Abstract
The use of Bcl-2 inhibitor Venetoclax (VEN) combined with hypomethylating agents or chemotherapy has shown efficacy in treating acute myeloid leukemia (AML) as frontline treatment and for relapse, allowing more patients to bridge to allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, the influence of VEN-based therapy on the prognosis of subsequent allogeneic HSCT remains unknown. We retrospectively collected data from patients who proceeded to allo-HSCT between November 2018 and November 2020 after VEN-based therapy at five transplant centers in Zhejiang Province, China. A total of 39 patients were analyzed. Thirty-one patients were diagnosed with AML (28 de novo, 3 secondary to MDS), 6 with MDS, and 2 with CMML. The majority (74.4%) of patients received VEN-based therapy for the treatment of relapse (38.5%) or refractory disease (35.9%); 5 (12.8%) received it as an initial treatment, and 5 (12.8%) patients who were already in complete remission (CR) received VEN for further consolidation or deep remission before HSCT. Twenty-seven (69.2%) patients were in CR at the time of HSCT. Day + 100 cumulative incidences of grade I–IV acute graft-versus-host disease (aGVHD) and grade II–IV aGVHD were 43.6% and 15.4%, respectively. Of 34 evaluable patients, 6.4% and 25.6% developed chronic GVHD at 1 year and 2 years. The 100-day cytomegalovirus (CMV) reactivation occurred in 76.3% of patients and Epstein-Barr virus (EBV) reactivation occurred in 29.7% of patients. With a median follow-up of 14.7 months, overall survival, progression-free survival, relapse, and non-relapse mortality incidence at 1 year were 75.5%, 61.6%, 16.7%, and 21.7%, respectively. Both univariate and multivariate analysis revealed that relapsed/refractory (R/R) disease was associated with inferior PFS (HR 4.849, 95% CI 1.009–23.30; p = 0.049). Prior poor response to VEN was found to be a significant factor predicting higher risk of relapse (HR 4.37, 95% CI 1.130–16.9; p = 0.033). Our results showed that VEN-based regimen therapy followed by allo-HSCT in AML patients is feasible and does not increase the risk of transplant-related mortality and toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-cell leukemia/lymphoma-2 (BCL-2) is the main anti-apoptotic protein and is frequently overexpressed in various hematologic malignancies (HMs) to inhibit tumor cell apoptosis [1, 2]. Venetoclax (VEN), also called ABT-199, is a highly selective small-molecule Bcl-2 inhibitor, which facilitates tumor cell apoptosis by freeing pro-apoptotic proteins and thereby promoting mitochondrial outer membrane permeabilization and release of caspases [3]. Compared with conventional treatments, it is relatively safer with less toxic effects. The combination of the selective Bcl-2 inhibitor VEN and hypomethylating agents (VEN-HMA) has marked activity in acute myelogenous leukemia (AML) in both the de novo and relapsed/refractory (R/R) settings [4,5,6,7,8]. This combination was considered as frontline therapy for elderly or patients unfit for conventional chemotherapy and produced a complete remission (CR) + CR with incomplete count recovery (CRi) of 66.4% in the pivotal trial that led to its approval [5]. Since then, VEN-based therapy in HMs including myelodysplastic syndromes (MDS), multiple types of leukemia, and B cell malignancies has been increasingly applied [9,10,11,12,13]. Given the fact that allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the sole curative option for leukemia, the high CR rates achieved with VEN sole or in combination therapies would be expected to allow more patients to proceed to allo-HSCT with curative intent. However, considering that the major toxicity of the VEN is peripheral blood cytopenia, the potential for increased risk of infections or transplant-related complications after allo-HSCT is a concern.
Herein, our study aims to evaluate the potential carry-over effect of VEN pretreatment on outcomes of subsequent allo-HSCT in patients diagnosed with AML and MDS. We focused on early transplant outcomes including engraftment, the incidence of graft-versus-host-disease (GVHD), OS, relapse, and non-relapse mortality (NRM) throughout the first year.
