Abstract
Non-transfusion-dependent thalassaemia (NTDT) is associated with a hypercoagulable state with thrombotic risk highest after splenectomy. Various mechanisms have been proposed. Although an antiplatelet agent is commonly recommended as thromboprophylaxis in NTDT, the role of platelets contributing to this hypercoagulable state is not well-defined. This study aims to evaluate the role of platelets contributing to hypercoagulability in NTDT patients using thrombin generation (TG). Platelet-rich (PRP) and platelet-poor plasma (PPP) were collected from NTDT patients (n = 30) and normal controls (n = 20) for TG measurement and compared. Controls had higher endogenous thrombin potential (ETP) in PPP (1204.97 nM.min vs 911.62 nM.min, p < 0.001) and PRP (1424.23 nM.min vs 983.99 nM.min, p < 0.001) than patients. Patients’ mean normalized ETP ratio [{PRP ETP (patient)/PPP ETP (patient)}/{mean PPP ETP (controls)/mean PPP ETP (controls)}], demonstrated that the presence of platelet does not alter ETP (mean ratio 0.97, 95% CI 0.93–1.02, equivalence defined as 10%). Types of thalassaemia, splenectomy, and severity of liver iron overload did not significantly influence patients’ ETP in PPP and PRP by multivariate analysis. Platelets did not increase the TG potential of NTDT patients. Instead of being hypercoagulable, our NTDT patients were hypocoagulable by ETP measurement, although this could not be conclusively demonstrated to correlate with their iron overloading state giving rise to reduced synthesis of coagulation factors. The guideline recommendations for thromboprophylaxis with antiplatelet agents in similar NTDT patients should be re-examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thrombosis and less commonly arterial thrombosis are cited as major complications of non-transfusion-dependent thalassaemia (NTDT) which encompasses beta thalassaemia intermedia, HbE/beta thalassaemia and HbH disease. Thrombotic events have been reported to account for up to 10% of mortality amongst these patients with the highest risk in patients who have undergone splenectomy [1,2,3,4,5]. To reduce thrombotic events, the Thalassaemia International Federation guidelines for the management of NTDT currently recommends consideration of aspirin therapy in splenectomized NTDT patients with elevated platelet counts above 500 × 109/L [6]. Robust evidence supporting the use of antiplatelet therapy in NTDT patients are however currently limited and wanting. As antiplatelet therapy is not innocuous, more compelling scientific data to define the role of platelets and support targeting platelets for reducing thrombotic events is necessary.
A hypercoagulable state in patients with NTDT has previously been demonstrated by using thromboelastography (TEG) [7]. While TEG measures the global haemostatic functions with respect to the dynamics of clot formation, stabilization and dissolution in whole blood, it however does not definitively identify the contributions of the different components in whole blood towards this state. Studies using assays to measure thrombin generation (TG) in platelet-poor plasma (PPP) from NTDT however did not find any increase in TG [7, 8]. By inference, this would suggest that this hypercoagulability was possibly conferred by red cells and/or platelet factors rather than plasma coagulation factors. Further efforts to elucidate a direct role of platelets have not been attempted. Because TG can also be measured using platelet-rich plasma (PRP), TG assays offers an opportunity to investigate the role of platelets. Hence, a comparison of TG between PRP and PPP specimens may be used to determine the contributions of platelets and plasma components in hyper- or hypocoagulable state. In essence, this provides a measure of the functional state of coagulation albeit indirectly.
Based on the hypothesis that NTDT patients are hypercoagulable because of an increase in platelet-dependent TG, we performed a study to compare TG in PRP and PPP using a calibrated automated thrombogram (CAT) system in NTDT patients and a population of controls. This paper reports the findings of our study.
Materials and methods
This study protocol was approved by our local Institutional Review Board and all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation of our institution. Informed consent was obtained from all the subjects for being included in the study. Recruitment of subjects was conducted between June 2014 and May 2015.
