Abstract
Central nervous system (CNS) relapse of diffuse large B-cell lymphoma (DLBCL) is a rare but devastating event. Intravenous high-dose methotrexate (HD-MTX) is recommended as CNS prophylaxis, but the optimal timing and dose has not been elucidated. Here, we report a multicenter analysis of prophylactic HD-MTX administration for DLBCL. Two hundred eighty-four patients receiving HD-MTX either concurrent with each induction chemotherapy cycle (n = 221) or at the end of induction therapy (EOI, n = 63) were included. Patients with CNS-IPI scoring 4–6, and/or testicular involvement, and/or double/triple hit lymphoma, were stratified into the high-risk group and the others into the moderate-risk group. Concurrent HD-MTX was associated with increased risk of grade 3/4 treatment-related toxicity (OR,1.49; P = 0.006) and subsequent chemotherapy delays (OR, 1.87; P = 0.003) in multivariate analysis. With a median follow-up of 36.0 months, no significant difference in CNS relapse rate was identified between the concurrent and EOI groups (3.2% vs 4.8%, P = 0.34), even in the high-risk group. Analysis on systemic MTX dose suggested that high-dose MTX (≥ 2 g/m2) was associated with better CNS relapse control only in the high-risk group, but not in the moderate-risk group. This study may elucidate the superiority of EOI HD-MTX to some extent. High MTX dose (≥ 2 g/m2) may not be necessary for the moderate-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin’s lymphoma [1]. Despite the significant improvement in outcomes of DLBCL since the introduction of rituximab [2,3,4,5], a minority of patients still suffer from relapse within the central nervous system (CNS). CNS relapse of DLBCL is an uncommon but devastating event, with a median survival of 2–5 months [6].
The poor outcome of CNS relapse highlights the need to identify high-risk patients at diagnosis. A validated clinical risk model consisting of the five International Prognostic Index (IPI) factors plus kidney and/or adrenal gland involvement (CNS-IPI) can identify a high-risk group with a > 10% risk of CNS relapse [7]. Involvement of certain extranodal sites, especially testicular involvement, also confers increased risk even with early-stage disease [8,9,10,11,12]. Adverse biomarkers include dual expression of MYC and BCL2 by immunohistochemistry (DEL) [13], as well as high-grade B-cell lymphoma with translocation of MYC, BCL2, and/or BCL6 (double/triple hit lymphoma (DHL/THL)) [14,15,16].
CNS prophylaxis should be considered for patients with high risk factors of CNS relapse. Intrathecal methotrexate (MTX) has been widely employed, but no significant preventive benefit favoring this approach could be identified based on available data [17,18,19,20,21,22,23]. Substantial evidences from retrospective studies suggest that intravenous high-dose MTX (HD-MTX) could be effective as prophylaxis for CNS relapse [22, 24,25,26]. Although there has been some debate about this prophylaxis according to recent studies [27, 28], HD-MTX is still considered a routine therapy for high-risk patients at present and recommended by the National Comprehensive Cancer Network (NCCN) guidelines. However, the optimal timing and dose of HD-MTX prophylaxis are incompletely elucidated. CNS events typically occur within 5–6 months after diagnosis [29, 30], which forms the rationale for concurrent HD-MTX delivery with induction chemotherapy. The NCCN guidelines also recommend systemic MTX given at the end of induction therapy (EOI) in selected patients. However, the decision of delivery timing is usually made by clinicians according to patients’ performance status, organ function, and prognostic factors, instead of being guided by recognized criteria. Besides, current recommended dose of prophylactic MTX is 3–3.5 g/m2, which is largely extrapolated from the treatment experience of primary CNS lymphoma [31, 32], and prophylactic strategies of Burkitt lymphoma [33, 34] and acute lymphoblastic leukemia [35]. The dose-dependent effect of systemic MTX has not been validated with clinical data. It is also unclear whether the optimal dose is different among patients with different risk levels. Therefore, we conducted this multicenter, retrospective study to investigate the optimal timing and dose, as well as the risk-adapted strategy of prophylactic MTX in DLBCL.
