Abstract
Purpose
Cisplatin has been widely accepted as an effective chemotherapy drug with various side effects, including nephrotoxicity. The mechanisms of cisplatin-induced acute kidney injury (AKI) are complex, and there are limited renoprotective approaches. Leonurine is the main active compound of a Chinese herb and has recently been reported to have a protective effect on the kidneys. This study aimed to verify the renoprotective effect of leonurine in attenuating cisplatin-induced AKI and explore the potential associated mechanisms.
Methods
C57BL/6 mice were divided into four groups (Sham, Cisplatin, Leonurine, and Cisplatin + Leonurine). Mice in the leonurine-treated groups were pretreated with a daily intraperitoneal injection of 25 mg/kg leonurine. AKI was induced by injecting cisplatin once intraperitoneally at 20 mg/kg body weight. Mice were killed on day 5. Kidney injury was assessed using a serum biochemical and histological assay. Apoptosis was evaluated using a terminal deoxyribonucleotide transferase-mediated dUTP nick-end labeling (TUNEL) staining assay and Western blot. Antioxidant enzymes were detected using commercial kits. The improvement in inflammasome activation, mitochondrial dysfunction, and endoplasmic reticulum stress (ERS) were assessed by polymerase chain reaction (PCR) and Western blot, respectively.
Results
Leonurine treatment improved kidney function by preventing renal tubular injury and apoptosis. Expression of nucleotide-binding leucine-rich repeat and pyrin domain containing protein 3 (NLRP3) inflammasome components and inflammatory cytokines, mitochondrial dysfunction, and ERS were all alleviated by leonurine.
Conclusion
The results indicate that leonurine plays a protective role in cisplatin-induced AKI and may represent an effective multi-targeted intervention strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is an inorganic chemotherapy drug that has been widely used for the treatment of several types of malignant tumors [1]. As a standard component of treatment regimens with a high cure rate in specific cancers [2], its use is frequently limited by various significant side effects, including neurotoxicity, ototoxicity, and nephrotoxicity. One of the most common presentations of nephrotoxicity is acute kidney injury (AKI), which was first reported in 1971 [3] with a 20–30% incident rate in patients undergoing cisplatin treatment [4, 5]. Several mechanisms are known to be involved, including mitochondrial dysfunction, inflammation, and endoplasmic reticulum stress (ERS) [6]. Although numerous approaches have been reported to provide renoprotection, its clinical use remains limited.
Growing evidence has demonstrated that mitochondrial dysfunction plays an important role in renal tubular injury. The overproduction of reactive oxygen species (ROS) and impaired mitochondrial redox balance induced by the accumulation of cisplatin in proximal tubular cells have been shown to damage mitochondrial deoxyribonucleic acid (mtDNA) [7]. In addition, cisplatin has been shown to interact with transcription factors that regulate mitochondrial biogenesis [8]. The expression of endogenous antioxidant enzymes (e.g., superoxide dismutase [SOD] and glutathione [GSH]) are simultaneously suppressed [9].
The NLRP3 inflammasome is a multi-protein complex that can be activated by a wide variety of stimuli, including ROS. The trigger of inflammatory cascades involving pro-inflammatory cytokines promotes programmed cell death and pyroptosis [10]. Our previous studies have demonstrated that NLRP3 up-regulation leads to aldosterone and angiotensin II-induced renal tubular cell apoptosis [11, 12]. It is believed that the NLRP3 inflammasome has also recently been involved in cisplatin nephrotoxicity, which requires further research [13,14,15,16].
The endoplasmic reticulum is an intracellular organelle associated with protein synthesis, folding, and degradation. Pathophysiological conditions can disrupt the normal protein folding processes, and subsequent ERS and activation of the unfolded protein response (UPR) are trigged by the accumulation of these misfolded proteins [17]. Although the mechanism of cisplatin-induced ERS is currently unknown, possible hypotheses include oxidative stress or the covalent binding of cisplatin to key microsomal proteins [18], leading to the activation of procaspase 12 and ERS markers, such as glucose regulatory protein 78 (GRP78/BiP) and C/EBP homologous protein (CHOP) [19, 20].