Materials and methods
Patients and venetoclax-based therapy
The study was conducted in five transplant centers in Zhejiang Province, China. Patients who received VEN before the first transplantation at five centers between November 2018 and November 2020 were enrolled. Baseline information and transplant data from patients who fulfilled the eligibility criteria for this study were collected. The study was approved by the institutional review board at each participating center, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
The doses of VEN in each cycle depend on the hematologic and clinical toxicities, and the duration is mainly based on the response evaluated by bone marrow examination and hematological examinations during their treatment period, generally following the regimens used in clinical trials. For HMA drugs as part of combination therapies, the doses of azacytidine and decitabine were 75 mg/m2 (d1 to d7) and 20 mg/m2 (d1 to d5). Whether to use prophylactic antifungal therapy depends on the patient’s condition.
HSCT
The conditioning regimen was defined as myeloablative or reduced-intensity regimens, which patients choose based on the age at HSCT, performance status, co-morbidities, and prior treatment strategies. For patients with a matched sibling donor (MSD) or a matched unrelated donor (MUD), the main myeloablative conditioning regimen was BuCy (busulfan 3.2 mg/kg/day i.v. on days − 7 to − 4 and cyclophosphamide 60 mg/kg/day i.v. on days − 3 to − 2). Rabbit antithymocyte globulin (ATG, Thymoglobulin; Genzyme, Cambridge, MA) was also administered to patients undergoing MUD HSCT (4.5 to 6 mg/kg total dose). For recipients of HLA-haploidentical related donor (HRD) HSCT, the conditioning regimen was Ara-BuCy-Me-CCNU-ATG, which included cytarabine (4 g/m2/day i.v. on days − 10 to − 9), Bu (3.2 mg/kg/day i.v. on days − 8 to − 6), Cy (60 mg/kg/day i.v. on days − 5 to − 4), Me-CCNU (250 mg/m2 orally on day -3), and anti-T lymphocyte globulin (ATG-F; Fresenius, Bad Homburg, Germany) (2.5 mg/kg/day i.v. on days − 5 to − 2) or rabbit ATG (1.5 mg/kg/day i.v. on days − 5 to − 2). Reduced-intensity conditioning (RIC) included Flu-Bu-ATG regimen (fludarabine 30 mg/m2/day i.v. on days − 10 to d − 5, busulfan 3.2 mg/kg/day i.v. on days − 6 to − 5, ATG 5 mg/kg/day i.v. on days − 4 to − 1). The EBV- and CMV-DNA loads in the blood were measured regularly using real-time quantitative polymerase chain reaction and monitored weekly for the first 3 months after HSCT, every 2 weeks from the fourth month to the sixth month after transplantation, and then monthly from the seventh month to the 12th month. The threshold for EBV-DNA and CMV-DNA copies provided by the manufacturer (ZJBio-Tech, Shanghai, China) was 500 copies/mL. Ganciclovir was administered when CMV-DNA in the blood was found to be positive. Absolute neutrophil count (ANC) > 0.5 × 109/L and platelet count > 20 × 109/L without platelet transfusions were defined as neutrophil and platelet recovery, respectively.
GVHD prophylaxis consisting of cyclosporin A (CSA), short-term methotrexate (MTX), and mycophenolate mofetil was performed as described previously [14]. Patients who survived ≥ 100 days were analyzed for chronic GVHD (cGVHD). Acute and chronic GVHD were evaluated according to the National Institutes of Health consensus guidelines [15]. Minimal residual lesion (MRD) was monitored by institutional standards according to established methods and MRD negativity was defined as MRD levels < 0.01%.
Definitions and statistical analysis
Remission criteria were assessed by the International Working Group (IWG) response criteria [16, 17]. Patients with a failure to achieve CR after 2 courses of induction or an insufficient response to the first induction—defined as a less than 50% proportional reduction in blasts and the presence of more than 15% blasts—were classified as having primary refractory disease [18]. OS was calculated from the day of allo-HSCT to the last follow-up visit or death from any cause. Progression-free survival (PFS) was defined as the time from HSCT to disease relapse/progression or death from any cause, whatever came first. Relapse was defined as disease relapse/progression after HSCT. NRM was defined as any death without relapse or progression after HSCT.