Patients
All NTDT patients managed by the Department of Haematology, Singapore General Hospital, were screened for the study. NTDT, as defined by the Thalassaemia International Federation, includes beta thalassaemia intermedia, HbE/beta thalassaemia (mild and moderate forms) and HbH disease (deletional and non-deletional). The exclusion criteria were pregnancy and patients on active anticoagulation.
Demographic and clinical parameters collected included diagnosis (type of thalassaemia), age, gender, splenectomy, red cell transfusion, chelation, history of arterial and venous thromboembolism, pulmonary hypertension, measures of iron overload (serum ferritin, liver and myocardial T2*), medication (including oral contraceptive pills and hormone replacement therapy, anti-platelets, anticoagulants) and malignancy.
A cohort of 20 healthy individuals was recruited as normal controls. Those with splenectomy, history of thrombotic or bleeding disorder, malignancy, use of anticoagulation, antiplatelet or oral contraceptives or have other conditions known to alter the haemostatic balance were excluded.
Blood sampling and plasma preparation
After obtaining informed consent, blood samples from patients and healthy subjects were collected into vacuum tubes using 21G needle without tubing. Samples were first collected into a vacuum tube (BD, Franklin Lakes, NJ, USA) containing K2 EDTA 5.4 mg for the measurement of full blood count. Three subsequent samples were collected in vacuum tubes (BD, Franklin Lakes, NJ, USA) containing 0.109 M sodium citrate as an anticoagulant at a proportion of 9:1 (blood:anticoagulant). The samples were then centrifuged within 30 min at room temperature using two separate procedures:
-
1.
Centrifugation for 5 min at 2000g, followed by 5 min at 2000g (Hettich Rotofix 32A, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) to obtain PPP. The PPP was then quick-frozen and stored at − 80 °C until testing for TG, which were performed not later than 6 months after blood collection.
-
2.
Centrifugation for 15 min at 150g (Hettich Rotofix 32A, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) to obtain the PRP. Supernatant plasma was harvested and platelets were counted using the Sysmex XN9000 automated haematology analyzer. Platelet numbers from each specimen were then adjusted by dilutions of the autologous platelet-rich plasma into autologous PPP to a standard platelet count of 150 × 10(9)/L. TG measurement was performed on PRP specimens within 1 h of preparation.
Thrombin generation
TG in PPP and PRP were analyzed using CAT ® (Diagnostica Stago S.A.S, Asnières sur Seine Cedex, France) system with PPP Reagent™, PRP Reagent™, Thrombin Calibrator™ and FluCa kit™ (Diagnostica Stago S.A.S, Asnières sur Seine Cedex, France). TG was assessed as endogenous thrombin potential (ETP) as previously described [9, 10]. Briefly, the CAT assay was performed in a prewarmed plate fluorometer. To each well, 80 μL of plasma was added in combination with 20 μL of the trigger: for PPP, the final concentrations of the trigger were 5pM tissue factor and 4 μM phospholipid; for PRP, the final concentration of tissue factor in the trigger was 0.5pM without added phospholipids. TG was then started and simultaneously measured by dispensing 20 μL of a mixture of fluorogenic substrate and buffer containing calcium chloride to the plasma. Data are obtained using ThrombinoscopeTM software (Thrombinoscope BV, Maastricht, The Netherlands). CAT parameters generated were (1) lag time (min): the initiation phase of clotting which equals to the clotting time; (2) peak height (nM): the maximal amount of thrombin formed; (3) ETP (nM*min): the area under the curve representing TG and decay in time; and (4) time to peak (min): the time needed to achieve the peak height.
Laboratory tests
Full blood count was performed using the Sysmex XN9000 automated haematology analyzer (Sysmex Corporation, Kobe, Japan) while prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured using the Sysmex CS2100i automated coagulometer (Sysmex Corporation, Kobe, Japan) with the Dade Innovin and Dade Actin FSL reagents (Siemens Healthcare, Marburg, Germany) respectively for all the subjects. Based on interim analysis of data, factor V and factor VII levels were subsequently measured using the Sysmex CS2100i coagulometer with the factor V deficient plasma (Diagnostica Stago, S.A.S, Asnières sur Seine Cedex, France) and factor VII deficient plasma (Siemens Healthcare, Marburg, Germany).
