Abstract
Recurrent problems of patients with myelofibrosis (MF) are cytopenias, debiliating disease-related symptoms and splenomegaly. Whereas the latter are usually addressed by the JAK1/2 inhibitors ruxolitinib and fedratinib, cytopenias often remain critical. Momelotinib, a JAK1/2 inhibitor recently approved for the treatment of anemic MF patients, was shown to improve anemia via a direct inhibition of activin A receptor type I. In this German-wide, multicenter, retrospective analysis the safety and efficacy profile of momelotinib was evaluated in a real world setting within a cohort of 60 MF patients independent of pre-treatment. The median duration of treatment was 12 weeks. As a new, but manageable safety finding, creatinine increase (CTC°1–2) was detected in 10/60 patients (17%). Interestingly, not only hemoglobin levels increased in 84% of patients, but also platelet values (67%). In the cohort of transfusion-dependent individuals (n = 38), transfusion requirement improved in 15 patients (39%) with 8 reaching transfusion independency (21%). Transfusion independency was achieved within a median of 4 weeks (range 2–12). Spleen size decreased in 13/53 individuals (25%) with a median response time of 6 weeks. Thereof, 11 patients had been pre-treated with JAK inhibitor(s) (85%). Clinical improvement was detected in 24/51 symptomatic individuals (47%) with a median response time of 4 weeks. 5 patients stopped treatment due to side effects (8%), 6 patients due to a worsening of clinical symptoms (10%). Taken together, the MoReLife analysis identifies momelotinib as potent and safe therapeutic option also for heavily pre-treated cytopenic MF patients under real world conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelofibrosis (MF) is a chronic, BCR: ABL1–negative myeloproliferative neoplasm (MPN) that is characterized by bone marrow fibrosis, disease-associated symptoms, splenomegaly, and changes in blood cell count often including progressive anemia. It is a clonal hematopoietic stem-cell disorder and deregulation of the Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) signaling pathway is a hallmark of MF [1]. MF can either present itself de novo (primary MF) or develop through the transformation of essential thrombocythemia (ET, post-ET MF) or polycythemia vera (PV, post-PV MF) [2]. The mechanisms underlying anemia are only partly understood. However, increasing evidence suggests that besides progressive fibrosis in the bone marrow, inflammatory processes disrupt iron distribution, leading to functional iron deficiency, aggravating anemia [3]. Furthermore, anemia is not only caused by the disease itself but is often related to the hematological side effects of therapeutic JAK inhibition [4,5,6]. Here, anemia is dose-dependent and usually managed with dose-reduction and/or blood transfusions.
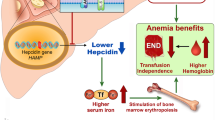
Momelotinib is a selective small molecule JAK1/2 inhibitor. It was recently approved by FDA (Food and Drug Administration) and EMA (European Medicines Agency) for use in patients with MF and anemia regardless of prior therapy. Momelotinib not only inhibits JAK1/2 but also targets the protein receptor kinase activin A receptor type I (ACVR1). Inhibition of ACVR1 leads to a decrease in circulating hepcidin, which is elevated in MF and contributes to anemia [3]. Improving anemia by inhibition of ACVR1 is a critical feature of momelotinib addressing an often unmet medical need [7,8,9,10,11]. The MOMENTUM study [12,13,14] and the analysis of a subpopulation of adult patients with anemia from the SIMPLIFY-1 phase III trial [15] provided the pivotal clinical data leading to drug approval. In MOMENTUM momelotinib was compared to danazol in pre-treated patients and in SIMPLIFY-1 momelotinib was compared to ruxolitinib in JAK Inhibitor (JAKi)-naïve patients. No real-world data for patients on momelotinib treatment have been presented yet.
MoReLife (Momelotinib in Real-Life) is a German-wide, multicenter, retrospective analysis to evaluate the impact of the drug on anemia, transfusion dependency, splenomegaly and total symptom burden in a cohort of 60 cytopenic MF patients under real world conditions. This study, conducted before the drug’s approval, also includes an assessment of its safety profile.
