Abstract
Prognosis for relapsed or refractory (R/R) acute myeloid leukemia (AML) despite salvage therapy is dismal. This phase I dose-escalation trial assessed the safety and preliminary clinical activity of selinexor, an oral exportin-1 (XPO1) inhibitor, in combination with FLAG-Ida in younger R/R AML patients. The aim was to find the recommended phase 2 dose (RP2D) and maximum tolerated dose (MTD). Fourteen patients were included, and selinexor dosage was 60 mg (3 patients), 80 mg (3 patients), and 100 mg (7 patients) weekly. No dose-limiting toxicities were reported. Grade ≥3 non-hematologic adverse events (AEs) occurred in 78.6% of patients. Two patients were non MTD evaluable due to early death, and overall, 3 out of 14 patients (21.4%) had fatal AEs. Five out of 12 (42%) response and MTD evaluable patients achieved a complete remission (CR; n=4) or CR with incomplete hematologic recovery (CRi, n=1), and 4 patients (33%) subsequently underwent allogeneic transplantation. The median overall survival (OS) and event-free survival (EFS) were 6.0 (range 0.9-19.3) and 1.1 months (range 0.7-19.3), respectively. Using selinexor 100 mg/weekly, CR/CRi rate of 66.7%, OS 13.6 months (range, 1.6-19.3), and EFS 10.6 months (range, 0.9-19.3). At last follow-up, 3 patients were alive. Selinexor 100 mg/weekly with FLAG-Ida combination in R/R AML showed acceptable tolerability and efficacy, establishing the RP2D of this regimen in future clinical trials. ClinicalTrials.gov Identifier: NCT03661515
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A sizable proportion of acute myeloid leukemia (AML) patients treated upfront with intensive approaches (e.g., with “7+3” regimens) are primarily refractory or relapse rapidly (R/R AML) after first complete remission (CR). Prognosis of R/R AML patients is poor, and there is no standard of care for salvage treatment [1]. The goal of salvage treatment is to achieve a second CR and serve as a bridge for allogeneic stem cell transplantation (allo-SCT), the only known curative therapy. In addition, due to the absence of standard effective therapies, enrollment into clinical trials of novel therapies whenever possible is recommended in this setting. The combination of fludarabine, cytarabine, G-CSF, and idarubicin (FLAG-Ida) is considered one of the most effective salvage therapies, reaching CR rates of up to 53% [1]. In a large Spanish study of patients <65 years with R/R AML treated with FLAG-Ida regimen, a CR/CR with incomplete hematologic recovery (CRi) rate of 51% was reported, with a 9% rate of induction deaths, mainly due to infections [2]. Long-term overall survival (OS) and event-free survival (EFS) after FLAG-Ida regimens in the salvage setting are 8.4 and 2.4 months [1, 2].
Selinexor is a novel, oral small molecule inhibitor that belongs to the group of selective inhibitors of nuclear export (SINE) compounds. Selinexor binds and inactivates the nuclear transport protein exportin 1 (XPO1). XPO1 is the major nuclear exporter of over 200 cargo proteins, including growth regulators and tumor suppressor proteins (TSPs) [3]. XPO1 is overexpressed in multiple tumors, including AML [4, 5], and leads to functional inactivation of TSPs through their aberrant cytoplasmic localization. The transient retention of TSP in the nucleus at high levels through inhibition of XPO1 activates TSP cell cycle checkpoint and genome inspection actions. This leads to apoptosis in malignant cells, while normal cells undergo transient cell cycle arrest and recovery when the export block is released.
The promising role of XPO1 inhibition in AML was demonstrated by preclinical studies showing that selinexor has potent cytotoxic activity in AML cell lines and in murine models, including its ability to kill noncycling leukemic stem cells with minimal effects on normal bone marrow [4,5,6]. In humans, the phase I trial demonstrated that selinexor monotherapy was safe and acceptably tolerated in 81 R/R AML patients, obtaining an objective response rate (ORR; consisting of CR or CRi) of 14%, and 31% of patients had a reduction of blasts of ≥50% [7]. A randomized study of selinexor monotherapy vs. physician choice in patients with R/R AML (median age 74 years, median 2 prior lines) showed an ORR of 12% vs. 3.5% respectively; but OS was not improved [8]. Several combinations of selinexor with intensive chemotherapy and with hypomethylating agents (HMA) have been explored based on the synergistic activity observed in murine models [9, 10]. Combinations of selinexor with intensive chemotherapy have demonstrated acceptable tolerance and CR/CRi rate of 38-48% in the R/R setting [11,12,13,14] and 53-85% in untreated patients with poor-risk AML [13, 15, 16]. In addition, a promising CR/CRi rate of 80% was observed in previously untreated elderly patients using selinexor in combination with decitabine [17]. Until now, the combination of selinexor with FLAG-Ida has not yet been evaluated as salvage therapy.
