urine albumin/creatinine, and renal expression of mRNAs encoding tumor necrosis factor-α, interleukin (IL)-1β, IL-6, intercellular adhesion molecule-1, monocyte chemoattractant protein-1, and transforming growth factor (TGF)-β were measured using real-time polymerase chain reaction. Histological changes were also evaluated.
Results
ANP treatment significantly attenuated cisplatin-induced increases in serum blood urea nitrogen and creatinine, urine albumin/creatinine, and renal expression of IL-1β, IL-6, intercellular adhesion molecule-1, and monocyte chemoattractant protein-1 mRNAs. Cisplatin-induced renal dysfunction and renal tubular necrosis were thus attenuated by ANP treatment.
Conclusions
Our results indicate that ANP exhibits a protective effect against cisplatin-induced AKI in mice. ANP may thus be of value in prophylactic strategies aimed at mitigating the adverse effects associated with chemotherapy agents, including cisplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is an effective chemotherapeutic agent used in the treatment of a wide variety of malignancies. Acute kidney injury (AKI) is the major toxicity associated with cisplatin, sometimes necessitating a reduction in dose or discontinuation of treatment [1, 2]. In addition, AKI induced by cisplatin is associated with high morbidity and mortality [1]. Although several therapeutic strategies have been proposed for preventing cisplatin-induced AKI, no specific treatments are currently recommended aside from vigorous hydration with normal saline [3]. Prophylactic management of AKI is therefore an important issue in chemotherapy with agents such as cisplatin.
Atrial natriuretic peptide (ANP) is secreted by the heart and mediates a wide range of biological activities, such as diuresis, natriuresis, vasorelaxation, and inhibition of the renin–angiotensin–aldosterone system through binding to the guanylyl cyclase-A (GC-A) receptor [4, 5]. The GC-A receptor is abundantly expressed in the heart, vascular endothelium, and kidney, indicating that the kidney would be one of the primary target organs for treatments using ANP [6]. ANP is also reportedly renoprotective, exhibiting both anti-inflammatory and anti-fibrotic activities [7, 8]. In addition, recent clinical studies reported that ANP treatment has beneficial effects on contrast-induced nephropathy [9], renal function, and postoperative cardio-renal events following cardiac surgery [10]. Therefore, we hypothesized that ANP may inhibit AKI induced by cytotoxic chemotherapy. In the present study, we investigated the protective effects of ANP on cisplatin-induced AKI.
Materials and methods
In vivo studies
Seven-week-old C57BL/6 mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animal experiments were performed according to the protocol approved by the Animal Care Ethics Committee of the National Cerebral and Cardiovascular Center Research Institute.
Cisplatin was purchased from Yakult Co. Ltd. (Tokyo, Japan). Mice were randomly divided into three groups: control, cisplatin/vehicle treatment, and cisplatin/ANP treatment. Cisplatin was administered as a single intraperitoneal dose of 20 mg/kg; an identical volume of sterile saline was administered to control mice. One day before cisplatin administration, ANP administration was started using an osmotic mini-pump, as previously reported [11]. ANP was purchased from Peptide Institute (Osaka, Japan). The osmotic mini-pump (Alzet Model 1003D, Duret Corporation, Cupertino, CA), containing either 5 % glucose (vehicle) or ANP in 5 % glucose (delivered at 1.5 μg/kg/min), was implanted subcutaneously under anesthesia in the upper back of each mouse. The ANP dose used had no effect on blood pressure or heart rate in the treated mice (data not shown). Mice were allowed free access to water and standard mouse chow and were killed 24 or 72 h after cisplatin administration, at which time the kidneys were removed and stored at −80 °C until analysis.