The final data cutoff for this study was September 14, 2021. Follow-up time was estimated by the reverse Kaplan–Meier method. Probabilities of OS and PFS were calculated by the Kaplan–Meier method and compared using the log-rank test for univariate analysis. All significant factors (P < 0.20) from the univariate analysis were included in the multivariate analysis calculated using Cox regression models. Cumulative incidence curves were used for relapse and NRM since death and relapse are competing events. Acute and chronic GVHD were estimated using cumulative incidence with death as a competing event. Univariate comparisons were done using Gray’s test for relapse, NRM, and GVHD, while a competing-risk regression model was performed for multivariate analysis. All statistical analyses were performed by SPSS R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 39 patients who had been exposed to VEN-based therapy before allo-HSCT were enrolled. The cohort had a median age of 46.2 years (ranging, 12.8 to 62.6 years), and the majority (n = 20, 51.3%) were male. Thirty-one patients were diagnosed with AML, including 28 de novo and 3 secondary to MDS. Six patients were diagnosed with MDS (1 with MDS-RAEB-I, 5 with MDS-RAEB-II) and 2 with chronic myelomonocytic leukemia (CMML). Among MDS patients, 5 (83.3%) were classified as high or very high risk based on IPSS-R.
Venetoclax therapy
The majority (n = 29, 74.4%) of patients received VEN for treatment of relapse (n = 15, 38.5%) or refractory diseases (n = 14, 35.9%) with a median of 3 cycles of prior chemotherapy (range, 1–9 cycles); 5 (12.8%) elderly patients who were unable to tolerate high-intensity chemotherapy received VEN as an initial treatment, and 5 (12.8%) patients who were already in CR received VEN for further consolidation or deep remission before HSCT considering previous chemotherapy toxicity and complications. Except for the one patient receiving VEN monotherapy, all other patients were treated with VEN-based combination therapy, with a median of 1 course (range, 1 to 6) and median duration of 28 days. Most patients received azacytidine in combination with VEN (n = 32, 76.9%), including 30 patients treated with VEN + azacytidine alone and 2 combined with azacytidine plus chemotherapy. Other VEN combination options included decitabine (n = 1, 2.6%), chemotherapy (n = 4, 10.2%), and sorafenib (n = 1, 2.6%).
Among 39 patients receiving VEN-based therapy, 30 patients (76.9%) had CR or CR with CRi, 3 patients (7.7%) PR, and 6 patients (15.4%) NR. Of the R/R 29 patients, 21 reached CR/CRi (72.4%), 2 PR (6.9), and 6 NR (20.7%). Among the 5 newly diagnosed patients, 4 achieved CR/CRi and 1 achieved PR. Details on patient characteristics are summarized in Table 1.
HSCT
Among 30 patients achieving CR/CRi after VEN-based therapy, 7 relapsed prior to HSCT, with a median time to relapse of 2.6 months (range, 0.5 to 5.7 months). Four of them received chemotherapy and achieved CR again before transplant, 2 failed to achieve CR after chemotherapy, and 2 proceeded directly to salvage HSCT. One MDS patient progressed to AML after 5.3 months in remission with VEN-based therapy and then underwent VEN combined with chemotherapy, achieving CR again before HSCT. The remission status and treatment process between VEN-based therapy and HSCT are presented in Fig. 1. The median time from the first initiation of VEN-based therapy to allo-HSCT was 3.1 months (range, 0.9 to 10.8 months). At the time of HSCT, 27 of the 39 patients were in CR (69.2%), 2 were in PR (5.1%), and 10 were in NR (25.7%). Of the 27 patients achieving CR, 20 were in MRD-negative CR and 7 were with MRD positivity pre-transplant.
Thirty patients (76.9%) underwent HRD HSCT, 5 (12.8%) underwent MUD HSCT, and 4 (8.9%) received MSD HSCT. Conditioning regimens included 32 (82.1%) myeloablative and 7 (17.9%) reduced-intensity. The median number of infused mononuclear cells and CD34 + cells was 9.5 × 108/kg (range, 1.7 to 31.4 × 108/kg) and 6.4 × 106/kg (range, 1.2 to 22.6 × 106/kg), respectively. The transplant information is shown in Table 2.
Engraftment, GVHD, and infection
Except for one patient who died at 3 days post-HSCT, all recipients achieved neutrophil engraftment with a median time of 12 days (range, 10–21 days). Thirty-six patients achieved platelet engraftment with a median time of 14 days (range, 10–28 days); one patient experienced primary platelet engraftment failure and two did not achieve platelet recovery due to early deaths. At day + 100, cumulative incidences of aGVHD I–IV and aGVHD II–IV were 43.6% and 15.4%, respectively (Fig. 2A and B). Of 34 evaluable patients, 16.4% and 25.6% developed chronic GVHD at 1 year and 2 years (Fig. 2C).