Herba Leonuri, a traditional Chinese herb, has been well accepted as a treatment for vaginal bleeding and metrorrhagia. Over the past several decades, leonurine, the main active compound of Herba Leonuri, has been verified to exhibit protective cardiovascular effects through various anti-apoptotic and anti-inflammatory mechanisms [21]. It has also recently been reported that leonurine can ameliorate podocyte injury, lipopolysaccharide (LPS)-induced AKI, and kidney fibrosis via suppressing ROS, which suggests a potential renoprotective effect [22,23,24]. In a model of gouty arthritis, leonurine was found to prevent NLRP3 inflammasome activation and decrease both interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) production [25]. However, it remains unclear whether leonurine protects the kidneys from cisplatin-induced nephrotoxicity.
Thus, the aim of the present study was to verify the renoprotective effect of leonurine in attenuating cisplatin-induced AKI. Furthermore, the potential mechanisms were explored to provide novel insight into therapeutic strategies.
Materials and methods
Reagents and antibodies
Leonurine was purchased from Herbpurify (Chengdu, China). Primary antibodies against CHOP (2895), GRP78/BiP (3177), glucose regulatory protein 94 (GRP94, 20292), B cell lymphoma 2 (Bcl-2, 3498), and Bcl-2-associated X (Bax, 2772) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against mitochondrial transcription factor A (TFAM, ab131607), peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α, ab54481), p62 (ab56416), autophagy-related gene 7 (ATG7, ab133528), dynamin-related protein 1 (Drp1, ab184247) and mitofusin 2 (Mfn2, ab124773) were obtained from Abcam (Cambridge, MA, USA). Anti-caspase-1 (56036) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against apoptosis-associated speck-like protein containing a CARD (ASC, AG-25B-0006-C100) and NLRP3 (AG-20B-0014-C100) were obtained from adipoGen (San Diego, CA, USA). Goat polyclonal IL-1β (AF-401-NA) antibodies were purchased from R&D Systems (Minneapolis, MN, USA). Monoclonal rat anti-interleukin-18 (IL-18, D046-3) antibodies were purchased from Medical&Biological Laboratories (Minato-ku, Tokyo, Japan). All horseradish peroxidase (HRP) conjugated-secondary antibodies for immunoblot analyses were obtained from Jackson ImmunoResearch (West Grove, PA, USA).
Animals
All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Eight-week-old male C57BL/6 mice were purchased from Shanghai SLAC Laboratory Animals (Shanghai, China) and housed in a stable environment with a regulated 12-h light–dark cycle, controlled temperature, and free access to food and water. All animals were randomly divided into four groups (Sham, Cis, Leo, and Cis + Leo) of six mice each. Kidney injury was induced in the Cis and Cis + Leo groups by injecting cisplatin once intraperitoneally at a dose of 20 mg/kg body weight, whereas the other groups (Sham and Leo groups) received saline. Leonurine was dissolved in a physiological saline solution. Mice in the Leo and Cis + Leo groups were pretreated with leonurine for 2 days before cisplatin injection and then another 5 days with an intraperitoneal injection dose of 25 mg/kg per day. Mice were killed on day 5 and blood samples were collected. The harvested kidneys were embedded in paraffin after fixing in 4% paraformaldehyde and the remaining samples were stored at − 80 °C for further analyses.
Serum biochemical measurements
Serum creatinine (Scr) and blood urea nitrogen (BUN) were measured at the clinical laboratory at the Shanghai Ninth People’s Hospital using standard laboratory methods.
Histological assay
Kidney tissues were sectioned into 3 μm slices and stained with hematoxylin and eosin (HE). Tubular injury was scored in accordance with the following semi-quantitative grades: normal (score = 0), below 25% (score = 1), 25–49% (score = 2), 50–74% (score = 3), and ≥ 75% (score = 4) of the damaged areas [15].
Western blot
Kidney tissues were collected and lysed in radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, China) according to the manufacturer’s protocol. Protein samples were loaded and separated by 10–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with primary antibodies (NLRP3 and IL-18 1 μg/ml, IL-1β 0.25 μg/ml, caspase-1 1:200, TFAM 1:2000, ATG7 1:10,000, others were all 1:1000) overnight at 4 °C after blocking in 5% nonfat milk for 2 h. The membranes were subsequently incubated with secondary antibodies for 1 h at room temperature. The immunoreactive band intensity was visualized by enhanced chemiluminescence (Amersham, Little Chalfont, UK) and densitometric analyses were quantified using Image J software.