Patients and methods
Sixteen clinical centers experienced in the care of MF patients provided data of a total of 60 patients. Data were collected pseudonymized using a standardized questionnaire (Supplementary Data Fig. 1). Momelotinib was provided by a Global Managed Access Program of the manufacturer (GSK), independently of our investigation. Here, a German-wide Compassionate Use Program (CUP) (protocol no 2,195,954,100,143) had been approved by the Federal Institute for Drugs and Medical Products in April 2023 and all participants had to provide written informed consent. Patients were at least 18 years of age and had to be diagnosed with a high-risk, intermediate-2, or intermediate-1 risk (as defined by the Dynamic International Prognostic Scoring System (DIPPS), or DIPPS-plus [16]) primary MF, post-PV MF or post-ET MF with a disease-related splenomegaly or symptoms and anemia as well as unresponsiveness to or ineligibility for available JAK inhibitor therapy. No washout period for previous treatment was required. Patients with a grade 2 or higher peripheral neuropathy, certain cancers (history or concurrent disease), uncontrolled infectious diseases (including hepatitis and HIV) or intercurrent illnesses that would limit study compliance as judged by the treating physician, or who were eligible for allogenic stem-cell transplantation, were excluded. Pregnant or breastfeeding women were excluded as well as patients with rare hereditary problems of galactose intolerance, lactase deficiency or glucose-galactose malabsorption, a current history of uncontrolled thrombotic or bleeding events, a peripheral blast count ≥ 10% or platelet count ≤ 25 × 109/L as well as patients with instable angina pectoris, symptomatic congestive heart failure or uncontrolled cardiac arrhythmia. Altogether, 98 patients were treated with momelotinib within the CUP and thereof 60 patients analyzed for MoReLife. Declared as an independent project, participation in the MoReLife analysis was offered to all treating physicians and their patients resulting in an inclusion of 60 patients.
As the analysis was retrospective and pseudonymized, the Research Ethics Committee of the Ludwig Maximilians University of Munich confirmed that no additional ethical approval was required. However, each center obtained an informed consent for the participation in this analysis. All data were analyzed descriptively.
Adverse events were described using The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 published in 2017 by the US National Cancer Institute.
Results
Baseline characteristics
Sixty patients (24 females, 36 males) with a median age of 69 years (range 52–84) with primary (39 patients) or secondary (21 patients) myelofibrosis treated with momelotinib were included in the MoReLife analyis (Table 1). The MPN driver mutations JAK2, CALR (Calreticulin) and MPL (myeloproliferative leukemia virus) were analyzed after exclusion of BCR: ABL1 in all patients. In 52 patients, additional molecular analysis had been performed and reported. The inclusion in the analysis was independent of pre-treatment status. In total, 51/60 patients (85%) were pre-treated with one (41/60) or more (10/60) JAKi. Only 9/60 patients (15%) were JAKi-naïve, of which 3 had no pre-treatment at all. Furthermore, 32/60 patients (53%) had been pre-treated with 2 or more regimens and 11 patients (18%) had a history of ≧ 3 lines of therapy as indicated (Table 1). A major inclusion criterion of the CUP was the presence of a clinically relevant anemia as defined by the investigator. No clear cut-off values were defined. The median hemoglobin (Hb) value at first dose was 8.7 g/dl (range 5.4–12.4). Baseline Hb value was ≤ 10 g/dl in 52/60 (87%) patients, 38/60 (63%) patients were transfusion dependent. Hence, transfusion dependency was not defined by a clear Hb cut off level but determined on an individual basis mainly influenced by patients` symptoms and comorbidities. The median platelet count was 115 × 103/µl at baseline (range 27–722).
Safety
Momelotinib has a favorable safety profile also in a real world setting
The mean duration of Momelotinib treatment within the analysis was 16 weeks with a median of 12 weeks (range 0.2–45). At data cut-off 41/60 (68%) patients were still on treatment. At the time of data closure, 34/41 (83% of all remaining patients) took a dosage of 200 mg, 1/41 (2,4%) patient 150 mg, 1/41 (2,4%) patient alternating doses of 100 mg and 200 mg, and 5/41 (12%) patients 100 mg of momelotinib. Hence, 19/60 (32%) patients stopped momelotinib treatment, thereof 5/60 (8%) due to side effects and 6/60 (10%) due to a worsening of symptoms (Table 2). In 3/60 patients (5%) medication was stopped on patient`s request. Detailed reasons for discontinuation are listed in Table 2. One patient died due to uncontrolled myeloproliferative disease after 6 weeks of treatment, one patient developed an MDS/MPN overlap syndrome and was taken off drug. Two patients got eligible for allogenic stem cell transplantation (ASCT) and stopped treatment after 13 and 20 weeks, respectively. One patient had an unexpectedly high increase in Hb values, thus momelotinib was stopped and phlebotomy started.
Creatinine increase is a new safety finding
Altogether, 14 patients (23%) reported no single adverse event. Table 3 shows the percentage of patients who encountered treatment-emergent adverse events. The percentage of adverse events (AE) CTC (Common Toxicity Criteria) grade 3 or 4 was low. Most common AEs CTC° 3 and 4 were hematotoxicity (thrombocytopenia or leukocytopenia), infections, gastrointestinal symptoms (nausea, diarrhea, abdominal pain), and fatigue. Two patients (3%) switched to a reduced dosage of 150 mg and 5 patients (8%) to a dosage of 100 mg due to side effects. As a new finding, creatinine increase (CTC grade 1/2) was detected in 10/60 (16.7%) of all patients with dose adjustment in 3/10 (30%) patients, discontinuation in 2/10 (20%) and no change of medication in 5/11 (50%) patients. In 4 patients, the adverse effect resolved, while in 5 patients, the creatinine increase persisted. Moreover, one patient died due to cardiac failure independently of momelotinib treatment after discontinuation of the medication.