Serum and urine analyses
On the day of the killing, mice were deeply anesthetized with inhaled isoflurane and the kidneys and urinary bladder were exposed through a midline abdominal incision. The urinary bladder was emptied by direct compression to obtain a urine sample. Blood samples were collected via the femoral vein and centrifuged (2,000×g for 5 min), and the resulting serum samples were stored at −80 °C until analysis. Serum blood urea nitrogen (BUN) and creatinine levels were measured using FUJI DRI-CHEM SLIDE BUN-PIII and CRE-PIII, respectively, with a DRI-CHEM 4000 chemistry analyzer (FUJIFILM Co., Ltd., Tokyo, Japan). Urine protein and creatinine levels were measured by Albuwell M ELISA and Creatinine Companion chemical analysis, respectively (Exocell Inc., Philadelphia, PA).
Histological evaluation of the kidney
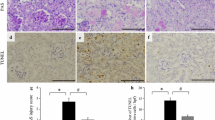
Kidneys were fixed with 4 % formaldehyde for 24 h, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin–eosin (HE) or periodic acid–Schiff (PAS) reagent for histological examination. Tubular damage was assessed microscopically in PAS-stained sections and scored based on the percentage of cortical tubules showing epithelial necrosis as follows: 0, normal; 1, <10 %; 2, 10–25 %; 3, 26–75 %; 4, >75 %. Tubular necrosis was defined as the loss of the proximal tubular brush border, blebbing of apical membranes, tubular epithelial cell detachment from the basement membrane, or intra-luminal aggregation of cells and proteins. Apoptosis in the kidney was assessed by terminal deoxynucleotidyl transferase-mediated uridine triphosphate nick-end labeling (TUNEL) assay using an ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, Bedford, MA) according to the manufacturer’s protocol. The number of apoptotic cells in each section was calculated by counting the number of TUNEL-positive cells in ten random, non-overlapping fields per slide at a 400× magnification. Histological and morphometric examinations were performed in a blinded manner.
Quantitative real-time PCR analysis
Total RNA was isolated from the kidneys using an RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA using a Quantitect Reverse Transcription Kit (Qiagen). PCR amplification was performed using SYBR Premix Ex Taq (Takara, Siga, Japan). Real-time PCR was performed in a 96-well plate using a Light Cycler 480 System II (Roche Applied Science, Indianapolis, IN) and the following primers: for monocyte chemoattractant protein-1 (MCP-1), sense 5′-GCAGGTGTCCCAAAGAAGCTGTAGT-3′ and antisense 5′-CAGAAGTGCTTGAGGTGGTTGTGGA-3′; for interleukin (IL)-6, sense 5′-CCAGTTGCCTTCTTGGGACTGATG-3′ and antisense 5′-GTAATTAAGCCTCCGACTTGTGAAG-3′; for IL-1β, sense 5′-AGCACCTTCTTTCCCTTCATCTTTG-3′ and antisense 5′-GAGGTGGAGAGCTTTCAGTTCATAT-3′; for tumor necrosis factor-alpha (TNF-α), sense 5′-TGGCCCAGACCCTCACACTCAGATC-3′ and antisense 5′-GCCTTGTCCCTTGAAGAGAACCTGG-3′; for intercellular adhesion molecule-1 (ICAM-1), sense 5′-GGCAAGAACCTTACCCTACGCTGCC-3′ and antisense 5′-GTTCAGTGCGGCACGAGAAATTGGC-3′; for transforming growth factor-beta (TGF-β), sense 5′-CAACTACTGCTTCAGCTCCACAGAG-3′ and antisense 5′-CAAGGACCTTGCTGTACTGTGTGTC-3′; and for 36B4, sense 5′-TCATTGTGGGAGCAGACAATGTGGG-3′ and antisense 5′-AGGTCCTCCTTGGTGAACACAAAGC-3′. The PCR conditions were as follows: Initial denaturation at 95 °C was followed by 35 cycles of amplification for 15 s at 95 °C and 20 s at 58–62 °C (optimized for each primer pair), with subsequent melting curve analysis, increasing the temperature from 72 to 98 °C. Quantification of gene expression was calculated relative to 36B4, which was used as a housekeeping gene.