Sixteen patients (41.0%) had 22 episodes of infection after transplantation. Pneumonia was most frequently observed (n = 12), followed by urinary tract infections (n = 5, 4 cystitis, and 1 infected by Pseudomonas aeruginosa). Two patients experienced intracranial infection caused by human herpesvirus 6 (HHV-6, 0.8 months from HSCT) and Nocardia farcinica (5.8 months from HSCT), respectively. Bloodstream infection occurred in 3 cases, including 1 with Enterococcus faecium, 1 with Pseudomonas aeruginosa, and 1 with both Escherichia coli and Klebsiella pneumonia. The incidence of infection at + 100 days was 25.6%, and 26.3% at 6 months after transplantation. The 100-day incidence of EBV reactivation was 29.7%, and CMV reactivation occurred in 76.3% of patients.
Outcome
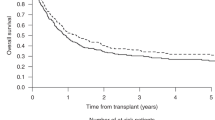
After a median follow-up of 14.7 months from allo-HSCT (range, 3.8 to 27.7 months), 28 patients were still alive. Eight patients experienced bone marrow relapse, with a median relapse time of 6.1 months (range, 1.7 to 14.7 months). Five relapsed patients received multiple courses of salvage chemotherapy, and one of them underwent the second HSCT to further consolidate the efficacy. Donor lymphocyte infusion was administrated in two relapsed patients. One relapsed patient achieved CR following VEN + HMA treatment but relapsed again after 3 courses. A total of 11 patients died. Six patients died of severe infection, 2 died of disease relapse, 1 died of multiple organ failure on day + 3, 1 died of thrombotic microangiopathy associated with infection, and 1 died of cerebral herniation. The probabilities of OS and PFS at 1 year were 75.5% (95% CI, 62.6%–91.0%) and 61.6% (95% CI, 47.5%–79.9%), and the cumulative incidence of NRM and relapse was 21.7% and 16.7% (Fig. 3A). Among the R/R cohort, 1-year incidences of OS, PFS, NRM and relapse were 70.4%, 51.3%, 25.5%, and 23.2%, respectively (Fig. 3B).
Risk factor for HSCT outcomes
Hazard ratios of prognostic factors associated with OS, PFS, and cumulative incidence of NRM relapse and II–IV aGVHD obtained using univariate and multivariate analysis are summarized in Table 3. In the multivariable analysis for OS, RIC was an independent prognostic factor for OS (HR 5.304, 95% CI 1.40 to 20.01; p = 0.014). R/R AML independently indicated poor PFS in the multivariable analysis (HR 4.849, 95% CI 1.009–23.30; p = 0.049), while it did not reach statistical significance for OS (HR 8.671, 95% CI 0.99–75.46; p = 0.05). In addition, prior poor response to VEN was found to be a significant factor predicting a higher risk of relapse (HR 4.37, 95% CI 1.130–16.9; p = 0.033). The longer interval from VEN discontinuation to HSCT was associated with a lower risk of II–IV aGVHD, but this did not reach statistical significance in the multivariable (HR 0.315, 95% CI 0.062–1.60; p = 0.16).
Discussion
Patients with R/R leukemia may lose their chance of transplantation due to the high tumor burden and poor general condition, even though 3-year survival with salvage transplantation is only 16–19% [19]. How to make these patients regain remission to bridge HSCT is the focus of attention. In recent years, the introduction of VEN significantly improved the landscape of treatment for multiple types of leukemia and other HMs [20]. Among R/R AML patients, VEN-based combination therapies showed a promising response rate of 21% to 74%, with a median OS of 3–11 months [21]. Even for patients with adverse cytogenetic risk and high-risk molecular mutations, OS and response rate can also be significantly improved with VEN treatment [5]. In the present study, our results have shown that among the R/R patients, 72.4% achieved CR/CRi after VEN-based therapy, with the potential to undergo HSCT. We also included a small number of newly diagnosed elderly patients with HMs who were considered unable to tolerate high-intensity chemotherapy. All newly diagnosed patients responded to VEN-based treatment, and the majority (80%) achieved CR/CRi and could then bridge to HSCT.