Quantitative real-time PCR
The total ribonucleic acid (RNA) was isolated and reverse-transcribed according to the instructions of the PrimeScript RT reagent kit (Takara, Japan). Real-time PCR was performed using an ABI Prism 7500 Sequence Detection System (Foster City, CA, USA). The relative expression was normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or 18S. The following primer sequences were used: interleukin-6 (IL-6), forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′, reverse 3′-TTGGTCCTTAGCCACTCCTTC-5′; TNF-α, forward 5′-CCCTCACACTCAGATCATCTTCT-3′, reverse 3′-GCTACGACGTGGGCTACAG-5′; monocyte chemoattractant protein 1 (MCP-1), forward 5′-CACTCACCTGCTGCTACTCA-3′, reverse 3′-GCTTGGTGACAAAAACTACAGC-5′; mtDNA, forward 5′-TTTTATCTGCATCTGAGTTTAATCCTGT-3′, reverse 3′-CCACTTCATCTTACCATTTATTATCGC-5′; NAHD dehydrogenase subunit 1 (ND-1), forward 5′-ATCCTCCCAGGATTTGGAAT-3′, reverse 3′-ACCGGTAGGAATTGCGATAA-5′; adenosine triphosphate (ATP), forward 5′-TCCATCAAAAACATCCAGAAAA-3′, reverse 3′-GAGGAGTGAATAGCACCACAAA-5′; GAPDH, forward 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse 3′-TGTAGACCATGTAGTTGAGGTCA-5′; 18S, forward 5′-TTCGGAACTGAGGCCATGATT-3′, reverse 3′-TTTCGCTCTGGTCCGTCTTG-5’.
TUNEL assay
Apoptosis in the renal tissue was detected using a TUNEL assay (Roche, Netley, NJ, USA) according to the manufacturer’s protocol. Briefly, after the tissues were incubated with the TUNEL reaction mixture in the dark for 60 min, 10 fields were randomly selected for each slide and the TUNEL-positive cells were counted.
SOD, glutathione peroxidase (GSH-Px) and catalase (CAT) activity measurement
The SOD, GSH-Px and CAT activity in renal tissue was measured by commercial kits and micro-plate reader according to the manufacturer’s protocol (Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
All data were presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using a Student’s t test or one-way analysis of variance (ANOVA). A threshold p value < 0.05 was considered to be statistically significant.
Results
Leonurine ameliorates cisplatin-induced kidney injury
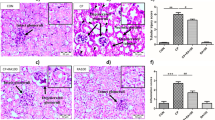
AKI was induced by a cisplatin injection, which was characterized by a significant increase in Scr and BUN as shown in Fig. 1A, B. Compared with the cisplatin group, treatment with leonurine successfully decreased the level of Scr and BUN. To estimate the renoprotective effect of leonurine, renal tissues were stained with HE to evaluate the level of histopathology (Fig. 1C). The loss of a brush border, cast formation, lumen dilation of the renal tubules, and degeneration of tubular epithelial cells in the cisplatin-treated group were substantially ameliorated by leonurine in accordance with the decrease in tubular injury score (Fig. 1C, D).
Leonurine ameliorates cisplatin-induced kidney injury. A Blood urea nitrogen levels. B Serum creatinine levels. C Representative histological photomicrographs of HE-stained kidney sections (× 400). D Tubular injury scores. Data are represented as the mean ± SEM (n = 6). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Leonurine decreases cisplatin-induced renal apoptosis
We examined the effect of leonurine on the degree of renal apoptosis induced by cisplatin in vivo using a TUNEL staining assay. Significant apoptosis was observed in the cisplatin-treated kidney tissue and was markedly attenuated in the leonurine group (Fig. 2A, B). These results were also verified by Western blot. Cisplatin also increased the level of Bax expression and reduced Bcl-2 expression. In contrast, treatment with leonurine prevented cisplatin-mediated Bcl-2 down-regulation, as well as Bax up-regulation, and synchronously increased the Bcl-2/Bax ratio (Fig. 2C, D).
Leonurine decreases cisplatin-induced apoptosis. A Representative TUNEL staining images (× 400, scale bars 20 μm). B Average percentage of TUNEL-positive cells. C Western blot analyses of Bax and Bcl-2 expression in the four treatment groups. D Densitometric analyses of Bcl-2/Bax expression normalized to GAPDH. Data are represented as the mean ± SEM (n = 3 or 6). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Leonurine suppresses cisplatin-induced oxidative stress
The oxidative stress induced by cisplatin in kidney tissues was detected by SOD activity, levels of GSH-Px and CAT. Cisplatin treatment significantly reduced SOD activity in renal, accompanied by decreases in levels of GSH-Px and CAT activity. The inhibitions of enzymatic activities were all improved by leonurine injection compared with cisplatin alone (Fig. 3A–C).