Methods
Patient population and treatment
Clinical data of all DLBCL patients consecutively treated with intravenous HD-MTX between 2005 and 2020 at the Sun Yat-sen University Cancer Center, Guangdong Provincial People’s Hospital, Sun Yat-sen Memorial Hospital, and ZhuJiang Hospital of Southern Medical University in China were reviewed, and eligible patients were included. The inclusion and exclusion criteria are listed in the Supplemental Method. All patients underwent CT/PET-CT scanning for staging. Craniocerebral MR and cerebrospinal fluid (CSF) examinations were performed for patients with clinical suspicion of CNS disease. The project was approved by the institutional review board of all participating centers.
Patients with high risk factors of CNS relapse, including CNS-IPI scoring 4–6, involvement of certain extranodal sites (e.g., testes, kidney, adrenal gland), DHL/THL, and DEL, were subjected to HD-MTX prophylaxis. HD-MTX prophylaxis was given either concurrent with each induction cycle or at the EOI. The delivery timing, dose, and number of cycles were mainly decided by clinicians. Since this is a retrospective study with a long time span, the criteria for MTX delivery could not be completely unified. Patients were pretreated with hydration and urine alkalinization, and then received leucovorin rescue 24 h after the initiation of MTX infusion. All the patients underwent CT/PET-CT scanning as response assessment. CNS disease was diagnosed according to radiologic findings and/or detection of lymphoma cells in CSF. Patients submitted their written informed consents in accordance with the Declaration of Helsinki.
Statistical analyses
For patients with high-risk CNS-IPI, testicular involvement, and DHL/THL, there is a > 10% risk of CNS relapse [7,8,9,10,11,12, 14,15,16]. Thus, we stratified the patients into two risk groups: patients with CNS-IPI scoring 4–6, and/or testicular involvement and/or DHL/THL, formed the high-risk group; the others formed the moderate-risk group. This risk stratification criteria were also applied in a Canadian research [27].
The clinical features of subgroups were compared using Mann–Whitney U test, χ2 test, and Fisher’s exact test. Logistic-regression analysis was used to estimate odds ratios (OR) for toxic events, chemotherapy delays, and complete remission (CR) rate of induction therapy. CNS relapse-free survival (CRFS), progression-free survival (PFS), and overall survival (OS) were calculated using Kaplan–Meier analysis and compared using the log-rank test. A P value < 0.05 was considered statistically significant.
We also used the combination of Cox regression and restricted cubic splines (RCS) [36, 37] with three knots at the 10th, 50th, and 90th percentiles to flexibly model the non-linear relationship of MTX dose and other covariates with progression and mortality. This analysis was performed using the rms package in R software, version 3.6.3. The significance of non-linear association was determined by a P for non-linearity < 0.05.
Results
Patient characteristics and treatment overview
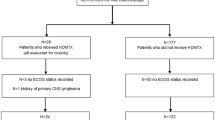
A total of 1056 patients were reviewed, and 284 eligible patients were included. The baseline characteristics of patients classified by MTX delivery timing and risk level of CNS relapse are summarized in Table 1 and Table 2. Two hundred twenty-one patients received concurrent HD-MTX and 63 received EOI HD-MTX. The median number of HD-MTX cycles in the concurrent and EOI groups were 4 (range, 1–8) and 2 (range, 1–4), respectively. Two hundred thee patients received MTX with the dose < 3 g/m2, and 81 patients with the dose ≥ 3 g/m2. The median time between the start of chemotherapy cycles and HD-MTX delivery in the concurrent group was 4 days (range, 0–17). Two hundred ten (74%) patients also received intrathecal methotrexate and cytarabine as prophylaxis, and the median number of intrathecal cycles was 5 (range, 1–8). Seventy-three patients received < 4 cycles of concurrent intrathecal prophylaxis, and 137 patients received ≥ 4 cycles. In terms of first-line therapy, 207 patients were treated with R-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab), 15 patients with CHOP, 59 patients with DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), and 3 patients with DA-EPOCH. The median numbers of CHOP ± R and EPOCH ± R cycles were both 6 (range, 2–8). One hundred four patients were stratified into the high-risk group, including 42 with CNS-IPI 4–6 points, 42 with testicular involvement, 8 with DHL/THL, and 12 with two risk factors simultaneously.