Western blot analysis
Kidneys were lysed with RIPA buffer containing protease inhibitors. Total cell lysates were loaded on a 10–20 % gradient gel (10 μg/lane) (Bio-Rad, Hercules, CA) and transferred onto polyvinylidene difluoride membranes (Millipore) after electrophoresis. The following antibodies were used: anti-nuclear factor-kappa B (NF-κB) and anti-pS536-NF-κB (93H1) (both purchased from Cell Signaling Technology, Beverly, MA).
Statistical analysis
Results are presented as mean ±SE. Stimulated samples were compared with controls using the unpaired Student’s t test. One-way ANOVA followed by the post hoc Tukey’s test was used for multiple-group comparisons. A P value <0.05 was considered significant.
Results
ANP inhibits cisplatin-induced AKI
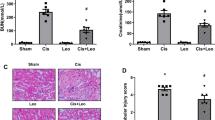
Figure 1 shows the mean body weight, serum BUN and creatinine levels, and urine albumin/creatinine levels for each group. Three days after cisplatin administration, significant weight loss was observed in the vehicle- and ANP-treated groups compared with the control group; however, there was no significant difference in body weight between the vehicle- and ANP-treated groups after cisplatin administration (Fig. 1a). The levels of serum BUN/creatinine and urine albumin/creatinine increased significantly after cisplatin administration, whereas ANP pretreatment significantly attenuated the increase in serum BUN/creatinine and urine albumin/creatinine levels observed after cisplatin administration (Fig. 1b–d). Expression of the genes encoding MCP-1, IL-6, IL-1β, TNF-α, ICAM-1, and TGF-β was significantly upregulated in the kidneys after cisplatin administration, but ANP pretreatment significantly attenuated the upregulated expression of the genes encoding MCP-1, IL-6, IL-1β, and ICAM-1 (Fig. 2). Although expression of the genes encoding TNF-α and TGF-β was lower in the ANP group compared with the vehicle group, the difference was not significant.
ANP protects kidney morphology and function after cisplatin administration
Histological examination of the kidneys of mice treated with vehicle after cisplatin administration revealed more extensive renal tubular injury, including tubular cell necrosis, loss of the brush border membrane, tubular dilatation, and cast formation, compared with the control group (Fig. 3). Compared with vehicle, ANP pretreatment significantly attenuated renal tubular injury in the kidneys resulting from cisplatin administration (Fig. 3a–c). Semi-quantitative assessment of the severity of tubular injury in PAS-stained tissue sections demonstrated significantly more extensive injury in vehicle-treated mice than in control mice. Compared with vehicle, ANP pretreatment significantly attenuated renal injury resulting from cisplatin administration (Fig. 3d). TUNEL analyses revealed significant apoptosis of tubular epithelial cells in cisplatin-treated mice. The extent of tubular epithelial cell necrosis following cisplatin administration was significantly attenuated in mice pretreated with ANP relative to vehicle-treated mice (Fig. 4a–d).
Effect of ANP on morphology in a mouse model of acute kidney injury induced by cisplatin. Kidney tissue sections from control (a), cisplatin/vehicle-treated (b), and cisplatin/ANP-treated (c) mice were prepared and stained with PAS 3 days after cisplatin administration. Representative images are shown at 400× magnification. Black arrows indicate PAS-positive parts. (d) Assessment of tubular injury score via PAS staining. Data are expressed as mean ±SE; n = 5 per condition; *P < 0.05
Effect of ANP on cisplatin-induced apoptosis in a mouse acute kidney injury model. Kidney tissue sections from control (a), cisplatin/vehicle-treated (b), and cisplatin/ANP-treated (c) mice were prepared and evaluated by TUNEL 3 days after cisplatin administration. Representative images are shown at 400× magnification. (d) Assessment of apoptotic cells via TUNEL assay. The number of apoptotic cells was counted in 10 high-power field (hpf) and is expressed as mean ±SE; n = 5 per condition; *P < 0.05
The renoprotective effects of ANP involve NF-κB signaling
Cisplatin-treated mice showed significantly higher levels of phosphorylated NF-κB (pNF-κB) in the kidneys 1 day after cisplatin administration (Fig. 5). Compared with vehicle, ANP pretreatment significantly attenuated the increase in kidney pNF-κB levels associated with cisplatin administration (Fig. 5).