Effects of leonurine on cisplatin-induced oxidative stress and expression of inflammatory cytokines. A–C Activities of SOD, CAT and levels of GSH-Px in kidney tissues. D Relative expression of IL-6, TNF-α, and MCP-1 mRNA normalized to GAPDH. Data are represented as the mean ± SEM (n = 3 or 6). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Leonurine inhibited the cisplatin-induced expression of inflammatory cytokines and inflammasome components
We also evaluated the level of inflammatory cytokine expression by real-time PCR. Compared with the control group, there was a substantial increase in the level of IL-6, TNF-α, and MCP-1 in the cisplatin group, whereas these levels were all suppressed by leonurine (Fig. 3D). To investigate the effect of leonurine on cisplatin-induced AKI, we analyzed the level of protein expression of various inflammasome components by Western blot. As shown in Fig. 4, activation of the NLRP3 inflammasome increased the level of pro-caspase-1 cleavage and ASC, leading to the induction of mature IL-1β and IL-18. Treatment with leonurine significantly decreased the level of cisplatin-induced activation of NLRP3, as well as the expression of ASC, caspase-1, mature IL-1β, and IL-18.
Leonurine inhibits cisplatin-induced expression of inflammasome components. A Western blot analyses of NLRP3, ASC, caspase-1, IL-1β, and IL-18 expression. B Densitometric analyses of NLRP3, ASC, and caspase-1 normalized to GAPDH. C Semi-quantitative analyses of IL-1β and IL-18. Data are represented as the mean ± SEM (n = 3). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Leonurine alleviates cisplatin-induced mitochondrial dysfunction
As shown in Fig. 5A–C, Western Blot results showed that the mitochondrial fission-related protein Drp1 and autophagy-related protein P62 were markedly increased after cisplatin treatment, expression of Mfn2 and ATG7 were significantly decreased. Simultaneously, the reverse trends were observed after leonurine treatment. The kidney tissues of cisplatin-treated mice exhibited down-regulated expression of PGC-1α and TFAM, proteins critical for the maintenance of mtDNA, mitochondrial biogenesis, and energetics (Fig. 5A, D). Moreover, these proteins were accompanied by a marked reduction in the level of ATP synthase, mtDNA, and ND-1 mRNA, which represented the occurrence of mitochondrial dysfunction (Fig. 5E–G); however, the decreased levels of protein and mRNA expression were reversed by leonurine treatment.
Effects of leonurine on cisplatin-induced mitochondrial dysfunction. A Western blot analyses of Drp1, Mfn2, ATG7, P62, PGC-1α and TFAM expression. B Relative protein expression of Drp1 and Mfn2 normalized to GAPDH. C Relative protein expression of ATG7 and P62 normalized to GAPDH. D Relative protein expression of PGC-1α and TFAM normalized to GAPDH. E–G Relative mRNA expression of mtDNA, ND-1 and ATP normalized to 18S. Data are represented as the mean ± SEM (n = 3–4). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Leonurine attenuates cisplatin-induced ERS
To further investigate the protective effect of leonurine, proteins related to ERS were analyzed by Western blot. The expression of GRP78/BiP, GRP94, and CHOP were notably up-regulated following cisplatin treatment. In contrast, leonurine alleviated the level of these proteins in the Cis + Leo group compared to that of the group that received cisplatin alone (Fig. 6).
Leonurine attenuates cisplatin-induced endoplasmic reticulum stress. A Western blot analyses of GRP78/BiP, GRP94, and CHOP expression. B Relative protein expression of GRP78/BiP, GRP94, and CHOP normalized to GAPDH. Data are represented as the mean ± SEM (n = 3). *p < 0.05 versus sham group; #p < 0.05 versus cisplatin group. Cis cisplatin group, Leo leonurine group
Discussion
Cisplatin-induced AKI is characterized by proximal tubular injury and vascular injury as the major histopathological features. The pathophysiological basis of the tissue injury was studied and different renoprotective interventions have been developed over past decades [4, 5]. Leonurine is the main active compound of the Chinese herb, Herba Leonuri, and has been reported to possess antioxidant properties in atherosclerosis, LPS-induced AKI, and chronic kidney disease models [21,22,23,24]. The findings of this study further confirmed the renal protective effect of leonurine in cisplatin-related AKI, which was represented as an improvement of kidney function and prevention of renal tubular injury and apoptosis, partially through alleviating the expression of the NLRP3 inflammasome components and inflammatory cytokines, mitochondrial dysfunction, and ERS.