Concurrent HD-MTX was associated with more frequent toxic events
A total of 1094 HD-MTX cycles were given in the concurrent (n = 953) and EOI groups (n = 141). Baseline characteristics were well-balanced between two groups (Table 1). The treatment-related toxic events after HD-MTX cycles are shown in Table S1. More hematological toxic events were observed in the concurrent group than the EOI group. No difference in the incidence of non-hematological toxicity was observed between two groups.
However, the hematological toxic events in the concurrent group might be caused by the inherent toxicity of induction regimens. In this study, the contribution of concurrent HD-MTX to the toxic events was the main area of concern. Thus, we compared the toxic events after induction cycles between the concurrent and EOI groups, and the latter group was regarded as a “control” to indicate how many toxic events were caused by induction therapy alone. Patients in the concurrent and EOI groups received a total of 1197 and 380 cycles of induction therapy, respectively. The toxic events after induction cycles are shown in Table S2. The concurrent group was still associated with more hematological toxicity than the EOI group, including grade 3/4 leucopenia (P < 0.001) and neutropenia (P < 0.001). The incidence of gastrointestinal events was also higher in the concurrent group (P = 0.009). Five patients suffered grade 1–2 nephrotoxicity in the concurrent group and none in the EOI group. The impact of concurrent HD-MTX on grade 3/4 toxicity after induction cycles was also evaluated in multivariate analysis. Concurrent HD-MTX was the only factor associated with higher incidence of grade 3/4 toxicity (OR, 1.49; 95%CI, 1.12–2.00; P = 0.006) (Fig. 1a).
Odds ratios of treatment-related toxicity, chemotherapy delays, and induction therapy response in patients receiving HD-MTX. Forest plot demonstrating the odds ratios (OR) and 95% confidence interval (CI) for treatment-related toxicity, subsequent chemotherapy delays, and response to induction therapy after multivariable logistic regression in the overall cohort. a Grade 3/4 treatment-related toxicity after induction therapy. b Subsequent chemotherapy delays. c Complete remission rate of induction therapy. ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; CrCl, creatinine clearance; EPOCH ± R, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, with or without rituximab; G-CSF, granulocyte colony-stimulating factor
Concurrent HD-MTX was associated with increased risk of chemotherapy delays
The toxicity-related delays of subsequent induction cycles between two groups were further compared, and the EOI group acted as a “control” again. Among the 1197 induction cycles in the concurrent group, 248 (21%) cycles were observed with toxicity-related delays and delays ≥ 7 days were seen in 55% (137/248) of all delayed cycles. Among the 380 induction cycles in the EOI group, 62 (16%) cycles were observed with toxicity-related delays and delays ≥ 7 days were seen in 37% (23/62) of all delayed cycles. Concurrent HD-MTX was the only factor independently associated with subsequent chemotherapy delays ≥ 7 days in multivariate analysis (OR, 1.87; 95%CI, 1.24–2.82; P = 0.003) (Fig. 1b).
The potential association between HD-MTX delivery timing and response to induction therapy was explored. However, patients who received EOI HD-MTX were selected only if they have complete or partial remission after induction therapy in clinical practice, but patients in the concurrent group were at continuous risk of progression throughout the treatment. To avoid this selection bias, we eliminated 9 patients with stable or progressive disease (SD/PD) during the induction therapy in the concurrent group before the comparison. The CR rate of the concurrent group was inferior to EOI HD-MTX group (74% vs 90%, P = 0.02, Table S3). In multivariate analysis, concurrent HD-MTX delivery (OR, 0.39; 95%CI, 0.16–0.94; P = 0.04) and induction therapy containing rituximab (OR, 2.97; 95%CI, 1.00–8.79; P = 0.03) were significantly associated with the CR rate of induction therapy (Fig. 1c).