We hypothesized that the combination FLAG-Ida with selinexor could result in improved response with an acceptable tolerability. The aim of this phase I clinical trial is to assess the tolerability and safety of selinexor in combination with FLAG-Ida for salvage therapy in younger R/R AML patients, as well as the efficacy of this combination.
Patients and methods
Eligibility
This phase I multicenter, open-label, non-randomized, single-arm trial was performed in four institutions from the PETHEMA group (NCT03661515). The key inclusion criteria included patients between 18 and 65 years of age with R/R AML defined as relapse, failure to achieve CR or CR with incomplete hematologic recovery (CRi) after 1 to 3 prior lines of treatment. Exclusion criteria included acute promyelocytic leukemia, Eastern Cooperative Oncology Group (ECOG) performance status > 2, pregnancy, recent treatment with radiotherapy, chemotherapy, immunotherapy or any other anticancer therapy ≤ 2 weeks, pretreatment with a SINE compound, recent major surgery, active infection (including hepatitis B or C and human immunodeficiency virus (HIV)), unstable cardiovascular function, liver dysfunction, severe renal dysfunction and patients unable to swallow tablets, and life-threatening disease (complete inclusion and exclusion criteria are provided in Supplemental Material). Written informed consent was obtained from all patients enrolled. This trial was approved by the Research Ethics Board of each participating hospital, according to the Declaration of Helsinki.
Treatment schedule
Treatment consisted of induction therapy with fludarabine 30 mg/m2/day intravenously on days 1 to 4, idarubicin 10 mg/m2/day intravenously on days 1 to 3, cytarabine 2 g/m2/day intravenously on days 1 to 4, G-CSF 300 mcg/m2/day subcutaneously from days −1 to 5, and oral selinexor (KPT-330) starting at the end of chemotherapy on days 5, 12, and 19. Based on previous clinical trials in multiple myeloma establishing selinexor 100 mg/weekly as the RP2D [18], we planned 100 mg/weekly as the maximum escalating dose for this combination trial. To avoid potential enhancement of myelosuppression, selinexor was administered during 3 weeks, three times per cycle, after completion of FLAG-Ida. Escalating doses of oral selinexor given once weekly for 3 weeks were tested in a 3+3 design, each with 3-6 patients until achieving the maximum tolerated dose (MTD). The following selinexor dosages were administered: 60 mg (level 1); 80 mg (level 2); and 100 mg (level 3). Subsequently, an extension cohort of 3 additional patients were treated with the established dose.
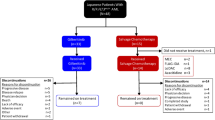
A new cycle was not started if there was an ongoing grade 3 or higher non-hematologic toxicity or persistent grade 3 neutropenia in patients achieving CR. Patients who obtained partial remission (PR) after the first cycle of treatment were treated with another cycle of identical induction therapy (Fig. 1). Patients who achieved CR/CRi after 1 or 2 cycles of FLAG-Ida plus selinexor proceeded to allo-SCT, if feasible, or consolidation therapy, if allo-SCT was not possible.
Consolidation therapy consisted of 1 or 2 cycles of cytarabine 1 g/m2/day intravenously (3 h) on days 1 to 6, combined with oral selinexor. Selinexor was given at the same dosage utilized for induction therapy. Maintenance therapy with selinexor for up to 6 additional cycles (3 weeks on selinexor at the same dose used during induction and 1 week off) was considered in patients in CR/CRi not undergoing allo-SCT.
Patients received concomitant medications to treat symptoms, adverse events (AEs), and intercurrent illnesses that are medically necessary as part of standard care. Use of antibacterial, antifungal, and antiviral agents was also recommended according to each institution’s guidelines. Prophylaxis against Pneumocystis jirovecii had to be administered to all patients as per standard of care for patients receiving FLAG-Ida combination therapy.