Statistical analysis
Results were analyzed using the SPSS version 23 (IBM Corporation, USA) with statistical significance set at p < 0.05. Descriptive parameters for numerical variables were presented as mean [standard deviation (SD)] and n (%) for categorical variables. Differences between patients and controls for numerical variables were analyzed using 2-sample t test when normality and homogeneity assumptions were satisfied. Otherwise, Mann-Whitney U was performed. Chi-square/Fisher’s exact tests were performed on categorical variables. Adjusted analyses for numerical outcomes were performed using general linear models (GLM).
Results
A total of 60 NTDT patients were screened. Two patients were excluded as they were on anticoagulation for indications not related to underlying NTDT (one for atrial fibrillation and one for anti-phospholipid syndrome). Twenty-eight patients declined to participate and 30 patients were eventually recruited. Twenty healthy subjects were recruited as normal control.
The baseline characteristics of NTDT patients and controls are shown in Table 1. While the gender distribution was similar, NTDT patients were significantly older than the controls. At baseline, both groups had similar platelet counts and aPTT but the patient group had significantly longer PT and corresponding lower factor V and factor VII than the control group. Their mean PT and factor VII were however within the normal reference ranges (9.9–11.4 s for PT, 70–170% for factor V and 40–180% for factor VII) of the local laboratory.
Clinical characteristics of the patients with NTDT are shown in Table 2. Almost half of the patients had HbH disease and approximately a quarter of the patients had splenectomy. Majority of the patients were below the age of 50. One patient had a history of malignancy but was treated and in remission during the time of the study. One patient was on antiplatelet for post-splenectomy primary prophylaxis while none was on anticoagulation. No patients had a history of arterial or venous thrombosis. Two patients admitted to taking traditional Chinese medicine infrequently. Such medicine was stopped for more than 1 week before blood sampling. The mean and median ferritin levels of the patients were 975.3μg/l and 558μg/l respectively (ranging from 74.6μg/l to 4967μg/l). The mean platelet count for patients with splenectomy was significantly higher than those without splenectomy (617 × 109/L vs 179 × 109/L, p < 0.01).
Approximately a quarter of the patients did not have a formal assessment of cardiac and liver iron load. These patients were older and aged above 50 years. For patients with cardiac/liver MRI T2* performed, the MRI T2* images were analyzed using CMRTools software (Cardiovascular Imaging Solutions Ltd. [CVIS], London, UK) and their liver iron concentrations (LIC) were estimated using validated formula of conversion from T2* values (LIC = 31.94(T2*)− 1.014) [11] based on the latest MRI T2* report at the point of enrolment to the study. They had mean LIC of 14.93 mg/g dw (SD = 10.19 mg/d dw, min = 3.52 mg/g dw and max = 35.54 mg/g dw). More than 90% of these patients had clinically significant liver iron overload (LIC of more than 5 mg/g dw) but only one patient had established liver cirrhosis from secondary haemochromatosis. None, except one, had cardiac iron loading on their latest MRI-T2* at the time of the study. That particular patient had a cardiac T2* of 16.9 ms.
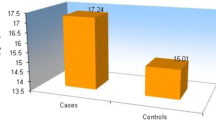
The representative TG parameters in both PPP and PRP samples of the patients and controls are shown in Fig. 1. With adjustment for age and gender, patients with NTDT had significantly less mean ETP compared to controls in both PPP and PRP (Table 3). There was no significant difference between patients and controls for the rest of the TG parameters in both PRP and PPP (Table 3). Overall, the mean ETP in PRP of all subjects were higher than that in PPP (1172.33 ± 316.55 nM.min vs 1039.14 ± 261.28 nM.min, p < 0.001).