Efficacy
Relevant outcomes in our real world analysis are increase in Hb and platelet values on momelotinib treatment in MF patients and the impact of momelotinib on transfusion dependency, splenomegaly and total symptom burden (Fig. 1).
Shows the change in Hb (A) and platelet values (B) as well as transfusion dependency (C), splenomegaly (D) and total symptom burden (E) on momelotinib treatment. (A) In 55/60 patients the Hb value at baseline and the maximum Hb value reached under treatment were reported. For each patient the delta of the indicated values (g/dl) is shown in one column. Green indicates patients without pre-treatment, yellow indicates that the pre-treatment included one JAKi (ruxolitinib) and orange indicates that pre-treatment included two JAKis (ruxolitinib and fedratinib). (B) In 55/60 patients the platelet value at baseline and the maximum platelet value reached under treatment were reported. One patient with an excessive platelet count > 1000 × 109/l at baseline was excluded from the analysis. The patient had a histologically confirmed myelofibrosis but showed extremely high platelet counts. Here, a reduction of the platelet count from 3170 × 109/l to 1570 × 109/l was detected over time. For each patient the delta of the indicated values (x109/l) is shown in one column. Green indicates patients without pre-treatment, yellow indicates that the pre-treatment includes one JAKi (ruxolitinib) and orange indicates that pre-treatment included two JAKis (ruxolitinib and fedratinib). (C) Transfusion dependency was reported in 38 patients. Worsen of transfusion dependeny was correlated to a value of “-1”, stable to a value of “0”, improvement to “+1” and transfusion independency to “+2”. (D) In 53 patients, relevant splenomegaly was reported. Worsen of splenomegaly was correlated to a value of “-1”, stable to a value of “0” and improvement to “+1”. (E) A relevant symptom burden was reported in 51 patients. Worsening of total symptom burden was correlated to a value of “-1”, stable to a value of “0” and improvement to “+1”
Momelotinib has a positive impact on cytopenia in MF patients independent of pre-treatment
First, we focused on the impact of momelotinib on anemia. The mean baseline hemoglobin value was 8.6 g/dl with a median of 8.7 g/dl (range 5.5–12.4). As shown in Fig. 1A, in the majority of patients (84%), a hemoglobin increase was observed on momelotinib treatment with a mean increase of 1.97 g/dl and a median increase of 1.5 g/dl (range 0.3–9.5). In line with this finding the mean Hb value on momelotinib treatment (maximum value measured) was 10 g/dl with a median of 9,8 g/dl (range 7–16.8).
Compared to other JAKi no decreases in hemoglobin values were detected in patients treated for the first time. In all 3 treatment-naive patients, Hb levels increased. One transfusion-dependent patient reached a stable situation on momelotinib. In the cohort of pre-treated, but JAKi-naïve patients, 5 patients were initially transfusion-dependent, with 2/5 (40% reaching transfusion independency, 1/5 (20%) with an improvement of transfusion dependency and 2/5 (40%) remaining stable on momelotinib. In all 6 JAKi-naïve patients Hb values increased.
Interestingly, in our real-world analysis, also platelet values have been shown to potentially increase upon momelotinib treatment over time. As shown in Fig. 1B, the majority of patients (67%) showed an increase in platelet values on momelotinib treatment over time. The mean increase was 62 × 103/µl with a median of 25 × 103/µl (range − 439 to 1026). However, an initial decrease was reported in 76% patients. The mean platelet value at baseline was 185 × 103/µl with a median of 115 × 103/µl (range 27–722). Platelet values ≤ 50 × 103/µl were detected in 11/60 (18%) patients. The mean value of the minimum platelet values reported under treatment was 149 × 103/µl with a median of 92 × 103/µl (range 3–506). The mean value of the maximum platelet values reported under treatment was 258 × 103/µl with a median of 166 × 103/µl (range 26–1469). As shown in Fig. 1B in 15 patients the maximum platelet value on momelotinib treatment was below baseline. In 36 patients the maximum platelet value was higher as baseline value. Thereof, 9 patients had a decrease in spleen volume.
Momelotinib improves transfusion dependency also in heavily pre-treated patients
As shown in Fig. 1C, in the cohort of transfusion-dependent individuals (n = 38), transfusion requirement improved in 15/38 (39%) patients and 8/38 (21%) patients became transfusion independent within median 4 weeks (range 2–12). An improvement of the transfusion frequency was also reached within median 4 weeks (range 2–6) in those patients not reaching transfusion independency.