Study definitions and endpoints
Dose-limiting toxicity (DLT) was defined as follows: (1) grade 4 non-hematologic toxicity during the induction phase (except in case of disease progression or DLT resolved with optimal therapy), (2) grade 4 neutropenia duration > 56 days from the end of the FLAG-Ida regimen and not attributable to persistent leukemia. If no DLT was observed in 3 evaluable patients, the next selinexor dose level was used in the following 3 patients’ cohort. If a DLT was observed in one patient, 3 additional subjects had to be treated using the same dose, and if ≤2 of these 6 patients experienced DLT, further dose escalation was allowed.
The safety analyses included the following variables: incidence, severity, duration, causality, and type of AE. Analytical changes, deaths (including 30-day mortality), severe AEs, and withdrawals due to AEs were also analyzed. AEs were classified according to Common Terminology Criteria for Adverse Events (CTCAE v4.03). AEs were classified as severe AEs, drug-related AEs, AEs of special concern (nausea, vomiting, neurological toxicity), and AEs that lead to discontinuation of treatment. The frequency, severity, and causal relationship of AEs were analyzed by the system organ class.
The primary objective was to find the recommended phase 2 dose (RP2D) and MTD of selinexor in combination with FLAG-Ida regimen. Secondary objectives were to assess the following: (i) safety and tolerability of selinexor in combination with FLAG-Ida; (ii) hematological and non-hematological toxicity; (iii) CR/CRi after induction treatment; and (iv) preliminary efficacy of selinexor in combination with FLAG-Ida.
Patients were considered evaluable for the safety analysis of selinexor when they met all inclusion criteria, had received at least one dose of selinexor, and had no deviations from the protocol. Only patients with an evaluable bone marrow sample after induction were considered for the efficacy evaluation. Flow cytometry was used to assess response and minimal residual disease (MRD). MRD negativity was defined as a level of blasts <0.01%.
OS was defined as the time from the first selinexor dose until death from any cause, whereas event-free survival (EFS) was defined as the time from the first selinexor dose until treatment failure, relapse, or death due to any cause.
Baseline cytogenetic/molecular and correlative studies
Cytogenetic study of leukemic cells by conventional karyotype was performed, preferably in bone marrow. Conventional karyotype studies with G bands were carried out using conventional methods. Karyotype was analyzed after 24h and 48h of culture. Molecular cytogenetic analysis performed by FISH for the detection of the most relevant chromosome abnormalities included core-binding factor (CBF) alterations t(8; 21), inv(16), t(15; 17), alterations of the chromosomes 5 and 7, and 11q23 anomalies.
Next-generation sequencing (NGS) was performed according to collaborative PETHEMA group recommendations (NGS-LMA NCT03311815) with Oncomine Myeloid Research Assay (Thermo Fisher Scientific), including 42 DNA target genes (hotspot genes: ABL1, BRAF, CBL, CSF3R, DNMT3A, FLT3, GATA2, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, MYD88, NPM1, NRAS, PTPN11, SETBP1, SF3B1, SRSF2, U2AF1, WT1 and full genes: ASXL1, BCOR, CALR, CEBPA, ETV6, EZH2, IKZF1, NF1, PHF6, PRPF8, RB1, RUNX1, SH2B3, STAG2, TET2, TP53 ZRSR2) and 29 RNA fusion transcript driver genes (ABL1, ALK, BCL2, BRAF, CCND1, CREBBP, ETV6, EGFR, FGFR1, FGFR2, FUS, HMGA2, JAK2, KMT2A, MECOM, MET, MLLT10, MLLT3, MYBL1, MYH11, NTRK3, NUP214, PDGFRA, PDGFRB, RARA, RBM15, RUNX1, TCF3, TFE3) in a broad fusion panel [19].
Statistical analysis
Quantitative and qualitative variables were summarized using descriptive statistics. Continuous variables were described with the number of observations (N), the mean and/or the median, the standard deviation, the minimum, and the maximum. Categorical variables were presented using frequencies and percentages. All data have been numbered and used to the greatest extent possible, without any imputation of missing data. Any deviation from the statistical methods given in the study protocol has been described and justified. Regarding the efficacy variables, CR, CRi, PR, therapeutic failure, and disease recurrence were evaluated separately, according to the recommendations for the response criteria of the International Working Group [20]. OS and EFS were analyzed descriptively using Kaplan-Meier methods.
Results
Patient characteristics
Between July 2018 and August 2019, 16 patients were screened for eligibility and 14 patients were enrolled (Fig. 2). All of them have R/R AML from a previous de novo AML. Twelve patients were assessed for efficacy and MTD. Two patients were not evaluable due to early death from unrelated causes before completing the DLT period.