As the ETPs in both PPP and PRP of the patients were lower than the controls, the ETP ratio (ETP in PRP/ETP in PPP) for each subject was calculated to allow for more robust evaluation of the role of the platelets between patients and controls in generating TG. The mean ETP ratio of the controls was 1.1677. To evaluate the contribution of patients’ platelet to ETP, a normalized ETP ratio (individual patient’s ETP ratio/1.1677) was established. A normalized ratio of 1 would mean the extent of reduction of a patient’s ETP in PPP and in PRP, respectively, compared to the controls is similar, i.e. the thrombin-generating function of the platelets is similar between patients and controls. A ratio of > 1 would mean there is more ETP generated in patients’ PRP and with the converse being the case if the ratio is < 1. Taking into account the standard deviation of ETP of our general population (based on 145 normal subjects, our locally established mean ETP in PPP was 1329.2 nM.min with SD of 164.9 nM.min; mean ETP in PRP of 1421 nM.min with SD 259 nM.min were established based on the 20 normal subjects in this study), equivalence was pre-determined to be 10% (95% confidence interval of patients’ normalized ETP ratio of 0.9 to 1.1). The mean normalized ETP ratio of the patients was 0.97 (95% CI 0.93–1.02) and this falls within the equivalence range.
Subgroup analysis of ETPs by type of thalassaemia, splenectomy status and severity of liver iron overload is shown in Table 4. Notably, patients with different types of thalassaemia had varying ETP levels although none reached statistical significance. In PPP, patients with beta thalassaemia intermedia had a mean difference of ETP of approximately between 160 nM.min and 200 nM.min lower compared to the other types of thalassaemia. A similar trend of ETP results amongst patients with beta thalassaemia intermedia was however not demonstrated in PRP. In PRP, patients with HbH disease had higher ETP than the rest (mean difference of between 100 nM.min and 160 nM.min).
For the single patient on aspirin, patient’s ETP in PRP and PPP were 1100 nM.min (marginally above the 95% CI of patients’ unadjusted mean) and PPP 857 nM.min (within 95% CI of patients’ unadjusted mean) respectively. For the only patient with established liver cirrhosis, the ETP in both PRP and PPP were 1092 nM.min and 884 nM.min, respectively (both within the 95% CI of patients’ adjusted mean).
Amongst patients whose iron overload status had been formally assessed, the severity of liver iron overload (as estimated by LIC) was inversely correlated to ETP in PPP (rs = − 0.487, p = 0.019) and in PRP (rs = − 0.390, p = 0.066) (Fig. 2). However, there was no significant correlation observed for patients’ LIC to factor V (rs = −0.094, p = 0.669), factor VII (rs = −0.011, p = 0.959) and PT (rs = 0.195, p = 0.371).
Multivariate analysis involving existing clinical variables (age, gender, type of thalassaemia, splenectomy status and severity of liver iron overload) demonstrated that none of these factors significantly correlated with ETPs in both PPP and PRP amongst the patients (p > 0.05) (Table 5).
Discussion
Increased platelet aggregation and expression of activation markers as well as the presence of platelet morphologic abnormalities have been described in NTDT patients [12,13,14,15]. Although this implies that their platelets are likely to be in the activated state, it was unclear if this is a primary phenomenon or an effect of increased TG. Activated platelets amplify TG but thrombin also serves as an activator of platelets. Regardless, our study sought to determine if the state of platelets in NTDT contributed to a more hypercoagulable state. A comparison of TG potential in both PRP and PPP samples from NTDT patients and normal controls offers an approach to evaluate the role of these platelets in NTDT patients. In having a normal control population, we were able to calculate a normalized ratio which was then used to determine if TG was enhanced in the presence of platelets in the studied patient population.