Apoptosis and necrosis can be induced by cisplatin both in vivo and in vitro [26, 27]. Apoptosis is the further focus of the mechanistic investigation of cisplatin-induced AKI. Several complex pathways and molecules have been implicated, including the extrinsic pathway mediated by death receptors, the intrinsic pathway concentrated on mitochondrial dysfunction, and the ERS pathway. In addition, the Bcl-2 family of proteins is known to play a critical role in mitochondrial dysfunction. The translocation of Bax to mitochondria induced by cisplatin results in porous defects to the outer membrane, the release of apoptogenic factors (e.g., cytochrome c, apoptosis-inducing factor, endonuclease G, and others) from the mitochondria [28,29,30,31,32]. A blockade of Bax via Bcl-2 expression has been shown to attenuate mitochondrial injury [30, 33]. Both a decrease in the release of Cytochrome c and apoptosis are mirrored in Bax-deficient mice and in vitro [31]. p53-dependent and independent activation of the caspase family emerges during the execution phase of cisplatin-induced apoptosis [33, 34]. Numerous pharmacological agents have been shown to diminish apoptosis by inhibiting Bax activation [35, 36]. Similarly, the findings of our study demonstrate the anti-apoptotic effect of leonurine on cisplatin nephrotoxicity by reducing the ratio between Bax and Bcl-2, in line with an improvement in kidney function, histopathology, and TUNEL staining.
ROS has been reported to be an important mechanism contributing to cisplatin-induced nephrotoxicity [37]. Despite reactions with cellular antioxidants [38] and the formation of ROS via the cytochrome P450 pathway [39], cisplatin can cause mitochondrial dysfunction and increase ROS production through damaging the respiratory chain [40]. The exposure of cultured proximal tubule cells to cisplatin can specifically inhibit mitochondrial complexes I to IV of the respiratory chain via decreased intracellular ATP levels [40]. Mitochondrial energetics are also disrupted, which were represented by the inhibition of fatty acid oxidation in vitro [41, 42]. It was reported that both the DNA-binding activity of peroxisome proliferator-activated receptor α (PPAR-α) and the availability of its coactivator, PGC-1, were reduced by cisplatin. In addition to mitochondrial biogenesis, mitochondrial dynamics that presented as the net balance between fission and fusion were also critical in maintenance of mitochondrial stability. Brooks et al. have demonstrated mitochondrial fragmentation in cisplatin-induced nephrotoxicity with changes of Drp1 [43]. Further evidence suggests that the antioxidant capacity of leonurine is protective against kidney injury [42]. In 2014, the renal protective effect of leonurine was first reported in an LPS-induced AKI model with the recovery of the redox balance [22]. Moreover, treatment with leonurine significantly reduced the level of ROS production in unilateral ureteral obstruction mice and ameliorated adriamycin-induced podocyte injury both in vivo and in vitro [23, 24]. In line with previous reports, our data showed that leonurine could ameliorate oxidative stress and improve cisplatin-induced mitochondrial dysfunction by protecting mtDNA and increasing the level of ND-1 and ATP. Leonurine can also promote the level of PGC-1α and TFAM expression, proteins critical for the replication, transcription, and maintenance of mtDNA [44]. The critical mitochondrial fission protein Drp1 was attenuated while fusion of mitochondria was improved at the same time.