In patients with an amelioration of transfusion dependency, the final dosage of momelotinib at data closure was 200 mg in 10/15 (66%) patients, 100 mg in 1/15 (7%) patient and 100 mg/200 mg alternating in another patient (7%). Three of the 15 patients (20%) discontinued treatment, 1 due to an exorbitant increase in hemoglobin, 1 due to side effects and 1 due to an increase in symptom burden.
Momelotinib treatment ameliorates splenomegaly and total symptom burden
Within our analysis splenomegaly and symptom burden were not analyzed as detailed as the anemia response. This is mainly due to the real world setting and the retrospective nature of our analysis. The investigators documented whether splenomegaly and total symptom burden were relevant findings and whether a worsening, a stable finding or an improvement was detected during momelotinib treatment. The results are shown in Fig. 1D. In 13/53 (25%) individuals with splenomegaly the spleen size decreased. Thereof 8/13 patients (62%) were pre-treated with one JAKi (ruxolitinib) and 3/13 patients (23%) with ruxolitinib and fedratinib. The median time to improvement was 5.7 weeks (range 2–13).
A relevant symptom burden was present in 51/60 patients (85%). As shown in Fig. 1E, an improvement of symptoms on momelotinib was detected in 24/51 (47%). In 18 of these 24 patients (75%) pre-treatment included one JAKi (ruxolitinib) and in 3 patients (17%) also fedratinib. The median time to improvement was 4 weeks (range 2–11).
Individual treatment journeys illustrate the diversity of the disease and the impact of momelotinib despite various pre-treatments
To illustrate the efficacy of momelotinib, detailed discussions of the individual courses of three patients follow. The first patient (Fig. 2) is an example for achievement of a sustained transfusion independency in a pre-treated patient.
Hemoglobin (g/dl), platelet and leucocyte values (10e9/l) over time (days) in a 64-year-old male patient on Momelotinib treatment. Figure 1 shows the course of hemoglobin levels of a 64-year-old male patient with post-ET-MF after initiation of momelotinib treatment. The patient had been pre-treated with hydroxyurea and ruxolitinib (last dosage 15 mg BID). Because of progressive anemia the patient received erythropoietin at a dosage of 10,000 units/week until 05/23 and a total number of 16 red blood cell units from 08/22 to 09/23.After start of Momelotinib at a daily dosage of 150 mg, 12/23 dosage was increased up to 200 mg due to a favorable toxicity profile. His hemoglobin values increased from 8 to 12 g/dl within 36 days, reaching and maintaining transfusion independency. Also, platelet values increased. Total symptom burden improved and splenomegaly remained stable
Figure 3 illustrates the efficacy and the safety profile of momelotinib and how to handle adverse events and dose modifications in an older patient with primary myelofibrosis. Despite infectious complications and hematotoxicity a long-term benefit was reached by careful titration of the drug
Hemoglobin (g/dl), platelet and leucocyte values (10e9/l) over time (days) in a 70 year old male patient on Momelotinib treatment (mg/day). Adverse events leading to an interruption of therapy as shown in white. He received ruxolitinib treatment (15 mg/day), and following two red blood cell transfusions, his hemoglobin level increased to 11.2 g/dl. We subsequently transitioned him to momelotinib on 06/16/23, which led to a further increase in his hemoglobin to 13 g/dl. On 08/10/23 the patient developed fever leading to a momelotinib treatment interruption for 3 weeks which was then reinitiated on 08/28/23. On 09/22/23 another infection necessitated a second treatment interruption. On 10/31/23, treatment was resumed with a dosage of 100 mg/day, followed by an alternating dosage of 100/200 mg/day starting from 11/28/23. Under this regimen the patient’s hemoglobin value was above 12 g/dl not needing any further transfusions. His spleen volume decreased considerably from 2280 to 1600 ml. Platelets initially dropped from 114.000 to 68.000 and rose after dose modification and overcoming the infection to 159.000/µl
The third patient (Fig. 4) is an example for a patient with a markedly hyperproliferative disease and a relevant increase of hemoglobin on momelotinib treatment even leading to phlebotomy.