The median age of patients was 52.5 years (range, 25-64), 50% were male, and all of the patients had Caucasian origin. Half of the patients had ECOG performance status of 0, while 43% patients had ECOG 1 and 7% ECOG 2. Eight (57%) patients received 1 prior line of treatment (range 1-3), whereas four (29%) received ≥2 prior therapies. According to the 2017 ELN guidelines [21], 85.7% and 14.3% were classified as adverse and intermediate genetic risk, respectively. The most frequently mutated genes were FLT3 (36%), TP53 (21%), IDH2 (21%), and WT1 (21%). Fusion transcript KMT2A-ELL was identified in only one case. Baseline characteristics are summarized in Table 1 (complete biological characteristics of patients are provided in Supplemental Table 1).
All patients treated with selinexor 100 mg received only 1 prior therapy (100% vs. 33% and 50% in the 60 mg and 80 mg cohorts), had more intermediate risk disease (29% vs. 0% and in 60 mg and 80 mg cohorts), and had lower median bone marrow blast (53% vs. 84% and 81% in 60 mg and 80 mg cohorts).
Dose escalation and treatment
Three patients were included in the dose level 1 cohort (selinexor 60 mg/week), four patients in the dose level 2 (selinexor 80 mg/week), as the first patient had early progression during the induction period and was withdrawn from the trial. The dose level 3 (selinexor 100 mg/week) was completed with the remaining 7 patients (Fig. 2). All patients received full dose of FLAG-Ida without delay, and 12 patients received the 3 complete doses of induction selinexor dose (no additional induction cycles were received). Four patients proceeded to consolidation (all received the complete cycle), and no patient received maintenance and 4 patients underwent allo-SCT.
Two patients did not complete the treatment regimen, per protocol. One patient had premature death during induction, and another patient discontinued treatment due to a diagnosis of multiple myeloma after 3 doses of consolidation cycle 1. One patient receiving dose level 3 (100 mg) interrupted selinexor during the third week of cycle 1 due to grade 3 hepatic adverse event.
All patients received antifungal prophylaxis, 7 with posaconazole, 6 with voriconazole, and one with isavuconazole. Eleven of 14 patients received prophylaxis for Pneumocystis jirovecii with trimethoprim/sulfamethoxazole.
Safety
Hematological toxicity was analyzed in the 12 patients who responded to treatment, since treatment-resistant patients did not recover peripheral blood counts at any time. The median duration of grade 4 neutropenia (<0.5 × 109/L) was 40 days (range, 22-63) during induction and 15 days (range, 13-57) during consolidation. The median duration of grade 4 thrombocytopenia (<20 × 109/L) for induction was 21 days (range, 0-41), while in consolidation, it was 18 days (range, 6-50).
All patients received transfusions with red blood cell and platelet concentrates. The mean number of red cell units transfused was 8 (range, 3-11) at induction and 6 (range, 1-8) at consolidation, while the mean number of platelet concentrates transfused during induction was 5 (range, 5-9), and 4.5 (range, 1-8) during consolidation.
All the 14 patients had AEs, being severe in 11 patients (78.6%). The total number of non-hematological AEs was 141, with a mean of 10 AEs per patient (Table 2). In 14 induction cycles administered, 121 AEs were recorded, representing an average of 8.6 AEs per cycle (range, 4-18). In the 4 cycles of consolidation, 20 AEs were recorded with a mean of 5 AEs per cycle (range, 0-8). No DLTs were reported in the R/R AML patient during dose escalation of selinexor and, therefore, the MTD was not established. Thus, 100 mg of selinexor was the RP2D in combination with FLAG-Ida.
Most of the AEs were mild, with 76.6% classified as grades 1-2 and 23.4% as grade ≥ 3. There were 3 fatal AEs (2.1%), two pulmonary infections and one cerebral hemorrhage. None of them was related to selinexor treatment. The most common non-hematologic AEs occurring in over 20% of patients were as follows (Table 2): febrile neutropenia (57.1%), diarrhea (50.0%), nausea/vomiting (57.1%), mucositis (50.0%), pulmonary infection (50.0%), fever (35.7%), enteritis (35.7%), headache (28.5%), anorexia (21.4%), abdominal pain (21.4%), exanthema/rash (21.4%), and pain (21.4%).