There has been a growing recognition of the pro-inflammatory nature of cisplatin due to the involvement of a large number of cytokines and chemokines. The NLRP3 inflammasome is an important member of the innate immune system that is activated by several inducers, including ROS and mtDNA release [45], and subsequently interacts with the ASC, resulting in caspase-1 activation and the secretion of IL-1β and IL-18. The NLRP3 inflammasome in cisplatin-induced AKI is associated with numerous concerns [16]. For instance, Zhang et al. showed that NLRP3/ASC/caspase-1 expression was significantly up-regulated in renal tissues of the cisplatin-treated mice, which was accompanied by an increase in the production of IL-1β and IL-18 [46]. In addition, TNF-α is a typical pro-inflammatory cytokine believed to be the trigger of the cytokine response, with increased concentrations at both in the serum and protein level in cisplatin-induced renal injury [47, 48]. The up-regulation of other cytokines (e.g., macrophage inflammatory protein-2, MCP-1, and IL-6) has also been observed in cisplatin-induced AKI [48, 49]. Consistent with previous results, the findings of this study revealed a significant increase in the expression of NLRP3 inflammasome components and inflammatory cytokines following cisplatin injection. Notably, this is the first study to show that leonurine could attenuate kidney injury through the suppression of the NLRP3 inflammasome.
Autophagy is the degradation process of damaged organelles such as mitochondria. In nephrotoxic models of AKI, autophagy was activated by cisplatin within hours in different renal proximal tubular cells [50, 51]. Contrary to majority of the results, mitophagy-related genes Atg5, Atg7 and proteins LC3 could also be lower in cisplatin-treated rats with increased p62 expression in recent study [52]. Despite the biphasic autophagic response in cisplatin-induced AKI, inhibition of autophagy all increased apoptosis, suggesting the protective role of autophagy in cisplatin nephrotoxicity [50, 51]. Previous studies have demonstrated the connection between autophagy and NLRP3 inflammasome. Autophagy ameliorated inflammatory responses by inhibiting NLRP3 inflammasome activation [53, 54] and cisplatin could activate NLRP3 inflammasome by inhibiting autophagy in kidney [55]. We also found the inhibition but not activation of autophagy after cisplatin treatment and the difference might attribute to different cisplatin dose and time. Similarly, leonurine treatment promoted autophagy and suppressed the activation of the NLRP3 inflammasome.
In addition to the extrinsic pathway and mitochondrial dysfunction, the ERS pathway is also involved in cisplatin-induced apoptosis. After being activated by various different stimulators (e.g., metabolic dysfunction and mutant protein accumulation), ERS can be initiated by the accumulation of unfolded or misfolded proteins. Protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) are the three main pathways related to UPR. Chaperone GRP78 binds to PERK, ATF6, and IRE1 to maintain the inactivated status of the UPR, whereas disassociation occurs upon the accumulation of unfolded or misfolded proteins followed by downstream signaling [56, 57]. In vitro, the expression of the GRP78/BiP and PERK pathways was found to be notably up-regulated following cisplatin treatment [58]. Furthermore, the over-expression of ERS can lead to apoptosis, which manifests as the increased expression of apoptosis proteins, including CHOP and Caspase 12, in cisplatin-treated LLC-PK1 cells [19]. In agreement with these findings, GRP78/BiP, GRP94 and CHOP were all found to be up-regulated in the cisplatin groups, whereas leonurine alleviated the ERS in the cisplatin-treated mice.
Conclusion
These results indicate that leonurine plays a protective role in cisplatin-induced AKI and may represent an effective intervention strategy. The renoprotective effect of leonurine against apoptosis is likely exerted by alleviating the expression of the NLRP3 inflammasome components and inflammatory cytokines, ameliorating mitochondrial dysfunction, and suppressing ERS (Fig. 7); however, the potential mechanism and signaling cascade pathway of leonurine remains incompletely understood and further studies are warranted.