Hemoglobin (g/dl), platelet and leucocyte values (10e9/l) over time (days) in a 81-year-old female patient on Momelotinib and Hydroxyurea treatment (mg/day). Figure 3 shows the treatment response in this 81-year-old female patient with post-PV-MF and multiple lines of previous therapies. At baseline she was transfusion-dependent and received hydroxyurea due to elevated white blood cell counts. Over time the hemoglobin values increased to 16.8 g/dl leading to a subsequent reduction in the momelotinib dosage and eventually its discontinuation. Phlebotomy was started as indicated by the arrow. Hemoglobin values remained high even without momelotinib. Hydroxyurea was given at a tailored dosage depending on leukocyte and platelet values. On momelotinib treatment, total symptom burden and splenomegaly remained stable
Discussion and conclusions
Anemia plays a pivotal role in patients with myelofibrosis (MF). At the time of first diagnosis about 40% of patients have a moderate to severe anemia. Moreover, during the course of the disease, nearly all patients develop low hemoglobin levels [17,18,19]. Furthermore, hematological toxicity is one of the main reasons for the discontinuation of JAKi treatment [20]. Until now, there were only very limited options available to treat anemia in patients with myelofibrosis such as red blood cell transfusions or off-label use of erythropoietin stimulating agents. Improving anemia by inhibition of ACVR1 is therefore a critical feature of momelotinib addressing an unmet medical need [7,8,9,10, 21]. Long-term outcomes will be especially interesting, as anemia is a negative prognostic factor in patients with MF [22] and transfusion-dependency is associated with a poor prognosis and shortened survival [23,24,25,26,27,28]. In addition, iron overload has a negative impact on hematopoiesis [29]. Noteworthy, also the JAK2/IRAK1 inhibitor pacritinib targets the same kinase resulting in an anemia benefit [30].
The pivotal clinical trials MOMENTUM and SIMPLIFY-1 have shown a high efficacy of momelotinib in MF patients especially focusing on the group of patients with transfusion dependency [5, 15]. In JAKi naïve patients, the impact of momelotinib on splenomegaly was comparable to ruxolitinib (reduction in spleen size at week 24: 26.5% vs. 29%), whereas ruxolitinib had a higher impact on symptom burden (42.2% compared to 28.4%) defined as a ≥ 50% reduction in the total symptom score. However, the transfusion rate and transfusion independency were significantly improved by momelotinib compared to ruxolitinib [15]. In the SIMPLIFY-2 study, 43% of patients achieved transfusion independency at week 24 compared to 21% with best available therapy (BAT) [31]. BAT included ruxolitinib, chemotherapy, anagrelide, corticosteroids, hematopoietic growth factors, immunomodulating agents, androgen, interferon-α, or no treatment. The authors discussed that most commonly ruxolitinib was merely continued. Transfusion independency was defined as the absence of red blood cell transfusions and Hb levels > 8 g/dL in the previous 12 weeks. However, the use in MF patients not participating in clinical studies has been only poorly described. Often, the “real world” is a strong contrast to the highly controlled environment of clinical trials, which often have narrow, predefined patient criteria. Here, efficacy and safety of drugs are analyzed in a pre-selected, homogeneous cohort often lacking relevant comorbidities. Therefore, the aim of our study was to analyze the impact of momelotinib treatment on anemia, transfusion dependency, splenomegaly and symptom burden as well as a thorough analysis of side effects in MF patients in a real-world scenario independently of pre-treatment.
Overall, in this limited number of patients, momelotinib had an acceptable safety profile comparable to other known JAK inhibitors. An increase in creatinine values was detected in 17% of patients and classified as a relevant, but manageable new safety finding. As this side effect was not described in detail before, we would recommend close monitoring for the first couple of weeks after starting momelotinib. In the SIMPLIFY-1 study (comparing momelotinib with ruxolitinib), the most common adverse reactions were thrombocytopenia, hemorrhage, bacterial infections, fatigue, dizziness, diarrhea, and nausea [15]. Here, the safety profile was comparable to the pivotal clinical trials, but no life-threatening bleeding events were reported. In one patient weight gain, needle-like irregular pain, rash, lethal candida sepsis, hyperuricemia, and hypertensive derailment were reported. Interestingly, two patients underwent stem cell transplantation after momelotinib treatment. They are both alive (as reported at day + 250 and + 237 respectively) and in good general conditions.
Hb increase (mean 2 g/dl) was detected in the majority of patients (84%) independently of pre-treatment. A Hb value < 10 g/dl was recommended as cut-off value for treatment initiation. The baseline mean Hb value was 8.6 g/dl. Therefore, the mean hemoglobin value in our cohort was lower than in SIMPLYFY-1/2, respectively This might be one reason why the rate of patients reaching transfusion independency in our analysis was lower (21%). Duration of Momelotinib treatment might also impact achievement of transfusion independency: trials were designed with a primary endpoint to be reached with a treatment period of 24 weeks [12, 15, 31]. Our analysis has a significantly shorter follow up with a median duration of Momelotinib treatment of 12 weeks (range 0.2–45). Transfusion independency was reached after median 4 (range 2–12) weeks. We therefore hypothesize, that a 12-week observational period might be sufficient for efficacy analysis per se but additional cases of transfusion independency are expected after completion of a 12 week treatment period in all patients with improvement in transfusion dependency. However, final conclusions regarding momelotinib impact on transfusion need are clearly limited in our analysis due to a potentially heterogeneous definition of “transfusion-dependency” as defined by physician´s discretion.