A total of 35 AEs (24.8%) were considered related with selinexor. The relation of these toxicities with selinexor was considered definite in 12 AEs, possible in 16 AEs, and probable in 7 AEs. The most frequent possible/probable/definite AEs selinexor-related were diarrhea (35.7%), nausea/vomiting (28.6%), and mucositis (28.6%). The majority of selinexor-related AEs (75.8%) were completely resolved; however, in 6 cases, there were some sequelae (17.1%) and 3 AEs continued to the end of the trial (8.6%). There were a total number of 33 serious AEs, and no association between selinexor dose and severe AE incidence was observed (Table 3).
One patient with grade 3 elevation of liver enzymes required a dose interruption of selinexor 100 mg and a 15-day hospitalization. The patient recovered with sequelae, maintaining an enzymatic basal state equivalent to a grade 1 AE. However, it was assessed by his/her physician as related to a previous autoimmune disease of the patient.
Efficacy
Twelve of the 14 patients included in the trial were evaluable for response, achieving CR in 4 patients (33.3%) and CRi in 1 patient (8.3%) for a total CR/CRi of 41.7% (Supplemental Table 2, Fig. 3). Two patients were not evaluable: one due to early death during induction (day +17) due to cerebral hemorrhage and another died on day 40 with morphologic leukemia-free state in bone marrow before further evaluation. Out of 5 patients with CR/CRi, 4 received a median of 1 cycle of consolidation (range 1-1) and 4 subsequently received an allo-SCT. MRD was negative in all 4 patients who achieved initial CR (Supplemental Table 2). The patient with CRi was not assessed for MRD because the sample was not evaluable.
Four of 6 patients (66.7%) treated with the 100 mg weekly dose of selinexor achieved a response, while 1 of 6 patients in the 60 and 80 mg dose cohorts responded (Table 4). CR/CRi was observed in 1 out of 2 patients with intermediate cytogenetic risk and 4 out of 10 patients with adverse cytogenetic risk. All 5 responders received 1 prior therapy. Bone marrow blast levels of >70% were associated with worse CR/CRi (16.7%; 1 of 6 patients) compared to patients with blasts ≤70% (66.7%; 4 of 6 patients). No correlations were observed between mutations and response (data not shown).
Three patients (25%) were alive at the time of the last follow-up (median 6.4 months, range 1.1-19.6). The median OS and EFS were 6.0 months (range 0.9-19.3; Fig. 4a) and 1.1 months (range 0.7-19.3; Fig. 4b), respectively. Patients treated with selinexor 100mg had better median OS and EFS than patients treated with other doses (60 mg: OS 5.1 months (range, 2.2-7.0) and EFS 0.8 months (range, 0.8-5.1), Fig. 5; 80 mg: OS 2.1 months (range, 0.9-13.3) and EFS 1.0 months (range, 0.7-1.0), Fig. 5; 100 mg: OS 13.6 months (range, 1.6-19.3) and EFS 10.6 months (range, 0.9-19.3), Fig. 5). Two out of 4 patients who received an allo-SCT in CR/CRi were alive at the time of analysis follow-up (median follow-up post allo-SCT 3.9 months, range 1.7-4.3; Fig. 3).
Discussion
The combination of selinexor with FLAG-Ida for induction in AML was tolerable and effective in adult R/R AML patients, with a CR/CRi of 41.7%. No DLTs occurred during the trial, and the MTD was not reached, as in previous trials [7, 15]. We established an RP2D of selinexor 100 mg dosed once weekly for 3 weeks for future phase II clinical trials in combination with FLAG-Ida.