Few studies to date have examined the efficacy and influence of VEN-based therapy on subsequent HSCT outcomes. In recent retrospective research, Sandhu et al. described the first experience regarding the impact of VEN therapy on the outcome of HSCT [22]. Of 32 patients with R/R and naïve AML who received VEN and HMA and bridged to allo-HSCT, 68.8% of patients achieved a CR/CRi, and the OS, PFS, and relapse rate at 1 year were 62.5%, 43.8%, and 37.5%, respectively. The cumulative incidences of aGVHD and cGVHD were 43.8% and 31.3%, respectively. A similar result was obtained by Pratz et al., who found a 1-year OS of 68% in older AML patients following VEN therapy [23]. Pollyea and his group conducted a retrospective analysis about the clinical outcomes of allo-HCT following VEN-HMA combination therapy in the newly diagnosed AML settings [24]. They found that significantly better OS was observed in patients bridging to allo-HCST, compared to those who deferred allo-HSCT. In our study, we provide comparable outcomes with 1-year OS, PFS, and relapse rates of 75.5%, 61.6%, and 16.7%, respectively, which was an encouraging outcome and similar to results previously reported. The cumulative incidences of grade 2 or greater aGVHD at 3 months post-HSCT and cGVHD were similar to historical data from our center (aGVHD, 15.4% vs 15–42%; cGVHD, 25.6% vs 24–41%) [14]. Mukherjee et al. recently reported that patients who discontinued VEN ≤ 2 weeks had a higher incidence of II–IV aGVHD (55% vs 17%, p = 0.02) [25]. Our results showed that the longer interval from VEN discontinuation to HSCT was associated with a lower risk of II–IV aGVHD, but this did not reach statistical significance (HR 0.315, 95% CI 0.062–1.60; p = 0.16). The relationship between the interval from VEN discontinuation to HSCT and aGVHD deserves further evaluation in subsequent clinical trials.
On the other hand, VEN has immunosuppressive effects that might alter the safety profile of subsequent allo-HSCT. Treatment-related hematological toxicities and infectious adverse events are typically observed, which have the potential to increase the risk of infection for HSCT recipients [26]. A previous study by Masarova et al. showed that 84% of AML patients experienced grade 3 or higher infection during the VEN-HMA treatment [27]. In our study, we found that the rate of infection at 3 and 6 months after HSCT did not increase post-transplant infection rate, compared to previous studies among allo-HSCT recipients (20–30% of infection incidence at the early stage of transplantation) [28, 29]. CMV reactivation occurred in 76.3% of patients during the first 3 months after allo-HSCT, comparable to previous studies among seropositive patients (30–80%) [30]. The impact of VEN on post-transplant viral or bacterial infections has yet to be clarified, and more clinical trials are required to further demonstrate.
The timing of bridging transplantation after VEN treatment has not been determined yet. Although VEN-based therapy offers superior OS compared with conventional chemotherapy, some patients still eventually progress or relapse, even if VEN therapy is maintained. The median duration of response in R/R AML patients treated with VEN-based therapy is reportedly approximately 4.8–10.8 months. In our study, of 30 patients with CR/CRi after VEN, 8 progressed or relapsed during the waiting period for a transplant, with the time from CR to relapse ranging from 0.5 to 5.7 months. Patients with non-remission at HSCT had a poorer PFS. Therefore, subsequent HSCT should be performed as soon as possible in transplant-eligible patients with remission to further consolidate VEN-induced responses and improve outcomes of transplant, especially in patients with high risk and R/R HMs.
Our study has several limitations. The number of cases in this retrospective study was small and varied significantly across centers. There was some heterogeneity among the study population, mainly among AML patients. Because patients were treated at different centers, there was no unified protocol of VEN-based therapy. Further investigations and prospective trials are required to confirm the efficacy of VEN on the subsequential HSCT.
In conclusion, our study showed VEN-based therapy is a potent strategy to achieve remission in AML patients, especially in R/R patients. VEN-based therapy followed by HSCT could improve the survival of patients without increased risk of transplant-related mortality or complications.
