Abstract
The combination of hypomethylating agents and venetoclax has revolutionized the therapeutic landscape of acute myeloid leukemia (AML), especially for patients previously deemed unfit for curative–intent treatment. Some of these patients undergo allogeneic hematopoietic cell transplant (alloHCT); yet, there are scarce data regarding transplantation outcomes. We conducted a multicenter nationwide retrospective cohort study, including patients with AML who underwent alloHCT in CR1 after frontline treatment with azacitidine plus venetoclax only (aza-ven group). We collected a historical control group of patients who achieved CR1 after first-line intensive chemotherapy only, followed by alloHCT (intensive group). Patients in the aza-ven group (n = 24) were transplanted between 2019 and 2021. Compared to the intensive group, patients in the aza-ven group were older (median age 71.7 vs. 58.4 years), had higher incidence of therapy-related AML and AML with antecedent hematologic disorder and had more often adverse cytogenetics. They had a higher percentage of allografts from matched-unrelated donors, and reduced intensity conditioning was more commonly used. The estimated 12 months non relapse mortality was 19.1% in the aza-ven group and 11.8% in the intensive group. The estimated 12 months relapse-free survival and overall survival were 58% and 63% in the aza-ven group and 54% and 70% in the intensive group, respectively. The cumulative incidence of acute GVHD at 6 months and of chronic GVHD at 12 months were 58% and 40% in the aza-ven group and 62% and 42% in the intensive group, respectively. Analysis of the aza-ven group revealed that HCT-CI score and ELN risk category were predictive of RFS in both univariate analysis as well as multivariate analysis. Our data suggests that alloHCT for AML patients achieving first CR with aza-ven appears feasible, with short-term post-transplant outcomes similar to those expected after traditional intensive chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, upfront treatment paradigm for newly diagnosed acute myeloid (AML) consisted of intensive induction chemotherapy for patients deemed “fit,” and low-intensity or palliative therapy for elderly patients or those deemed too frail to withstand such intensive regimens. Traditional induction chemotherapy yields complete remission rates of approximately 60–80% in younger patients and 40–60% in those older than 60 years [1]. Allogeneic hematopoietic cell transplant (alloHCT) at first complete remission (CR1) is offered when risk of leukemia relapse outweighs transplant-related morbidity and mortality, most often utilized in patients with intermediate- or poor-risk AML [2]. Advanced age and comorbidities have traditionally been the limiting factors for offering alloHCT [3]; yet, innovations in leukemia therapeutics, transplant protocols, and alternative donors have continued to fuel the debate on the role of alloHSCT in CR1 [4].
Regimens based on hypomethylating agents (HMA) plus venetoclax, without conventional chemotherapy, have been recently incorporated into the armamentarium available for patients with AML, expanding the population receiving curative–intent treatment. A large prospective randomized trial of first-line 5-azacitidine plus venetoclax yielded a 66.4% rate of CR or CR with incomplete hematologic recovery (CRi) in patients unfit for intensive chemotherapy [5]. Some of these patients undergo alloHCT; yet, there are scarce data in the literature regarding their post-transplant outcomes. Here, we report the post-transplant outcomes of 24 AML patients transplanted in CR1 after receiving 5-azacitidine plus venetoclax.
Materials and methods
Study population
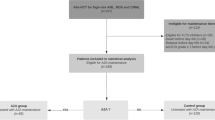
We conducted a multicenter nationwide retrospective cohort study in four academic centers in Israel (Rabin medical Center, Sorasky Medical Center, Chaim-Sheba Medical Center, and Hadassah medical Center). The use of 5-azacitidine plus venetoclax before alloHCT was identified by searching the computerized systems of all participating centers and crossing these data with the departments’ AML database. We included all consecutive patients with AML who had documented CR1 following first-line treatment with 5-azacitidine plus venetoclax only, and proceeded to alloHCT between January 2019 and March 2021 (aza-ven group). In addition, we collected a historical control group of consecutive patients who achieved CR1 after first-line intensive chemotherapy only followed by alloHCT between 2016 and 2019 at Rabin Medical Center (intensive group). Patient, disease, and transplant characteristics were collected using the electronic medical record system. Data regarding comorbidities included ischemic heart disease (IHD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), and chronic kidney disease (CKD). Hematopoietic cell transplantation–comorbidity index (HCT-CI) scores were calculated [6]. Conditioning intensity (MAC vs. RIC) was defined as previously described [7]. The study was approved by the Institutional Review Board of each center.