References
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4(4):307–320
Kociba RJ, Sleight SD (1971) Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother Rep 55(1):1–8
Goldstein RS, Mayor GH (1983) Minireview. The nephrotoxicity of cisplatin. Life Sci 32(7):685–690
Madias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65(2):307–314
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73(9):994–1007
Bajwa A et al (2015) Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol 26(4):908–925
Guo Y et al (2018) MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J Am Soc Nephrol 29(2):449–461
Un H et al (2020) Phloretin and phloridzin guard against cisplatin-induced nephrotoxicity in mice through inhibiting oxidative stress and inflammation. Life Sci 266:118869
Swanson KV et al (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489
Xu Y et al (2019) Prohibitin 2-mediated mitophagy attenuates renal tubular epithelial cells injury by regulating mitochondrial dysfunction and NLRP3 inflammasome activation. Am J Physiol Renal Physiol 316(2):F396–F407
Bi X et al (2018) MnTBAP treatment ameliorates aldosterone-induced renal injury by regulating mitochondrial dysfunction and NLRP3 inflammasome signalling. Am J Transl Res 10(11):3504–3513
Jiang S et al (2020) Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J Steroid Biochem Mol Biol 206:105789
Yang SK et al (2020) Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed Pharmacother 130:110521
Li S et al (2019) NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp Cell Res 383(1):111488
Gao H et al (2020) Omeprazole attenuates cisplatin-induced kidney injury through suppression of the TLR4/NF-kappaB/NLRP3 signaling pathway. Toxicology 440:152487
Cybulsky AV (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13(11):681–696
Foufelle F, Fromenty B (2016) Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect 4(1):e00211
Liu H, Baliga R (2005) Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16(7):1985–1992
Peyrou M et al (2007) Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci 99(1):346–353
Zhang Y et al (2012) SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis 224(1):43–50
Xu D et al (2014) Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia 97:148–155
Cheng H et al (2015) Leonurine ameliorates kidney fibrosis via suppressing TGF-beta and NF-kappaB signaling pathway in UUO mice. Int Immunopharmacol 25(2):406–415
Liu X et al (2018) Leonurine ameliorates adriamycin-induced podocyte injury via suppression of oxidative stress. Free Radic Res 52(9):952–960
Liu Y et al (2018) Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol 351:1–11
Lieberthal W et al (1996) Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol 270(4 Pt 2):F700–F708
Ramesh G, Reeves WB (2003) TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285(4):F610–F618
Cilenti L et al (2005) Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Renal Physiol 288(2):F371–F379
Park MS et al (2002) Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13(4):858–865
Jiang M et al (2006) Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25(29):4056–4066
Wei Q et al (2007) The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72(1):53–62
Yin X et al (2007) Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J Am Soc Nephrol 18(9):2544–2553
Jiang M et al (2004) Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287(6):F1140–F1147
Kaushal GP et al (2001) Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int 60(5):1726–1736
Nagothu KK et al (2005) Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int 68(6):2680–2693
Wang J et al (2004) Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279(19):19948–19954
Baliga R et al (1997) Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis 29(3):465–477
Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22(47):7265–7279
Liu H, Baliga R (2003) Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int 63(5):1687–1696
Kruidering M et al (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 280(2):638–649
Li S et al (2004) PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286(3):F572–F580
Portilla D et al (2002) Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int 62(4):1208–1218
Brooks C et al (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119(5):1275–1285
Larsson NG et al (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18(3):231–236
Abais JM et al (2015) Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22(13):1111–1129
Zhang Y et al (2014) P2X7 receptor blockade protects against cisplatin-induced nephrotoxicity in mice by decreasing the activities of inflammasome components, oxidative stress and caspase-3. Toxicol Appl Pharmacol 281(1):1–10
Liu M et al (2006) A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol 17(3):765–774
Ramesh G, Reeves WB (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110(6):835–842
Faubel S et al (2007) Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322(1):8–15
Periyasamy-Thandavan S et al (2008) Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74(5):631–640
Yang C et al (2008) Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294(4):F777–F787
Yu X et al (2020) Nuclear receptor PXR targets AKR1B7 to protect mitochondrial metabolism and renal function in AKI. Sci Transl Med 12:543
Zhong Z et al (2016) NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164(5):896–910
Han J et al (2016) Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy 12(12):2326–2343
Qu X et al (2018) Autophagy inhibition-enhanced assembly of the NLRP3 inflammasome is associated with cisplatin-induced acute injury to the liver and kidneys in rats. J Biochem Mol Toxicol 33:e22208
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Wang M, Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529(7586):326–335
Huang Z et al (2020) Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomed Pharmacother 126:110056
Funding
This work was supported by grants from the National Natural Science Foundation of China (81970609); “The Belt and Road international cooperation project” (18410741500) and “Interdisciplinary program of Shanghai Jiao Tong University” (YG2017MS07) and “Science and Technology Innovation Fund of Shanghai Ninth People’s Hospital” (CK2019010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
All the animal experiments were approved by the Animal Care Committee at Shanghai Jiao Tong University and carried out in accordance with institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Sun, Q., Tong, Y. et al. Leonurine attenuates cisplatin nephrotoxicity by suppressing the NLRP3 inflammasome, mitochondrial dysfunction, and endoplasmic reticulum stress. Int Urol Nephrol 54, 2275–2284 (2022). https://doi.org/10.1007/s11255-021-03093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03093-1