References
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT (2013) BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12(3):329–341. https://doi.org/10.1016/j.stem.2012.12.013
Konopleva M, Letai A (2018) BCL-2 inhibition in AML: an unexpected bonus? Blood 132(10):1007–1012. https://doi.org/10.1182/blood-2018-03-828269
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang HC, Humerickhouse RA, Rosenberg SH, Elmore SW (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, Kaiser J, Lyle L, Boggs C, Halsema K, Schowinsky JT, Rosser J, Ewalt MD, Siegele B, Rana V, Schuster S, Abbott D, Stevens BM, Jordan CT, Smith C, Pollyea DA (2019) Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv 3(20):2911–2919. https://doi.org/10.1182/bloodadvances.2019000243
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Dohner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hajek R, Porkka K, Illes A, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW (2020) Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383(7):617–629. https://doi.org/10.1056/NEJMoa2012971
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1):7–17. https://doi.org/10.1182/blood-2018-08-868752
DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, Daver N, Covert W, Marx KR, Mace M, Jabbour E, Cortes J, Garcia-Manero G, Ravandi F, Bhalla KN, Kantarjian H, Konopleva M (2018) Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 93(3):401–407. https://doi.org/10.1002/ajh.25000
Zeidan AM, Pollyea DA, Garcia JS, Brunner A, Roncolato F, Borate U, Odenike O, Bajel AR, Watson AM, Gotze K, Nolte F, Tan PT, Hong WJ, Dunbar M, Zhou Y, Gressick L, Ainsworth W, Harb J, Salem AH, Hayslip J, Swords R, Garcia-Manero G (2019) A phase 1b study evaluating the safety and efficacy of venetoclax as monotherapy or in combination with azacitidine for the treatment of relapsed/refractory myelodysplastic syndrome. Blood 134:565. https://doi.org/10.1182/blood-2019-124994
Wei AH, Garcia JS, Borate U, Fong CY, Baer MR, Nolte F, Peterlin P, Jurcic JG, Garcia-Manero G, Hong WJ, Platzbecker U, Odenike O, Dunbar M, Zhou Y, Harb J, Tanwani P, Wolff JE, Jacoby M (2019) A phase 1b study evaluating the safety and efficacy of venetoclax in combination with azacitidine in treatment-naive patients with higher-risk myelodysplastic syndrome. Blood 134:568. https://doi.org/10.1182/blood-2019-124437
Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, Leonard J, Colace SI, Khaw SL, Fleming SA, Mattison RJ, Norris R, Opferman JT, Roberts KG, Zhao Y, Qu C, Badawi M, Schmidt M, Tong B, Pesko JC, Sun Y, Ross JA, Vishwamitra D, Rosenwinkel L, Kim SY, Jacobson A, Mullighan CG, Alexander TB, Stock W (2021) Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov 11(6):1440–1453. https://doi.org/10.1158/2159-8290.CD-20-1465
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374(4):311–322. https://doi.org/10.1056/NEJMoa1513257
Kaufman JL, Gasparetto C, Schjesvold FH, Moreau P, Touzeau C, Facon T, Boise LH, Alzate S, Macartney T, Pesko J, Salem AH, Ross JA, Hong WJ, Maciag PC, Pauff JM, Kumar SK (2019) Phase I/II study evaluating the safety and efficacy of venetoclax in combination with dexamethasone as targeted therapy for patients with t(11;14) relapsed/refractory multiple myeloma. Blood 134:926. https://doi.org/10.1182/blood-2019-125871
Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, Benboubker L, Facon T, Amiot M, Moreau P, Punnoose EA, Alzate S, Dunbar M, Xu T, Agarwal SK, Enschede SH, Leverson JD, Ross JA, Maciag PC, Verdugo M, Touzeau C (2017) Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130(22):2401–2409
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, Xie W, Zheng W, Zhu Y, Ye X, Yu X, Cai Z, Lin M, Huang H (2014) T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 124(17):2735–2743. https://doi.org/10.1182/blood-2014-04-571570
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wolfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE (2016) International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22(1):4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425. https://doi.org/10.1182/blood-2005-10-4149
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis SoRCTO, Reporting Standards for Therapeutic Trials in Acute Myeloid L (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649. https://doi.org/10.1200/JCO.2003.04.036