Outcomes
Efficacy outcomes included relapse-free survival (RFS), defined as the time from transplant to the date of either disease progression, last follow-up or death, and overall survival (OS), calculated as the time from transplant to the date of last follow-up or death. Safety data included hematological and non-hematological adverse events (AE), classified according to the CTCAE criteria version 5.0 [8]. Acute GVHD was classified according to the MAGIC criteria [9], and chronic GVHD according to the NIH criteria [10].
Statistics
Categorical variables are presented as numbers and percentages. Continuous variables are presented as mean and standard deviation for normally distributed variables and as median and range for non-normally distributed variables. Differences in continuous variables were estimated by t-test or Mann–Whitney test, as applicable. Differences in categorical variables were estimated by the Fischer exact test. The probability of OS and RFS were estimated by the Kaplan–Meier method. GVHD incidence analyses were performed through competing risk analysis with death and relapse as competing risks (Fine and Gray model).
In the aza-ven group, Cox proportional hazards regression models were fitted to predict effect of covariates on OS and RFS in univariable models. GVHD as a predictor was also tested in the model and treated as time dependent variable. Covariates with a P value ≤ 0.05 were retained in the cox regression multivariable model for OS and RFS. All statistics were performed with IBM SPSS, version 27.0 (SPSS, Chicago, IL) and SAS software version 9.4 (SAS Institute, North Carolina, USA).
Results
Patient characteristics
Twenty four AML patients were included in the aza-ven group and 24 patients in the intensive group. Patient, disease, and transplant characteristics are shown in Table 1.
Patients in the aza-ven group were older (median age 71.7 vs. 58.4 years), had more often therapy-related AML (t-AML) and AML with antecedent hematologic disorder (AHD) (21% and 42% vs. 13% and 0%) and adverse cytogenetics. Compared to patients in the intensive therapy group, patients in the aza-ven group were more often transplanted from matched unrelated donors (67% vs. 46%) and received more often reduced intensity conditioning regimens (88% vs. 17%).
Three patients in the aza-ven group received post-transplant maintenance therapy (azacitidine, n = 1; azacitidine + venetoclax, n = 2), compared to 8 patients in the intensive group (sorafenib, n = 5; midostaurin, n = 1; azacitidine + venetoclax, n = 2).
Outcomes
Entire cohort
The median follow-up was 8 (range, 0 to 25) months in the aza-ven group and 23 (range, 4 to 56) months in the intensive group.
The estimated median RFS was not reached in the aza-ven group and was 19.3 months (CI 95% 1–38) in the intensive group. The 12 months RFS was 58% and 54%, in the aza-ven (Fig. 1A) and the intensive group (Fig. 1B), respectively.
The estimated median overall survival of the aza-ven group was not reached and the 12 months OS rate was 63.2% (Fig. 2A). The estimated median survival of the intensive group was 50 months (CI 95% 5–96) and the 12 months OS rate was 70.8% (Fig. 2B).
The estimated 12 month non-relapse mortality (NRM) was 19.1% in the aza-ven group, and 11.8% in the intensive group. Relapse was the major cause of death in both groups (Table 2).
The cumulative incidence of aGVHD at 6 months was 58% in the aza-ven group and 62% in the intensive group. The cumulative incidence of cGVHD at 12 months was 40% and 42%, respectively (Table 2).
Aza-ven group analysis
In a subgroup Cox regression analysis of the aza-ven group, adverse ELN 2017 risk category and HCT-CI score ≥ 3 were predictive of decreased RFS, both in UVA and in MVA (HR 10.56, CI 95%1.64–68.1, p = 0.013 and HR 6.43, CR 95% 1.34–30.75, p = 0,02, respectively; Table 3).
Graft source (alternative vs. matched donor) and HCT-CI score ≥ 3 were predictive of decreased OS in UVA (HR 19.45, CI 95% 1.66–228.13, p = 0.018 and HR 5.93, CI 95% 1.13–31.05, p = 0.03, whereas age, ELN2 2017 risk stratification, GVHD, and maintenance therapy were not. In MVA, neither of these factors retained their predictive value.
Of note, neither aGHVD nor cGHVD as time-dependent variables were predictive of RFS or OS (Table 3).