Despite modest efficacy of selinexor monotherapy in R/R AML [7] and myelodysplastic syndromes (MDS) or oligoblastic AML refractory to HMA (ORR 26%) [22], several combination regimens containing selinexor have shown potential synergy with promising efficacy in AML. In our study, the combination of selinexor with FLAG-Ida resulted in a CR/CRi of 41.7% (62.5% in patients with only 1 prior regimen), similar to that observed in other R/R AML trials combining selinexor with intensive chemotherapy: 47% with fludarabine/cytarabine in a pediatric cohort of 15 patients [11]; 48% with idarubicin/cytarabine in a cohort of 45 adult patients [12]; 38% with HIDAC/mitoxantrone in the subset of 8 R/R AML patients [13], and 45% with cladribine, cytarabine, and G-CSF (CLAG) in a cohort of 40 patients [14]. Of note, the PETHEMA study reported 52% CR/CRi rate with FLAG-Ida with or without gemtuzumab ozogamicin used as the first salvage therapy [2], similar to the results described here for patients with one prior therapy. As first-line therapy, selinexor added to cytarabine and daunorubicin (7+3) obtained a 53% of CR/CRi in 19 evaluable patients with poor-risk AML [15]; and 85% with frontline treatment with cytarabine and daunorubicin (7+3) in 20 patients > 60 years [16]; and 83% with selinexor in combination with high-dose cytarabine and mitoxantrone [13]. Recently, a phase I trial suggested that selinexor maintenance therapy after allo-SCT is safe and feasible in a cohort of 12 patients with high-risk AML and MDS [23], with a median PFS and OS of 775 days and 872 days respectively. Maintenance therapy with selinexor was not tested in our study, although selinexor could be continued into consolidation.
The OS in our study with selinexor combined with FLAG-Ida (median, 6.0 months) was similar to that reported by other studies in R/R AML using combinations with idarubicin/cytarabine (median, 8.0-8.2 months) [12], CLAG (median, 7.8 months) [14], and decitabine (median, 5.9 months) [17]. We observed median EFS of 1.1 months, but EFS was only reported in the CLAG study (6.1 months) [14]. Survival rates in the overall population (all dose levels) were similar to historical control of patients with R/R AML treated with FLAG-Ida (median OS 8.4 months) [1]. We show acceptable OS and EFS (13.6 months and 10.6 months, respectively) in the 100 mg cohort, probably related to a relatively high CR/CRi rate (66.7%) in these patients (with a negative MRD in 3 out of 4 responders). A better response with higher selinexor doses was suggested previously in combination with HIDAC plus mitoxantrone [13], idarubicin/cytarabine [12], and decitabine [17], but it was not reproduced with selinexor monotherapy [7]. However, in our opinion, the higher efficacy observed in the 100 mg dose is likely related to more favorable baseline characteristics in this cohort (all patients with only 1 prior line, more intermediate risk, and lower bone marrow blast). It is noteworthy that other trials reported worse [13] or equal CR/CRi [7, 15] associated with adverse cytogenetic risk, and number of prior therapies was not related with response [7]. On the other hand, the correlation between lower bone marrow blast and better CR/CRi was also observed in a selinexor monotherapy trial [7]. Of note, 100 mg selinexor once weekly is approved in combination with bortezomib and dexamethasone in patients with previously treated multiple myeloma, and is the highest once-weekly dose recommended [24].
Selinexor plus FLAG-Ida showed an acceptable safety profile, with only 23.4% of patients experiencing severe AEs (grade ≥3); febrile neutropenia (57.1%), pulmonary infections (42.9%), and digestive tract disorders (28.5%) being the most frequently reported severe AEs. The non-hematologic AEs reported were similar to those in other studies combining selinexor with intensive and non-intensive therapy, notably febrile neutropenia [11, 13, 17], pulmonary infections [11, 13, 17], and digestive tract disorders [12,13,14,15, 17]. Most of these AEs were well-known and associated with intensive chemotherapy. Regarding selinexor-related AEs, diarrhea (35.7%), nausea/vomiting (28.6%), and mucositis (28.6%) were the main reported AEs in our study. Diarrhea and nausea/vomiting have been previously reported, but less frequently, as treatment emergent AEs with selinexor [7, 12,13,14,15, 17, 22,23,24]. However, mucositis has not been described in previous selinexor trials, and we can speculate that the high frequency here reported was mainly induced by FLAG-Ida regimen [25]. Other studies have reported asymptomatic hyponatremia as one of the most frequent selinexor-related AEs [11, 15, 17], but it was rarely reported in our trial. It should be noted that asthenia, anorexia, and even encephalopathy, which have been reported in older AML patients receiving selinexor monotherapy, were not observed in this trial. This seems to suggest that such AEs could occur mainly in elderly patients, given their susceptibility to neurological toxicities, and/or in those given twice weekly selinexor which has been used previously. A novel second-generation SINE has recently been developed, eltanexor (KPT-8602), with a potential reduction of severe AEs associated with a lower penetration in the central nervous system [26].