CNS relapse and survival outcomes of patients receiving HD-MTX
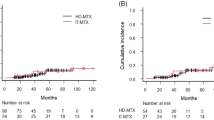
With a median follow-up of 36.0 months (range, 0.9–180.4), ten CNS relapse occurred (Table S4). Seven (70%) relapses occurred in brain parenchyma, 2 (20%) involved in leptomeninges, and 1 (10%) in both. Six patients developed isolated CNS relapse, two developed CNS relapse with concurrent systemic relapse, one developed CNS relapse after systemic relapse, and one developed CNS relapse with primary refractory systemic disease. The median time to CNS relapse was 23 months (range, 2.7–132.9). The incidence of CNS relapse for CNS-IPI low (0–1), intermediate (2–3), and high (4–6) risk group were 2.2%, 3.5%, and 5.7%, respectively. Three (5.6%) patients with testicular involvement developed CNS relapse, compared with 3.0% (n = 7) of patients without testicular involvement. Risk of CNS relapse in patients with other risk factors is presented in Table S5. The CNS recurrence rate of patients receiving additional intrathecal MTX was 2.8%, compared with 5.4% for patients omitting intrathecal MTX. The cumulative incidence of CNS relapse in the concurrent and EOI groups were 3.2% (n = 7) and 4.8% (n = 3), respectively. One patient developed CNS relapse during induction therapy in the concurrent group and was excluded before the comparison. The 3-year CRFS of patients in the concurrent and EOI groups was 97.4% (95%CI, 94.9–99.9%) and 95.9% (95%CI, 90.4–101.4%), respectively (P = 0.34) (Fig. 2a).
Control of CNS relapse and survival outcomes of concurrent and EOI HD-MTX groups. Kaplan–Meier estimates the CNS relapse-free survival (CRFS), progression-free survival (PFS), and overall survival (OS) according to MTX delivery timing. a CRFS, PFS, and OS of all patients. b CRFS, PFS, and OS of high-risk group. c CRFS, PFS, and OS of moderate-risk group. EOI, end of induction therapy
The prophylactic effect among patients with different risk levels was further investigated. Seven (6.7%) CNS relapses occurred in the high-risk group and three (1.7%) occurred in the moderate-risk group, including one with CNS relapse during induction therapy and excluded before subgroup analysis. The baseline characteristics were well-balanced between the concurrent and EOI groups in both risk subgroups (Table S6). In the high-risk group, the 3-year CRFS was 94.9% (95%CI, 89.2–100.6%) in the concurrent group, and 94.4% (95%CI, 83.8–105.0%) in the EOI group (P = 0.77) (Fig. 2b). In the moderate-risk group, the 3-year CRFS was 99.3% (95%CI, 97.9–100.7%) in the concurrent group, and 96.9% (95%CI, 90.8–103.0%) in the EOI group (P = 0.32) (Fig. 2c).
The potential association of HD-MTX delivery timing and systemic disease control was explored. SD/PD patients in the concurrent group were excluded before analysis to avoid the immortal time bias. In the high-risk subgroup, the 5-year PFS of the EOI group tends to be higher than that of the concurrent group (69.8% vs 34.1%, P = 0.09) (Fig. 2b). No difference of PFS between these two groups for patients with moderate risk was observed (Fig. 2c).
High MTX dose was associated with better CNS relapse control in the high-risk group, but not the moderate-risk group
To quantify the dose-dependent effect, we used restricted cubic splines to allow for non-linear relationships between MTX dose and survival outcomes. This model suggested that the risk (hazard ratio, HR) of disease progression decreased sharply until 1.5–3.0 g/m2 in the high-risk group (P for non-linearity = 0.042) (Fig S1a). However, the dose-dependent effect of MTX on PFS disappeared in the moderate-risk group (P for non-linearity = 0.864). After adjustment for all covariates (age, gender, ECOG PS, LDH, stage, and number of extranodal site involvement), the HR curves revealed similar trends for PFS (Fig S1b).
Since the HR approached 1 at 2 g/m2, we divided the patients into 2 groups: high dose (≥ 2 g/m2) and low dose(< 2 g/m2). The baseline characteristics were well-balanced between the high- and low-dose groups in both risk subgroups (Table S7). In the high-risk group, the median numbers of HD-MTX cycles of the low- and high-dose groups were 3.5 (range, 1–8) and 4 (range, 1–8), respectively. In the moderate-risk group, the median number of HD-MTX cycles of these two dose groups was both 4 (range, 1–8). In the high-risk group, low-dose MTX demonstrated an inferior CNS relapse control compared with high-dose MTX, with the 3-year CRFS of 85.8% (95%CI, 70.7–100.9) in the low-dose group vs 98.1% (95%CI, 94.4–101.8%) in the high-dose group (P = 0.04). Patients receiving low dose of MTX also tended to have inferior 5-year PFS (P = 0.07) and 5-year OS (P = 0.06) (Fig. 3a). However, in the moderate-risk group, CRFS (P = 0.49), PFS (P = 0.20), and OS (P = 0.82) were not dependent on MTX dose (Fig. 3b).