Discussion
Herein, we report the post-transplant outcomes of 24 AML patients transplanted in CR1 after first-line therapy with azacitidine plus venetoclax. With a median follow-up of 8 (range 0 to 25) months, 62.5% were alive and in remission. We also describe outcomes of a second group of 24 patients treated with intensive induction therapy prior to transplant. Although a direct comparison of outcomes between these two distinct groups is not possible due to different baseline patient characteristics, short-term outcomes, including RFS, OS, and GVHD rates seem similar. In subgroup analysis of the aza-ven group, HCT-CI score and ELN risk category were predictive of RFS in both UVA as well as MVA.
HMA agents primarily target DNA hypermethylation, thereby disrupting myeloid maturation and differentiation with a relatively favorable toxicity profile [11]. Venetoclax induces apoptosis by BCL2 inhibition [12]. Venetoclax-based combination therapy, mainly with HMAs or low-dose cytarabine (LDAC), has been shown to be safe and efficacious in AML, in both upfront as well as salvage setting [13]. In the study conducted by Dinardo et al., the addition of venetoclax to azacitidine increased the proportion of patients achieving a composite of CR or CR with incomplete hematologic recovery to 66.4%, compared to 28.3% in the placebo group [5]. Importantly, almost half of patients achieved their response prior to their second cycle of treatment. In our study, 46% of patients underwent alloHCT after just one or two cycles of aza-ven, having achieved CR.
Data regarding post-transplant outcomes after azacitidine and venetoclax treatment are limited. Pratz et al. presented in abstract form outcomes of 31 older AML patients included in phase 1/2 clinical trials who received first-line venetoclax–based therapy who underwent alloHCT. Post-transplant 1 year OS and PFS rates were 68% and 55%, respectively [14], similar to the rates observed in the present study. A real-life report of venetoclax-based combinations demonstrated a CR/Cri rate of 60% among AML patients ineligible for intensive chemotherapy [15]. The added drug was either a HMA or low-dose cytarabine (LDAC). Only 10 of the 133 patients included in the study underwent allogeneic HSCT, which was associated with improved survival. A study published by Sandhu et al. described post-transplant outcomes of 32 patients who received HMA and venetoclax as first-line treatment (N = 13) or for relapsed/refractory disease (N = 19) [16]. Although almost a third of patients were not in CR/Cri at transplant, results were encouraging, with a 1-year DFS and OS rates of 43.8% and 62.5%, respectively. Compared to these previous reports, the present study includes a rather homogenous study cohort, comprised of 24 patients who underwent allogeneic HSCT at CR1 after receiving only azacitidince and venetoclax.
In a study of 63 patients who received first-line HMA with venetoclax on or off clinical trials, the authors conducted a theoretical comparison with intensive induction chemotherapy using the AML SCORE calculator to evaluate each patients’ response and early death, revealing non-inferior response rates and lower death rates with the HMA and venetoclax combination [17].
For years, the mainstay of treatment for patients with AML has been intensive chemotherapy. As such, only younger fit patients have received curative–intent therapy, including alloHCT. Furthermore, high-risk features such as complex karyotype convey inferior remission rates with conventional chemotherapy [18]. Only 10–44% of patients with complex karyotype AML older than 60 years achieve CR with this approach [19]. In comparison, the efficacy of HMA-based therapy seems less dependent on leukemia cytogenetic characteristics [20], and this regimen causes a low rate of organ toxicity [5, 21]. Therefore, older patients with comorbidities and/or adverse-risk cytogenetics may now achieve CR and undergo allHCT. Indeed, Pollyea et al. recently showed that transplant confers a survival advantage in patients who responded to initial azacitidine and venetoclax therapy [22]. In the future a wider range of transplant-eligible AML patients, perhaps also the young and fit could potentially achieve remission and undergo transplant without traditional intensive chemotherapy, following the footsteps of tyrosine kinase inhibitor–based therapy in Philadelphia positive acute lymphoblastic leukemia [23, 24].
Our study has several limitations. First, due to the retrospective nature of the study, there is inherent selection bias, since all patients achieved CR after first-line treatment and were eligible for transplant. Yet, even though the aza-ven group had several characteristics which traditionally confer inferior outcomes (older age, secondary AML, and adverse cytogenetic features) [1, 18], outcomes were still similar to patients in the intensive group. OS and RFS in the latter group were comparable to those previously described in the literature [3]. Second, follow-up time in the aza-ven group was significantly shorter than the intensive group due to the novelty of this former combination, limiting the yield of chronic GVHD comparison. A further limitation is the lack of minimal residual disease (MRD) data for our patients, due to the lack of validated flow cytometry/next generation sequencing (NGS) MRD measurement for AML in Israel. Lastly, due to the distinct characteristics of the two groups, we were not able to conduct direct statistical comparisons.