Discussion
In the present study, we demonstrate for the first time that ANP has a prophylactic effect on cisplatin-induced AKI. ANP pretreatment significantly attenuated renal dysfunction and tubular epithelial cell necrosis induced by cisplatin in our mouse model. The present findings indicate that ANP administration is a valuable treatment option to prevent AKI induced by cytotoxic chemotherapy, including that using cisplatin.
Numerous studies have shown that ANP has inhibitory effects against cardiovascular events such as acute heart failure and arrhythmias [4, 5]. However, there have been few studies examining the effects of ANP on renal injury and the pathophysiology of renal disease. As the GC-A receptor is abundantly expressed not only in the cardiovascular system but also in the kidney, the kidney could be a primary target organ for ANP [6]. Previous studies have shown that ANP inhibits the growth of mesangial cells, vascular smooth muscle cells, and fibroblasts [12, 13]. Recently, it was reported that ANP inhibits early inflammatory responses and renal fibrosis through NF-κB signaling [7, 8]. More recently, clinical evidence has shown that ANP exerts a much broader range of renoprotective activities [9, 10, 14]. Morikawa et al. [9] reported the results of a prospective randomized controlled trial that showed ANP administration attenuates contrast-induced nephropathy after coronary angiography. In their study protocol, low-dose ANP administration (0.042 μg/kg/min) was started 4–6 h before angiography and continued for 48 h. As a result, the incidence of increases in creatinine of ≥25 % or ≥0.5 mg/dL over baseline was significantly lower in the ANP-treated group than in the control group (3.2 vs. 11.7 %, respectively; P = 0.015). Mori et al. [14] reported that low-dose (0.0125 μg/kg/min) ANP treatment reduces the incidence of AKI after aortic arch surgery. In their study protocol, ANP administration was started just before surgery and continued for 24 h postoperatively. As a result, the incidence of AKI was significantly lower in the ANP-treated group than in the placebo-treated group (30 vs. 73 %, respectively; P = 0.015). There were no significant differences in mean arterial pressure or number of events of hypotension between the groups. The results of these studies suggest that ANP reduces the incidence of renal events following chemical or surgical injury, without severe side effects.
However, there are some limitations to the efficacy of ANP with respect to attenuating the severity of renal failure. Allgren et al. [15] reported no beneficial effects resulting from ANP treatment with respect to the need for dialysis in patients with acute renal failure. In their study protocol, an excessive dose of ANP (0.2 μg/kg/min) was administered for condition-established patients with acute tubular necrosis due to recent ischemic or nephrotoxic insults. Furthermore, in their study population, ANP-treated patients had significantly lower blood pressure than placebo-treated patients. In another study, it was shown that low-dose ANP (0.02 μg/kg/min) initiated at the start of cardiopulmonary bypass decreases the incidence of post-surgery dialysis for acute renal failure in patients with chronic kidney disease, without significant hypotension [10]. It is possible that hypotension might account for the lack of beneficial effects of ANP treatment on renal function. In addition, it could also be very important that ANP is given as a “pretreatment” prior to any stimulation in order for it to be of benefit. In the present study, we showed that ANP pretreatment significantly attenuates renal dysfunction and the increases in inflammatory cytokine levels in the kidneys in AKI induced by cisplatin, without causing hypotension.
ANP is an endogenous peptide that has been approved for treatment of acute heart failure in Japan, and few patients have suffered severe side effects [16]. Therefore, the clinical safety of ANP has already been established [16]. The observed protective effects of ANP in attenuating AKI as demonstrated in this study may reduce the severity of side effects resulting from cytotoxic chemotherapy agents, including cisplatin.