HD-MTX improved the CRFS in high-risk group, but not moderate-risk group. Kaplan–Meier survival curves of CNS relapse-free survival (CRFS), progression-free survival (PFS), and overall survival (OS) according to MTX dose. a CRFS, PFS, and OS of the high-risk group. b CRFS, PFS, and OS of the moderate-risk group. EOI, end of induction therapy
Discussion
CNS prophylaxis in DLBCL has remained as a controversial issue for a long time. HD-MTX is widely employed but carries with the risk of toxic events including renal dysfunction, mucositis, and infection. Thus, it is important to estimate the effect of different administrations of HD-MTX in order to optimize its delivery. We used the EOI group as a reference to determine whether concurrent HD-MTX increased the incidence of toxicity and chemotherapy delays of induction therapy. Concurrent HD-MTX was associated with increased incidence of grade 3/4 toxicity and delays ≥ 7 days of subsequent chemotherapy. The clinical significance of these increased toxicity and delays is actually ambiguous. Although in this study, the EOI group was also associated with higher CR rate and tended to have better PFS in the high-risk group, we could not conclude that increased toxicity and delays of concurrent HD-MTX could result in inferior treatment response and systemic disease control. We acknowledge that the response to induction therapy and systemic disease control was influenced by many confounding factors. Also, the influence of immortal time bias between the EOI and concurrent groups could not be completely avoided, although we have controlled this by eliminating the PD/SD patients in the concurrent group before the comparison. Therefore, this study is not powered to prove this causality. However, considering the aggressive clinical course of DLBCL, delays ≥ 7 days could affect the maintenance of dose intensity, which may consequently influence the efficacy of induction therapy. Notably, patients with high risk of CNS relapse are also at a high risk of systemic recurrence, so the difference of CR rate and PFS observed in this study should still be concerning.
With a median follow-up of 36.0 months, the cumulative incidence of CNS relapse for concurrent HD-MTX and EOI HD-MTX groups were 3.2% and 4.8%. No statistical difference of CNS relapse rate between the concurrent and EOI groups was observed (P = 0.34), which is consistent with another study in British focusing on the timing of prophylactic HD-MTX [38]. It is difficult to conclude that HD-MTX delivered in two different timings has comparable prophylactic efficacy with a single study, given the low incidence of CNS event. However, the consistency across two studies may be the evidence to support this notion. Moreover, the concurrent group received more cycles of HD-MTX (median number, 4 vs 2), but there was still not a suggestion of improved efficacy with concurrent HD-MTX. According to the above analysis, EOI delivery is likely to be a better choice with acceptable prophylactic efficacy and lower incidence of toxic events.
The indications of HD-MTX prophylaxis varied among clinicians and centers in real-world practice, so the study population was heterogeneously constituted. We tried to screen out a patient group with higher risk of CNS relapse with recognized risk factors and evaluate whether the prophylactic measures should be given earlier in this group. In this study, patients stratified into the high-risk group showed a relatively higher CNS relapse rate (6.7%) than those stratified into the moderate-risk group (1.7%), but lower than the historical reports utilizing the same criteria for the high-risk stratification of CNS relapse [27]. This difference may result from the underdiagnoses of CNS relapses in the retrospective setting. The subgroup analysis failed to show the superiority of concurrent HD-MTX in the prevention of CNS events even for the high-risk group. However, considering the relatively small sample size of the high-risk group in this study and a reported CNS relapse incidence > 10%, decision of HD-MTX delivery timing for this patient cohort should still be made carefully in clinical practice.