In conclusion, for patients with AML who achieve CR1 after aza-ven therapy, alloHCT is a valid option, with short-term post-transplant outcomes that appear to be similar to those achieved after traditional intensive chemotherapy. Our results were collected in the real-world setting, and patients in the aza-ven group were older and had inherently worse leukemia characteristics, including more secondary AML and more adverse cytogenetic features. Therefore, future research is warranted to decipher the true spectrum of AML patients who could benefit from remission induction with this less intensive regimen prior to alloHCT.
Data availability
Upon request, from corresponding author.
References
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, Appelbaum FR, Dohner H, Antin JH, Soiffer RJ, Cutler C (2009) Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 301(22):2349–2361. https://doi.org/10.1001/jama.2009.813
Cornelissen JJ, Blaise D (2016) Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood 127(1):62–70. https://doi.org/10.1182/blood-2015-07-604546
Yanada M (2021) The evolving concept of indications for allogeneic hematopoietic cell transplantation during first complete remission of acute myeloid leukemia. Bone Marrow Transplant. https://doi.org/10.1038/s41409-021-01247-4
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Dohner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hajek R, Porkka K, Illes A, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW (2020) Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383(7):617–629. https://doi.org/10.1056/NEJMoa2012971
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919. https://doi.org/10.1182/blood-2005-05-2004
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15(12):1628–1633. https://doi.org/10.1016/j.bbmt.2009.07.004
Services UDoHaH (2017) Common terminology criteria for adverse events v5.0
(CTCAE) [Internet] Princ Pract Clin Trial Med. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. 2021
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wolfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE (2016) International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22(1):4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME (2015) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21 (3):389–401 e381. doi:https://doi.org/10.1016/j.bbmt.2014.12.001
Schuh AC, Dohner H, Pleyer L, Seymour JF, Fenaux P, Dombret H (2017) Azacitidine in adult patients with acute myeloid leukemia. Crit Rev Oncol Hematol 116:159–177. https://doi.org/10.1016/j.critrevonc.2017.05.010
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208. https://doi.org/10.1038/nm.3048
Aldoss I, Pullarkat V, Stein AS (2021) Venetoclax-containing regimens in acute myeloid leukemia. Ther Adv Hematol 12:2040620720986646. https://doi.org/10.1177/2040620720986646
Keith W, Pratz CDD, Arellano ML, Letai AG, Thirman M, Pullarkat VA, Roboz GJ, Becker PS, Hong W-J, Jiang Qi, Hayslip J, Potluri J, Pollyea DA (2019) Outcomes after stem cell transplant in older patients with acute myeloid leukemia treated with venetoclax-based therapies. Blood 134:264. https://doi.org/10.1182/blood-2019-127251
Apel A, Moshe Y, Ofran Y, Gural A, Wolach O, Ganzel C, Canaani J, Zektser M, Duek A, Stemer G, Hellman I, Basood M, Frisch A, Leibovitch C, Koren-Michowitz M (2021) Venetoclax combinations induce high response rates in newly diagnosed acute myeloid leukemia patients ineligible for intensive chemotherapy in routine practice. Am J Hematol. https://doi.org/10.1002/ajh.26190
Sandhu KS, Dadwal S, Yang D, Mei M, Palmer J, Salhotra A, Al Malki M, Aribi A, Ali H, Khaled S, Forman SJ, Snyder D, Nakamura R, Stein AS, Marcucci G, Aldoss I, Pullarkat V (2020) Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant 26(12):e322–e327. https://doi.org/10.1016/j.bbmt.2020.08.027
Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, Kaiser J, Lyle L, Boggs C, Halsema K, Schowinsky JT, Rosser J, Ewalt MD, Siegele B, Rana V, Schuster S, Abbott D, Stevens BM, Jordan CT, Smith C, Pollyea DA (2019) Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv 3(20):2911–2919. https://doi.org/10.1182/bloodadvances.2019000243
Daneshbod Y, Kohan L, Taghadosi V, Weinberg OK, Arber DA (2019) Prognostic significance of complex karyotypes in acute myeloid leukemia. Curr Treat Options Oncol 20(2):15. https://doi.org/10.1007/s11864-019-0612-y
Mrozek K (2008) Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin Oncol 35(4):365–377. https://doi.org/10.1053/j.seminoncol.2008.04.007