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, Keating A, Lazarus HM, Litzow MR, Marks DI, Maziarz RT, Rizzieri DA, Schiller G, Schultz KR, Tallman MS, Weisdorf D (2010) Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28(23):3730–3738. https://doi.org/10.1200/JCO.2010.28.8852
Yue X, Chen Q, He J (2020) Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int 20(1):524. https://doi.org/10.1186/s12935-020-01614-z
Aldoss I, Pullarkat V, Stein AS (2021) Venetoclax-containing regimens in acute myeloid leukemia. Ther Adv Hematol 12:2040620720986646. https://doi.org/10.1177/2040620720986646
Sandhu KS, Dadwal S, Yang D, Mei M, Palmer J, Salhotra A, Al Malki M, Aribi A, Ali H, Khaled S, Forman SJ, Snyder D, Nakamura R, Stein AS, Marcucci G, Aldoss I, Pullarkat V (2020) Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant 26(12):e322–e327. https://doi.org/10.1016/j.bbmt.2020.08.027
Pratz KW, DiNardo CD, Arellano ML, Letai AG, Thirman M, Pullarkat VA, Roboz GJ, Becker PS, Hong WJ, Jiang Q, Hayslip J, Potluri J, Pollyea DA (2019) Outcomes after stem cell transplant in older patients with acute myeloid leukemia treated with venetoclax-based therapies. Blood 134:264. https://doi.org/10.1182/blood-2019-127251
Pollyea DA, Winters A, McMahon C, Schwartz M, Jordan CT, Rabinovitch R, Abbott D, Smith CA, Gutman JA (2021) Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant 57(2):160–166. https://doi.org/10.1038/s41409-021-01476-7
Mukherjee A, Milton DR, Jabbour E, Daver N, Gulbis A, Ledesma C, Konopleva M, DiNardo CD, Ravandi F, Kadia TM, Alatrash G, Alousi AM, Daher M, Marin D, Olson AL, Oran B, Kebriaei P, Saini N, Srour SA, Popat UR, Im JS, Mehta R, Rondon G, Kantarjian HM, Champlin RE, Khouri IF (2020) Risk of GvHD and survival in patients with acute leukemia who were bridged to allogeneic stem cell transplantation (alloSCT) with venetoclax-based therapy. Blood 136:13–14. https://doi.org/10.1182/blood-2020-137097
Rausch CR, DiNardo CD, Maiti A, Jammal NJ, Kadia TM, Marx KR, Borthakur G, Savoy JM, Pemmaraju N, DiPippo AJ, Daver NG, Chew SM, Sasaki K, Issa GC, Short NJ, Takahashi K, Ohanian MN, Ning J, Xiao L, Alvarado Y, Kontoyiannis DP, Ravandi F, Kantarjian HM, Konopleva MY (2021) Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer 127(14):2489–2499. https://doi.org/10.1002/cncr.33508
Masarova L, DiNardo CD, Bose P, Pemmaraju N, Daver NG, Kadia TM, Chifotides HT, Zhou L, Borthakur G, Estrov Z, Konopleva M, Verstovsek S (2021) Single-center experience with venetoclax combinations in patients with newly diagnosed and relapsed AML evolving from MPNs. Blood Adv 5(8):2156–2164. https://doi.org/10.1182/bloodadvances.2020003934
Mikulska M, Del Bono V, Viscoli C (2014) Bacterial infections in hematopoietic stem cell transplantation recipients. Curr Opin Hematol 21(6):451–458. https://doi.org/10.1097/MOH.0000000000000088
Vu DL, Dayer JA, Masouridi-Levrat S, Combescure C, Boely E, Khanna N, Mueller NJ, Kleber M, Medinger M, Halter J, Passweg J, Muller AM, Schanz U, Chalandon Y, Neofytos D, van Delden C, Kaiser L, Swiss Transplant Cohort S (2020) Microbiologically documented infections after adult allogeneic hematopoietic cell transplantation: a 5-year analysis within the Swiss Transplant Cohort study. Transpl Infect Dis 22(4):e13289. https://doi.org/10.1111/tid.13289
Styczynski J (2018) Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther 7(1). https://doi.org/10.1007/s40121-017-0180-z
Acknowledgements
The authors acknowledge all collaborating institutions that contributed cases to this study.
Funding
This work was funded by the National Natural Science Foundation of China (grant nos. 82070179, 81970158, and 81970097) and Zhejiang Key R&D Program (Science and Technology Department, grant no. 2020C03G2013586).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by institutional review boards at each study site.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All patients consented to the research and its publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, TT., Song, XL., Zhao, YM. et al. Outcome after allogeneic hematopoietic stem cell transplantation following Venetoclax-based therapy among AML and MDS patients. Ann Hematol 101, 2731–2741 (2022). https://doi.org/10.1007/s00277-022-04983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04983-9