In the analysis of prophylactic MTX dose, we observed that high MTX dose was associated with improved prophylactic efficacy in the high-risk group, which may support the current use of high-dose MTX for this cohort. However, this dose-dependent effect was absent in the moderate-risk group, which may introduce a preliminary notion that high-dose MTX may not be necessary for this cohort. There is another implication that any dose of systemic methotrexate may not be beneficial for the moderate-risk patients. Notably, results from recent large-sample studies suggested that there may be no rationale to use MTX prophylaxis in the immunochemotherapy era, even for patients with high risk factors of CNS relapse [27, 28]. Whether MTX prophylaxis should be given is an ongoing debate right now. In this study, whether low-dose MTX offers a benefit as compared to no systemic MTX in moderate-risk patients remains unknown and should be further compared. Besides, the effect of systemic MTX dose on CNS relapse may be confounded by the use of intrathecal therapy. In the clinical practice, the choice of a low-dose MTX may also be related to elderly age, poor performance status, or pre-existing organ deficiency. Although the baseline characteristics and the proportion of patients receiving intrathecal therapy between the subgroups were balanced in this study, the selection bias may still exist. Overall, the dose-dependent effect of MTX needs to be further confirmed in prospective, randomized controlled trials.
This study has several limitations. First, as this study mainly focused on the administration of prophylactic HD-MTX, patients with risk factors of CNS events but omitting HD-MTX were not included. Therefore, whether HD-MTX could bring benefit to the control of CNS events in the immunochemotherapy era as mentioned above was not evaluated. Also, it would be better to select a matched cohort of DLBCL patients who did not receive HD-MTX as a “control” when addressing whether concurrent HD-MTX would increase the incidence of toxic events and delay. Second, as a retrospective study, it is difficult to ensure that the baseline characteristics were fully balanced between groups, although no significant difference was found in this study. The indication for CNS prophylaxis evolved over time and varied between centers, and ultimate decisions were commonly made by clinicians. The selection bias among patients assigned to HD-MTX prophylaxis is inevitable, so this study may be underpowered when estimating the survival outcomes. Third, the sample size of the EOI group is much smaller than the concurrent group, which was largely interpreted by the preference for concurrent HD-MTX in clinical practice. Given the rarity of CNS relapse, the observations reported in this study are possibly a random effect caused by low patient numbers of the EOI group.
In general, EOI HD-MTX was associated with fewer toxic events, chemotherapy delays, and comparable CNS relapse risk than concurrent HD-MTX. To a certain extent, our study provided some clinical data support for the priority of EOI HD-MTX. High-dose MTX (≥ 2 g/m2) was associated with decreased risk of CNS relapse only in the high-risk group, but not in the moderate-risk group, suggesting that high MTX dose may not be necessary for moderate-risk patients.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS (2015) Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 90(9):790–795
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242
Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB et al (2006) Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24(19):3121–3127
Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R et al (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23(22):5027–5033
Kridel R, Dietrich PY (2011) Prevention of CNS relapse in diffuse large B-cell lymphoma. Lancet Oncol 12(13):1258–1266
Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH et al (2016) CNS International prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 34(26):3150–3156
Kridel R, Telio D, Villa D, Sehn LH, Gerrie AS, Shenkier T et al (2017) Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol 176(2):210–221
Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, Vitolo U et al (2003) Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 21(1):20–27
Mazloom A, Fowler N, Medeiros LJ, Iyengar P, Horace P, Dabaja BS (2010) Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: the M. D. Anderson Cancer Center experience. Leuk Lymphoma. 51(7):1217–24
Fonseca R, Habermann TM, Colgan JP, O’Neill BP, White WL, Witzig TE et al (2000) Testicular lymphoma is associated with a high incidence of extranodal recurrence. Cancer 88(1):154–161
Cai QQ, Hu LY, Geng QR, Chen J, Lu ZH, Rao HL et al (2016) New risk factors and new tendency for central nervous system relapse in patients with diffuse large B-cell lymphoma: a retrospective study. Chin J Cancer 35(1):87
Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R et al (2016) Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 127(18):2182–2188
Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L et al (2014) Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 166(6):891–901
Kanungo A, Medeiros LJ, Abruzzo LV, Lin P (2006) Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol 19(1):25–33
Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N et al (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92(10):1335–1342
Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M (2009) CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 113(17):3896–3902
Gleeson M, Counsell N, Cunningham D, Chadwick N, Lawrie A, Hawkes EA et al (2017) Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol 28(10):2511–2516
Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E et al (2012) Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer 118(11):2944–2951
Tomita N, Takasaki H, Ishiyama Y, Kishimoto K, Ishibashi D, Koyama S et al (2015) Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 56(3):725–729
Schmitz N, Zeynalova S, Glass B, Kaiser U, Cavallin-Stahl E, Wolf M et al (2012) CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann Oncol 23(5):1267–1273
Ferreri AJ, Bruno-Ventre M, Donadoni G, Ponzoni M, Citterio G, Foppoli M et al (2015) Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol 168(5):654–662
Tai WM, Chung J, Tang PL, Koo YX, Hou X, Tay KW et al (2011) Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol 90(7):809–818
Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T et al (2010) Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 116(18):4283–4290
Holte H, Leppä S, Björkholm M, Fluge O, Jyrkkiö S, Delabie J et al (2013) Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol 24(5):1385–1392
Cheah CY, Herbert KE, O’Rourke K, Kennedy GA, George A, Fedele PL et al (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 111(6):1072–1079
Puckrin R, El Darsa H, Ghosh S, Peters A, Owen C, Stewart D (2021) Ineffectiveness of high-dose methotrexate for prevention of CNS relapse in diffuse large B-cell lymphoma. Am J Hematol 96(7):764–771
Orellana-Noia VM, Reed DR, Sen JM, Barlow C, Malecek MK, Kahl BS et al (2020) CNS prophylaxis during front-line therapy in aggressive non-Hodgkin lymphomas: real-world outcomes and practice patterns from 19 US academic institutions. Blood 136:6
Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M et al (2007) Incidence and risk factors of central nervous system recurrence in aggressive lymphoma–a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 18(1):149–157
Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H (2002) Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol 13(7):1099–1107
Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A et al (2002) A multicenter study of treatment of primary CNS lymphoma. Neurology 58(10):1513–1520
Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M et al (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 374(9700):1512–1520
Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J et al (1996) Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol 14(3):925–934
Rizzieri DA, Johnson JL, Byrd JC, Lozanski G, Blum KA, Powell BL et al (2014) Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: cancer and Leukemia Group B study 10 002. Br J Haematol 165(1):102–111
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC et al (2009) Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360(26):2730–2741
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8(5):551–561
Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA (2007) Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 26(20):3735–3752
Wilson MR, Eyre TA, Martinez-Calle N, Ahearne M, Parsons KE, Preston G et al (2020) Timing of high-dose methotrexate CNS prophylaxis in DLBCL: an analysis of toxicity and impact on R-CHOP delivery. Blood Adv 4(15):3586–3593
Funding
This work was supported by grants from the National Natural Science Foundation of China (81973384); the Special Support Program of Sun Yat-sen University Cancer Center (PT19020401), the Science and Technology Planning Project of Guangzhou, China (202002030205), and the Clinical Oncology Foundation of Chinese Society of Clinical Oncology (Y-XD2019-124).
Author information
Authors and Affiliations
Contributions
Y.F. and Q.C. designed the study. Y.F. did the statistical analysis. Y.F., N.S., and S.M. wrote the manuscript. J.C., H.H., Z.L., H.H., Y.X., P.L., L.Z., W.L., L.G., Z.L., Y.W., X.T., J.W., and Y.Z. collected clinical data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the review board of each participating institution. All the procedures were performed in accordance with the 1964 Declaration of Helsinki principles and its later amendments or comparable ethical standards.
Consent to participate
Patients submitted their written informed consents in accordance with the Declaration of Helsinki.
Consent for publication
The authors provided informed consent to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 Non-linear relationship between methotraxate dose and survival outcomes in high- and moderate-risk groups
Association of methotraxate dose with progression-free survival (PFS) and overall survival (OS) in high- and moderate-risk group. (A) Unadjusted nonlinear relationship between methotraxate dose and survival outcomes. B, Nonlinear relationship between methotraxate dose and survival outcomes adjusted with age, gender, ECOG PS, LDH, stage and number of extranodal site. Hazard ratios (HR) are indicated by solid lines and 95% confidence intervals (CI) is by shaded areas.
Supplementary file1 (TIF 943 KB)
Rights and permissions
About this article
Cite this article
Fang, Y., Su, N., Ma, S. et al. Optimization of high-dose methotrexate prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma: a multicenter analysis. Ann Hematol 101, 595–605 (2022). https://doi.org/10.1007/s00277-021-04739-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04739-x