The anemia benefit might also be underestimated in some patients as colleagues reported that the baseline Hb value had been measured after transfusion of red blood cells and stable values were reached without further transfusions.
Interestingly, Hb values increased in all 3 patients without prior treatment and in all 6 pre-treated but JAK2 inhibitor naïve individuals. These findings support the potential role of Momelotinib as JAKi first-line treatment in anemic patients.
Noteworthy, momelotinib seemed to have a positive long-term effect on thrombocytopenia in selected cases (67%). However, thrombocytopenia was also one of the most common adverse events leading to dose modification or treatment interruption. Thrombocytopenia CTC°1 was reported in 4 patients, CTC°2 for 3 patients, CTC°3 for 11 patients and CTC°4 for 4 patients. Partly, this might be explained by the high number of pre-treated patients with preexisting thrombocytopenia at baseline with > 25 × 109/µl platelet counts being sufficient for participation.
Momelotinib also had an impact on splenomegaly and symptom burden even in pre-treated patients. In 13/53 (25%) individuals with splenomegaly, the spleen size decreased, the majority (85%) being treated with ≧ 1 JAK-inhibitor. Again, as the mean time to improvement was 5.7 weeks with a minimum of 2 weeks and a maximum of 13 weeks, we hypothesize that a period of 12 weeks should be sufficient enough to evaluate therapeutic effects. However, this analysis lacks standardized assessment methods for spleen size reduction as they have been used in the aforementioned clinical trials. Furthermore, rapid clinical improvement on Momelotinib was observed in 47% of patients. In the cohort of symptomatic, JAK2 inhibitor pre-treated patients, symptom burden also rapidly improved in 41% patients.
In a recent subgroup analysis, Tefferi et al. analyzed the impact of CALR type 1/like mutation as predictive factor of survival and longevity without transplant on momelotinib therapy [32]. More biomarkers are clearly required to predict momelotinib response on hemoglobin value. In our cohort, ferritin values were collected in a number of patients. However, mainly due to the small number of the data set no predictive marker could be identified. Interestingly, the female patient reaching extremely high Hb values on momelotinib with need for phlebotomy had a high JAK2 V617F variant allele frequency of 97%. Therefore, a more thorough analysis of these molecular markers seems to be worth striving for in future.
In summary, our MoReLife analysis confirms that momelotinib is a safe and effective therapeutic option in daily practice in treatment-naïve and pre-treated cytopenic myelofibrosis patients in a limited number of patients treated within a real-life scenario.
A treatment period of 12 weeks should be sufficient enough to evaluate the impact on anemia, splenomegaly and symptom burden. Although transfusion independency may not be reached in all patients, we hypothesize, that improvement of anemia has a beneficial effect on the quality of life and disease outcome. Patient education has an important impact on compliance as some patients stopped treatment due to side effects such as nausea or ruxolitinib withdrawal syndrome just after a few days. Close monitoring of platelets is mandatory as the majority of patients showed an initial drop in platelet values. Here, it might be of special interest, that the number of patients with thrombocytopenia was limited and no severe bleeding events were reported (Table 3). Epistaxis CTC I° was reported only in 2 patients (3.3%) (Table 3). Both patients had a thrombocytopenia IV°. While one patient finally discontinued momelotinib due to a number of side effects including thrombocytopenia CTC° IV, epistaxis CTC° I, nausea CTC° I, infection CTC° I and increase in creatinine CTC° I, the other continued momelotinib treatment with a dosage of 200 mg without any further complications and was still under treatment at data cut-off.
Data availability
No datasets were generated or analysed during the current study.