Contrary to our expectations, the ETP measured did not support the notion of platelets playing a significant role in the touted hypercoagulability of NTDT patients. Firstly, the absence of any statistically significant difference in the mean normalized ETP ratio between PRP and PPP samples indicates that the platelet of NTDT patients did not enhance TG any differently than platelets in normal individuals. Therefore, if indeed NTDT patients are hypercoagulable compared to normal individuals, an alternative mechanism other than platelets or plasma must be in play. Evidently, this points towards the remaining component in the equation which is the role of the red cells in these individuals. Splenectomized NTDT patients who are not transfused adequately are reported to be at higher risk of thrombosis than splenectomized thalassaemia major patients who are transfused adequately [4]. This seems to suggest the procoagulant role of red cells in these patients with NTDT with the effect potentially negated by transfusion [12, 13]. Unfortunately, the current study cannot assess the role of NTDT red cells in TG nor are there readily available commercial kits to do so.
Another significant finding was that ETP in our patients in both PRP and PPP were lower than that in normal controls. Two previous studies have shown contrasting results in this respect. Tripodi et al. measured TG in the presence of thrombomodulin and reported similar ETPs amongst patients with NTDT and normal controls [7]. In contrast, another group of investigators demonstrated significantly reduced ETPs in PPP of thalassaemic patients without the addition of thrombomodulin compared to controls [8]. While these differences could be explained by the use of thrombomodulin, this reduction in ETP in NTDT patients may be accounted by additional findings in our study.
Firstly, more than 80% of our patients with measured liver iron had iron overload which could contribute to subtle impairment of liver function. This was reflected in the significantly longer PT in NTDT patients compared to controls, although most were numerically within normal ranges. As surrogates of liver synthetic functions, factor V and factor VII were measured in this study and were also found to be significantly reduced in NTDT patients. More directly, we demonstrated ETPs were inversely correlated to LIC amongst patients with measured liver iron. Although the extent of reduction in clotting factors do not fall outside of their normal ranges, the amalgamated effect of these reductions of procoagulant factors in the plasma produces a relative hypocoagulable state in NTDT patients which may best be explained by the presence of liver iron overload. This hypocoagulable state will not usually be obvious on routine global coagulation tests such as the PT and aPTT. In fact, only 30% of our patients had PT longer than the normal range while the distributions of aPTT were similar between our patients and controls.
Our postulation of iron overload as the cause for a hypocoagulable state however cannot be verified with other causes of primary and secondary liver iron overload as TG have not been well-studied in these populations. While we have demonstrated clotting factor reduction amongst patients with liver iron overload, the extent of which the production and functions of the natural anticoagulants system is affected could not be determined as direct measurement of these factors were not performed and the TG assay was performed without thrombomodulin. These would be the main limitations of our study together with the small sample size which did not permit accurate determination of clinical end points such as bleeding and thrombosis.
Consistent with existing data, we demonstrated that the patients’ splenectomy status did not influence their ETPs in PPP [7]. In addition, our data also suggested that the type of NTDT did not have any significant impact on the patients’ ETPs in both PPP and PRP samples. Albeit there were a limited number of patients in each type of NTDT, the biggest absolute difference in ETP observed between any two groups was not sufficiently large to signify any clinically relevant dissimilarity.
To our best knowledge, the other study evaluating TG in PRP in thalassaemia involved transfusion-dependent beta thalassaemia patients only [16]. Interestingly, the investigators demonstrated that in their four patients with splenectomy, the kinetics parameters of TG (lag time and time to peak) were significantly reduced compared to those without splenectomy and healthy controls, suggesting a potential increased platelet TG potential amongst those with splenectomy, although the peak TG and ETP were similar [16]. In our current study, the NTDT patients with splenectomy have a significantly shorter time to peak and higher peak TG in PRP but similar ETP and lag time compared to patients without splenectomy (results not shown). When compared to controls, despite having significantly shorter time to peak in PRP, our splenectomised NTDT patients have significantly reduced ETP (results not shown). While there is some similar trend of increased kinetic parameters of TG in PRP amongst splenectomized patients (compared to controls) between the earlier study and ours, the overall TG potential as reflected in ETP is dissimilar between transfusion dependent and NTDT. Hence, our study contributes important information on the overall procoagulant potential generated by platelets in NTDT. Although other aspects of platelet functions are not investigated, based on our present findings, the platelet TG potential is not increased in NTDT patients.