In this study, the effects of ANP on tumor-bearing mice with or without cisplatin have not been studied. In addition, we examined the effect of ANP on only NF-κB pathway; however, cisplatin-induced kidney injury has been reported to involve various pathways, such as mitochondrial pathway [17]. Therefore, further studies to identify the specific targets affected by ANP are required. After mechanism identification, we want to begin clinical trials examining the use of human ANP for preventing AKI in lung cancer patients undergoing chemotherapy.
In summary, we present the first report that ANP exerts a prophylactic effect on AKI induced by cisplatin. Additional studies are warranted to determine whether these effects can be observed in clinical cases and translated into improved clinical outcomes.
References
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334:115–124
Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M (2008) Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol 6:903–909
Nishikimi T, Maeda N, Matsuoka H (2006) The role of natriuretic peptides in cardioprotection. Cardiovasc Res 69:318–328
Saito Y, Nakao K, Nishimura K, Sugawara A, Okumura K, Obata K, Sonoda R, Ban T, Yasue H, Imura H (1987) Clinical application of atrial natriuretic polypeptide in patients with congestive heart failure: beneficial effects on left ventricular function. Circulation 76:115–124
Totsune K, Takahashi K, Murakami O, Satoh F, Sone M, Saito T, Sasano H, Mouri T, Abe K (1994) Natriuretic peptides in the human kidney. Hypertension 24:758–762
Nishikimi T, Inaba-Iemura C, Ishimura K, Tadokoro K, Koshikawa S, Ishikawa K, Akimoto K, Hattori Y, Kasai K, Minamino N, Maeda N, Matsuoka H (2009) Natriuretic peptide/natriuretic peptide receptor-A (NPR-A) system has inhibitory effects in renal fibrosis in mice. Regul Pept 154:44–53
Rosón MI, Toblli JE, Della Penna SL, Gorzalczany S, Pandolfo M, Cavallero S, Fernández BE (2006) Renal protective role of atrial natriuretic peptide in acute sodium overload-induced inflammatory response. Am J Nephrol 26:590–601
Morikawa S, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesugi M, Morita Y, Numaguchi Y, Okumura K, Murohara T (2009) Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol 53:1040–1046
Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, Kimura H, Shiono M, Takayama T, Hirayama A (2011) Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 58:897–903
Nojiri T, Hosoda H, Tokudome T, Miura K, Ishikane S, Kimura T, Shintani Y, Inoue M, Sawabata N, Miyazato M, Okumura M, Kangawa K (2014) Atrial natriuretic peptide inhibits lipopolysaccharide-induced acute lung injury. Pulm Pharmacol Ther 29:24–30
Wolf G, Thaiss F, Schoeppe W, Stahl RA (1992) Angiotensin II-induced proliferation of cultured murine mesangial cells: inhibitory role of atrial natriuretic peptide. J Am Soc Nephrol 3:1270–1278
Pandey KN, Nguyen HT, Li M, Boyle JW (2000) Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase. Biochem Biophys Res Commun 271:374–379
Mori Y, Kamada T, Ochiai R (2014) Reduction in the incidence of acute kidney injury after aortic arch surgery with low-dose atrial natriuretic peptide: a randomised controlled trial. Eur J Anaesthesiol 31:381–387
Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, Sweet RM, Genter FC, Kurnik BR, Conger JD, Sayegh MH (1997) Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med 336:828–834
Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S (2007) Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 370:1483–1493
Maimaitiyiming H, Li Y, Cui W, Tong X, Norman H, Qi X, Wang S (2013) Increasing cGMP-dependent protein kinase I activity attenuates cisplatin-induced kidney injury through protection of mitochondria function. Am J Physiol Renal Physiol 305:F881–F890
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (26861136) and a Grant from the Takeda Science Foundation, Japan Research Foundation for Clinical Pharmacology, Osaka Cancer Society, and Kobayashi Foundation for Cancer Research, Japan.
Conflict of interest
All authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nojiri, T., Hosoda, H., Kimura, T. et al. Atrial natriuretic peptide protects against cisplatin-induced acute kidney injury. Cancer Chemother Pharmacol 75, 123–129 (2015). https://doi.org/10.1007/s00280-014-2624-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2624-4