In summary, the results of the combination of selinexor with FLAG-Ida in adult patients with R/R AML suggest an acceptable tolerability and potent antileukemic efficacy. The dose of selinexor 100 mg weekly showed efficacy and acceptable tolerability. Despite the very small cohort of RP2D (100 mg/week), the results in terms of tolerability, responses, and feasibility compared to FLAG-Ida alone could justify further phase 2 trial with dose expansion in order to elucidate whether a phase 3 could be recommended, or not. Further clinical trials with selinexor 100 mg plus FLAG-Ida are needed to establish potential usefulness of this regimen in R/R AML patients younger than 65 years.
References
Megías-Vericat JE, Martínez-Cuadrón D, Sanz MÁ, Montesinos P (2018) Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol 97(7):1115–1153
Bergua JM, Montesinos P, Martinez-Cuadrón D, Fernández-Abellán P, Serrano J, Sayas MJ, Prieto-Fernandez J, García R, García-Huerta AJ, Barrios M, Benavente C, Pérez-Encinas M, Simiele A, Rodríguez-Macias G, Herrera-Puente P, Rodríguez-Veiga R, Martínez-Sánchez MP, Amador-Barciela ML, Riaza-Grau R, Sanz MA, the PETHEMA group (2016) A prognostic model for survival after salvage treatment with FLAG-Ida +/- gemtuzumab ozogamicine in adult patients with refractory/relapsed acute myeloid leukaemia. Br J Haematol 174(5):700–710
Fung HY, Chook YM (2014) Atomic basis of CRM1-cargo recognition, release and inhibition. Semin Cancer Biol 27:52–61
Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT et al (2013) KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol 161(1):117–127
Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, Walker A, Klisovic R, Blum W, Caligiuri M, Croce CM, Marcucci G, Garzon R (2012) Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 120(9):1765–1773
Etchin J, Montero J, Berezovskaya A, Le BT, Kentsis A, Christie AL et al (2016) Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia. 30(1):0–9
Garzon R, Savona M, Baz R, Andreeff M, Gabrail N, Gutierrez M, Savoie L, Mau-Sorensen PM, Wagner-Johnston N, Yee K, Unger TJ, Saint-Martin JR, Carlson R, Rashal T, Kashyap T, Klebanov B, Shacham S, Kauffman M, Stone R (2017) A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 129(24):3165–3174
Sweet K, Blum W, Donner H, Flinn I, Frankfurt O, Heuser M, Kota V, Liu H, Raffoux E, Roboz G, Rollig C, Savona M, Showel M, Strickland S, Vives S, Tang S, Unger T, Kauffman M, Shah J, Shacham S, Montesinos P (2019) A randomized, open-label, phase II study of selinexor versus physician’s choice (PC) in older patients with relapsed or refractory acute myeloid leukemia (AML). Hemasphere. 3:82–83
Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM (2014) Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol 27:62–73
Ranganathan P, Yu X, Santhanam R, Hofstetter J, Walker A, Walsh K, Bhatnagar B, Klisovic R, Vasu S, Phelps MA, Devine S, Shacham S, Kauffman M, Marcucci G, Blum W, Garzon R (2015) Decitabine priming enhances the antileukemic effects of exportin 1 (XPO1) selective inhibitor selinexor in acute myeloid leukemia. Blood. 125(17):2689–2692
Alexander TB, Lacayo NJ, Choi JK, Ribeiro RC, Pui CH, Rubnitz JE (2016) Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J Clin Oncol 34(34):4094–4101
Fiedler W, Chromik J, Amberg S, Kebenko M, Thol F, Schlipfenbacher V, Christine Wilke A, Modemann F, Janning M, Serve H, Ganser A, Bokemeyer C, Theile S, Deppermann U, Kranich AL, Heuser M (2020) A phase II study of selinexor plus cytarabine and idarubicin in patients with relapsed/refractory acute myeloid leukaemia. Br J Haematol 190(3):e169–e173
Wang AY, Weiner H, Green M, Chang H, Fulton N, Larson RA, Odenike O, Artz AS, Bishop MR, Godley LA, Thirman MJ, Kosuri S, Churpek JE, Curran E, Pettit K, Stock W, Liu H (2018) A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J Hematol Oncol 11(4):4