References
Vainchenker W, Kralovics R (2017) Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 129(6):667–679. https://doi.org/10.1182/blood-2016-10-695940
Pizzi M, Croci GA, Ruggeri M, Tabano S, Dei Tos AP, Sabattini E, Gianelli U (2021) The classification of Myeloproliferative neoplasms: Rationale, historical background and future perspectives with focus on unclassifiable cases. Cancers (Basel) 13(22). https://doi.org/10.3390/cancers13225666
Birgegard G, Samuelsson J, Ahlstrand E, Ejerblad E, Enevold C, Ghanima W, Hasselbalch H, Nielsen CH, Knutsen H, Pedersen OB, Sorensen A, Andreasson B (2019) Inflammatory functional iron deficiency common in myelofibrosis, contributes to anaemia and impairs quality of life. From the nordic MPN study Group. Eur J Haematol 102(3):235–240. https://doi.org/10.1111/ejh.13198
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366(9):787–798. https://doi.org/10.1056/NEJMoa1110556
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH Jr., Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366(9):799–807. https://doi.org/10.1056/NEJMoa1110557
Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, Sarlis NJ, Peng W, Sandor V, Gopalakrishna P, Hmissi A, Stalbovskaya V, Gupta V, Harrison C, Verstovsek S, Investigators C (2015) A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica 100(9):1139–1145. https://doi.org/10.3324/haematol.2014.119545
Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM, Druker BJ, Burns CJ, Fantino E, Deininger MW (2010) CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 115(25):5232–5240. https://doi.org/10.1182/blood-2009-05-223727
Gupta V, Mesa RA, Deininger MW, Rivera CE, Sirhan S, Brachmann CB, Collins H, Kawashima J, Xin Y, Verstovsek S (2017) A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica 102(1):94–102. https://doi.org/10.3324/haematol.2016.148924
Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, Hogan WJ, Litzow MR, Leontovich A, Kowalski M, Tefferi A (2013) Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 27(6):1322–1327. https://doi.org/10.1038/leu.2013.71
Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, Posch W, Nairz M, Maciejewski P, Fowles P, Burns CJ, Smith G, Wagner KU, Weiss G, Whitney JA, Theurl I (2017) Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood 129(13):1823–1830. https://doi.org/10.1182/blood-2016-09-740092
Gupta V, Oh S, Devos T, Dubruille V, Catalano J, Somervaille TCP, Platzbecker U, Giraldo P, Kosugi H, Sacha T, Mayer J, Illes A, Ellis C, Wang Z, Gonzalez Carreras FJ, Strouse B, Mesa R (2024) Momelotinib vs. ruxolitinib in myelofibrosis patient subgroups by baseline hemoglobin levels in the SIMPLIFY-1 trial. Leuk Lymphoma 1–13. https://doi.org/10.1080/10428194.2024.2328800
Verstovsek S, Gerds AT, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, Grosicki S, Iurlo A, Goh YT, Lazaroiu MC, Egyed M, Fox ML, McLornan D, Perkins A, Yoon SS, Gupta V, Kiladjian JJ, Granacher N, Lee SE, Ocroteala L, Passamonti F, Harrison CN, Klencke BJ, Ro S, Donahue R, Kawashima J, Mesa R, Investigators MS (2023) Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet 401(10373):269–280. https://doi.org/10.1016/S0140-6736(22)02036-0
Gerds AT, Verstovsek S, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, Grosicki S, Iurlo A, Goh YT, Lazaroiu MC, Egyed M, Fox ML, McLornan D, Perkins A, Yoon SS, Gupta V, Kiladjian JJ, Granacher N, Lee SE, Ocroteala L, Passamonti F, Harrison CN, Oh S, Klencke BJ, Yu J, Donahue R, Kawashima J, Mesa R (2023) Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis previously treated with a JAK inhibitor (MOMENTUM): an updated analysis of an international, double-blind, randomised phase 3 study. Lancet Haematol 10(9):e735–e746. https://doi.org/10.1016/S2352-3026(23)00174-6
Verstovsek S, Mesa R, Gupta V, Lavie D, Dubruille V, Cambier N, Platzbecker U, Hus M, Xicoy B, Oh ST, Kiladjian JJ, Vannucchi AM, Gerds A, Egyed M, Mayer J, Sacha T, Kawashima J, Morris M, Huang M, Harrison C (2023) Momelotinib long-term safety and survival in myelofibrosis: integrated analysis of phase 3 randomized controlled trials. Blood Adv 7(14):3582–3591. https://doi.org/10.1182/bloodadvances.2022009311
Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, McLornan D, Shimoda K, Winton EF, Deng W, Dubowy RL, Maltzman JD, Cervantes F, Gotlib J (2017) SIMPLIFY-1: a phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol 35(34):3844–3850. https://doi.org/10.1200/JCO.2017.73.4418
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Mesa RA, Demory JL, Barosi G, Rumi E, Tefferi A (2009) New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 113(13):2895–2901. https://doi.org/10.1182/blood-2008-07-170449