In conclusion, our study demonstrated that platelets did not further enhance TG in NTDT patients especially those with liver iron overload, regardless of their splenectomy status. Hence, these patients may not benefit from aspirin as prophylaxis against thrombotic events previously attributed to NTDT, as recommended by the Thalassaemia International Federation Guidelines. On the contrary, they may actually be hypocoagulable, especially in patients with liver iron overloading. Given the bleeding risk associated with aspirin therapy, its role should be reconsidered in NTDT patients with iron overload.
References
Al-Allawi NAS, Jalal SD, Mohammad AM et al (2014) β-thalassemia intermedia in Northern Iraq: a single center experience. Biomed Res Int 2014:262853
Taher A, Isma’eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz E, Cappellini M (2006) Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost 96:488–491
Musallam KM, Taher AT (2011) Thrombosis in thalassemia: why are we so concerned? Hemoglobin 35:503–510
Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci PM (2000) Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol 111:467–473
Borgna Pignatti C, Carnelli V, Caruso V, Dore F, de Mattia D, di Palma A, di Gregorio F, Romeo MA, Longhi R, Mangiagli A, Melevendi C, Pizzarelli G, Musumeci S (1998) Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta Haematol 99:76–79
Taher A, Vichinsky E, Musallam K et al (2013) Guidelines for the management of non transfusion dependent thalassaemia (NTDT)
Tripodi A, Cappellini MD, Chantarangkul V, Padovan L, Fasulo MR, Marcon A, Mannucci PM (2009) Hypercoagulability in splenectomized thalassemic patients detected by whole-blood thromboelastometry, but not by thrombin generation in platelet-poor plasma. Haematologica 94:1520–1527
Tatli Gunes B, Turker M, Gozmen S et al (2014) Procoagulant phospholipid activity, whole blood thromboelastography and thrombin generation assay to detect hypercoagulability in thalassemic children. Blood 124:4896
Chantarangkul V, Clerici M, Bressi C et al (2003) Thrombin generation assessed as endogenous thrombin potential in patients with hyper- or hypo-coagulability. Haematologica 88:547–554
Hemker HC, Al Dieri R, De Smedt E, Béguin S (2006) Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 96:553–561
Garbowski MW, Carpenter J-P, Smith G, Roughton M, Alam MH, He T, Pennell DJ, Porter JB (2014) Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J Cardiovasc Magn Reson 16:40
Taher AT, Otrock ZK, Uthman I, Cappellini MD (2008) Thalassemia and hypercoagulability. Blood Rev 22:283–292
Sirachainan N (2013) Thalassemia and the hypercoagulable state. Thromb Res 132:637–641
Eldor A, Lellouche F, Goldfarb A et al (1991) In vivo platelet activation in beta-thalassemia major reflected by increased platelet-thromboxane urinary metabolites. Blood 77:1749–1753
Eldor A, Rachmilewitz EA (2002) The hypercoagulable state in thalassemia. Blood 99:36–43
Trinchero A, Marchetti M, Giaccherini C et al (2017) Platelet haemostatic properties in β-thalassaemia: the effect of blood transfusion. Blood Transfus 15:413–421
Acknowledgements
The authors are very grateful to all the study participants and Xiao Zhang for her help with the co-ordination of study subjects.
Funding
This study was funded by the Singapore General Hospital Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tan, C.W., Wong, W.H., Idros, R. et al. Role of platelets in thrombin generation amongst patients with non-transfusion-dependent thalassaemia. Ann Hematol 98, 861–868 (2019). https://doi.org/10.1007/s00277-018-3579-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3579-z