Abboud R, Chendamarai E, Rettig MP, Trinkaus KM, Riedell PA, Abboud CN, Ghobadi A, Pusic I, Stockerl-Goldstein K, Schroeder MA, Vij R, Westervelt P, DiPersio JF, Uy GL (2020) Selinexor combined with cladribine, cytarabine, and filgrastim in relapsed or refractory acute myeloid leukemia. Haematologica. 105(8):e404–e407
Sweet K, Komrokji R, Padron E, Cubitt CL, Turner JG, Zhou J, List AF, Sallman DA, Dawson JL, Sullivan DM, Chavez J, Shah BD, Lancet JE (2020) Phase I clinical trial of selinexor in combination with daunorubicin and cytarabine in previously untreated poor-risk acute myeloid leukemia. Clin Cancer Res 26(1):54–60
Pardee TS, Pladna KM, Lyerly S, Dralle S, Manuel M, Ellis LR et al (2020) Frontline selinexor and chemotherapy is highly active in older adults with acute myeloid leukemia (AML). Blood 136(Supplement 1):24–25
Bhatnagar B, Zhao Q, Mims AS, Vasu S, Behbehani GK, Larkin K, Blachly JS, Blum W, Klisovic RB, Ruppert AS, Orwick S, Oakes C, Ranganathan P, Byrd JC, Walker AR, Garzon R (2020) Selinexor in combination with decitabine in patients with acute myeloid leukemia: results from a phase 1 study. Leuk Lymphoma 61(2):387–396
Bahlis NJ, Sutherland H, White D, Sebag M, Lentzsch S, Kotb R, Venner CP, Gasparetto C, del Col A, Neri P, Reece D, Kauffman M, Shacham S, Unger TJ, Jeha J, Saint-Martin JR, Shah J, Chen C (2018) Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 132(24):2546–2554
Sargas C, Ayala R, Chillón MC, Larráyoz MJ, Carrillo-Cruz E, Bilbao C, Yébenes-Ramírez M, Llop M, Rapado I, García-Sanz R, Vázquez I, Soria E, Florido-Ortega Y, Janusz K, Botella C, Serrano J, Martínez-Cuadrón D, Bergua J, Amigo ML, Martínez-Sánchez P, Tormo M, Bernal T, Herrera-Puente P, García R, Algarra L, Sayas MJ, Costilla-Barriga L, Pérez-Santolalla E, Marchante I, Lavilla-Rubira E, Noriega V, Alonso-Domínguez JM, Sanz MÁ, Sánchez-Garcia J, Gómez-Casares MT, Pérez-Simón JA, Calasanz MJ, González-Díaz M, Martínez-López J, Barragán E, Montesinos P (2020) Networking for advanced molecular diagnosis in acute myeloid leukemia patients is possible: the PETHEMA NGS-AML project. Haematologica., 0
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia (2003) Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21(24):4642–4649
Döhner H, Estey EH, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 129(4):424–447
Taylor J, Mi X, Penson AV, Paffenholz SV, Alvarez K, Sigler A, Chung SS, Rampal RK, Park JH, Stein EM, Tallman MS, Sen F, Gönen M, Abdel-Wahab O, Klimek VM (2020) Safety and activity of selinexor in patients with myelodysplastic syndromes or oligoblastic acute myeloid leukaemia refractory to hypomethylating agents: a single-centre, single-arm, phase 2 trial. Lancet Haematol 7(8):e566–e574
Cooperrider JH, Fulton N, Artz AS, Larson RA, Stock W, Kosuri S, Bishop M, Liu H (2020) Phase I trial of maintenance selinexor after allogeneic hematopoietic stem cell transplantation for patients with acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant 55(11):2204–2206
Karyopharm Therapeutics Inc (2020) XPOVIO® (selinexor): US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212306s005lbl.pdf Accessed 09 March 2021
de la Rubia J, Regadera A, Martín G, Cervera J, Sanz G, Martínez J, Jarque I et al (2002) FLAG-IDA regimen (fludarabine, cytarabine, idarubicin and G-CSF) in the treatment of patients with high-risk myeloid malignancies. Leuk Res 26(8):725–730
Hing ZA, Fung HY, Ranganathan P, Mitchell S, El-Gamal D, Woyach JA et al (2016) Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 30(12):2364–2372
Acknowledgements
We are grateful to all participating institutions and clinicians in the PETHEMA group, and all the patients included.
Funding
This investigator-sponsored study was granted by Karyopharm.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Martínez Sánchez, M.P., Megías-Vericat, J.E., Rodríguez-Veiga, R. et al. A phase I trial of selinexor plus FLAG-Ida for the treatment of refractory/relapsed adult acute myeloid leukemia patients. Ann Hematol 100, 1497–1508 (2021). https://doi.org/10.1007/s00277-021-04542-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04542-8