Kuendgen A, Muller-Thomas C, Lauseker M, Haferlach T, Urbaniak P, Schroeder T, Brings C, Wulfert M, Meggendorfer M, Hildebrandt B, Betz B, Royer-Pokora B, Gattermann N, Haas R, Germing U, Gotze KS (2018) Efficacy of azacitidine is independent of molecular and clinical characteristics - an analysis of 128 patients with myelodysplastic syndromes or acute myeloid leukemia and a review of the literature. Oncotarget 9(45):27882–27894. https://doi.org/10.18632/oncotarget.25328
Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, Mei M, Salhotra A, Khaled S, Nakamura R, Snyder D, O’Donnell M, Stein AS, Forman SJ, Marcucci G, Pullarkat V (2018) Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 103(9):e404–e407. https://doi.org/10.3324/haematol.2018.188094
Daniel A, Pollyea AW, Jordan CT, Smith C, Gutman JA (2020) Allogeneic transplant improves AML outcomes compared to maintenance venetoclax and azacitidine following response to initial venetoclax and azacitidine therapy. Blood 136:24. https://doi.org/10.1182/blood-2020-138821
Candoni A, Rambaldi A, Fanin R, Velardi A, Arcese W, Ciceri F, Lazzarotto D, Lussana F, Olivieri J, Grillo G, Parma M, Bruno B, Sora F, Bernasconi P, Saccardi R, Foa R, Sessa M, Bresciani P, Giglio F, Picardi A, Busca A, Sica S, Perruccio K, Zucchetti E, Diral E, Iori AP, Colombo AA, Tringali S, Santarone S, Irrera G, Mancini S, Zallio F, Malagola M, Albano F, Carella AM, Olivieri A, Tecchio C, Dominietto A, Vacca A, Sorasio R, Orciuolo E, Risitano AM, Leotta S, Cortelezzi A, Mammoliti S, Oldani E, Bonifazi F, Gitmo, (2019) Outcome of allogeneic hematopoietic stem cell transplantation in adult patients with philadelphia chromosome-positive acute lymphoblastic leukemia in the era of tyrosine kinase inhibitors: a registry-based study of the Italian Blood and Marrow Transplantation Society (GITMO). Biol Blood Marrow Transplant 25(12):2388–2397. https://doi.org/10.1016/j.bbmt.2019.07.037
Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, Paoloni F, Fazi P, Cimino G, Nobile F, Ferrara F, Castagnola C, Sica S, Leoni P, Zuffa E, Fozza C, Luppi M, Candoni A, Iacobucci I, Soverini S, Mandelli F, Martinelli G, Baccarani M, Party GALW (2011) Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 118(25):6521–6528. https://doi.org/10.1182/blood-2011-05-351403
Author information
Authors and Affiliations
Contributions
OP contributed to research design, acquisition of data, analysis and interpretation of data, drafted the manuscript and approved the submitted and final versions; SS contributed to research design, acquisition of data, analysis and interpretation of data, drafted the manuscript and approved the submitted and final versions; RR contributed to research design, acquisition of data, revised the manuscript critically and approved the submitted and final versions; AS contributed to research design, acquisition of data, revised the manuscript critically and approved the submitted and final versions; LS contributed to the acquisition of data, revised the manuscript critically and approved the submitted and final versions; BA contributed to the acquisition of data, revised the manuscript critically and approved the submitted and final versions; OW contributed to research design, analysis and interpretation of data, revised the manuscript critically and approved the submitted and final versions; TS contributed to research design, analysis and interpretation of data, revised the manuscript critically and approved the submitted and final versions; RY contributed to the acquisition of data, revised the manuscript critically and approved the submitted and final versions; OA contributed to the acquisition of data, revised the manuscript critically and approved the submitted and final versions; PR contributed to research design, analysis and interpretation of data, revised the manuscript critically and approved the submitted and final versions; MY contributed to research design, analysis and interpretation of data, drafted the manuscript and approved the submitted and final versions;
Ethics declarations
Conflicts of interest
None.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pasvolsky, O., Shimony, S., Ram, R. et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first complete remission after 5-azacitidine and venetoclax: a multicenter retrospective study. Ann Hematol 101, 379–387 (2022). https://doi.org/10.1007/s00277-021-04693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04693-8