Tefferi A, Lasho TL, Jimma T, Finke CM, Gangat N, Vaidya R, Begna KH, Al-Kali A, Ketterling RP, Hanson CA, Pardanani A (2012) One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc 87(1):25–33. https://doi.org/10.1016/j.mayocp.2011.11.001
Scherber RM, Mesa RA (2020) Management of challenging myelofibrosis after JAK inhibitor failure and/or progression. Blood Rev 42:100716. https://doi.org/10.1016/j.blre.2020.100716
Bassiony S, Harrison CN, McLornan DP (2020) Evaluating the Safety, Efficacy, and therapeutic potential of Momelotinib in the treatment of Intermediate/High-Risk myelofibrosis: evidence to date. Ther Clin Risk Manag 16:889–901. https://doi.org/10.2147/TCRM.S258704
Kuykendall AT, Shah S, Talati C, Al Ali N, Sweet K, Padron E, Sallman DA, Lancet JE, List AF, Zuckerman KS, Komrokji RS (2018) Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol 97(3):435–441. https://doi.org/10.1007/s00277-017-3194-4
Naymagon L, Mascarenhas J (2017) Myelofibrosis-related Anemia: current and emerging therapeutic strategies. Hemasphere 1(1):e1. https://doi.org/10.1097/HS9.0000000000000001
Elena C, Passamonti F, Rumi E, Malcovati L, Arcaini L, Boveri E, Merli M, Pietra D, Pascutto C, Lazzarino M (2011) Red blood cell transfusion-dependency implies a poor survival in primary myelofibrosis irrespective of IPSS and DIPSS. Haematologica 96(1):167–170. https://doi.org/10.3324/haematol.2010.031831
Chifotides HT, Bose P, Verstovsek S (2022) Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J Hematol Oncol 15(1):7. https://doi.org/10.1186/s13045-021-01157-4
Tefferi A, Hudgens S, Mesa R, Gale RP, Verstovsek S, Passamonti F, Cervantes F, Rivera C, Tencer T, Khan ZM (2014) Use of the Functional Assessment of Cancer therapy–anemia in persons with myeloproliferative neoplasm-associated myelofibrosis and anemia. Clin Ther 36(4):560–566. https://doi.org/10.1016/j.clinthera.2014.02.016
Tefferi A (2021) Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 96(1):145–162. https://doi.org/10.1002/ajh.26050
Rumi E, Trotti C, Vanni D, Casetti IC, Pietra D, Sant’Antonio E (2020) The genetic basis of primary myelofibrosis and its clinical relevance. Int J Mol Sci 21(23). https://doi.org/10.3390/ijms21238885
How J, Hobbs GS (2020) A practical guide for using myelofibrosis prognostic models in the clinic. J Natl Compr Canc Netw 18(9):1271–1278. https://doi.org/10.6004/jnccn.2020.7557
Nicolosi M, Mudireddy M, Lasho TL, Hanson CA, Ketterling RP, Gangat N, Pardanani A, Tefferi A (2018) Sex and degree of severity influence the prognostic impact of anemia in primary myelofibrosis: analysis based on 1109 consecutive patients. Leukemia 32(5):1254–1258. https://doi.org/10.1038/s41375-018-0028-x
Griffiths EA (2024) Transfusion avoidance in myelodysplastic neoplasms. Curr Opin Hematol 31(2):40–46. https://doi.org/10.1097/MOH.0000000000000794
Oh ST, Mesa RA, Harrison CN, Bose P, Gerds AT, Gupta V, Scott BL, Kiladjian JJ, Lucchesi A, Kong T, Buckley SA, Tyavanagimatt S, Harder BG, Roman-Torres K, Smith J, Craig AR, Mascarenhas J, Verstovsek S (2023) Pacritinib is a potent ACVR1 inhibitor with significant anemia benefit in patients with myelofibrosis. Blood Adv 7(19):5835–5842. https://doi.org/10.1182/bloodadvances.2023010151
Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, Passamonti F, Winton EF, Dong H, Kawashima J, Maltzman JD, Kiladjian JJ, Verstovsek S (2018) Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol 5(2):e73–e81. https://doi.org/10.1016/S2352-3026(17)30237-5
Tefferi A, Pardanani A, Begna KH, Al-Kali A, Hogan WJ, Litzow MR, Ketterling RP, Reichard KK, Gangat N (2024) Calr type 1/like mutation in myelofibrosis is the most prominent predictor of momelotinib drug survival and longevity without transplant. Blood Cancer J 14(1):51. https://doi.org/10.1038/s41408-024-01028-4
Acknowledgements
We thank Konstanze Pechloff for critical review.
Funding
There was no funding.
Author information
Authors and Affiliations
Contributions
SJ and PEP conceived the project, contributed, collected and analyzed the clinical data and wrote the paper.All other authors (JS, KS, CCC, VB, FS, MJ, DS, MM, SF, DG, MC, LLT, FH, HKA) contributed clinical data, gave conceptional advice, read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
As the analysis was retrospective and pseudonymized, the Research Ethics Committee of the Ludwig Maximilians University of Munich confirmed that no additional ethical approval was required. However, each center obtained an informed consent for the participation in this analysis. All data were analyzed descriptively.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jilg, S., Schwaab, J., Sockel, K. et al. MoReLife – real-life data support the potential of momelotinib as a safe and effective treatment option for cytopenic myelofibrosis patients. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05908-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05908-4