Abstract
Recently, there has been remarkable progress in basic and preclinical studies of acute myeloid leukemia (AML). The improved outcomes of AML can largely be attributed to advances in supportive care and hematopoietic cell transplantation as opposed to conventional chemotherapy. However, as the 5-year survival rate remains low due to a high incidence of relapse, novel and effective treatments are urgently needed. Increasing attention is focusing on identifying suitable immunotherapeutic strategies for AML. Here, we describe the immunological features, mechanisms of immune escape, and recent progress in immunotherapy for AML. Problems encountered in the clinic will also be discussed. Although current outcomes may be limited, ongoing preclinical or clinical efforts are aimed at improving immunotherapy modalities and designing novel therapies, such as vaccines, monoclonal antibody therapy, chimeric antibody receptor-engineered T cells (CAR-T), TCR-engineered T cells (TCR-T), and checkpoint inhibitors, which may provide promising and effective therapies with higher specificity and efficacy for AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acute myeloid leukemia (AML) is a malignant hyperplasia disease characterized by the ability of blasts to self-renew, continuously proliferate, and escape apoptosis, ultimately resulting in the inhibition of normal hematopoiesis. As blasts originate from leukemia stem cells, which exhibit diversity in expression, immunophenotype, and differentiation, AML is also a heterogeneous disease [1]. Furthermore, the major therapies for AML are chemotherapeutic approaches, yet only approximately one third of elderly patients are eligible for intensive chemotherapeutic treatments [2]. Due to the limitations of current therapies, effective and individualized treatments are urgently needed for patients with AML.
Forty years ago, the conventional chemotherapy regimen consisted of 7 days of cytarabine and 3 days of daunorubicin (“7 + 3”) [3]. However, no regimen can effectively protect against AML relapse and achieve sustained remission for patients. Given that AML typically arises from genetic defects and causes damage to the immune system, strategies to improve immune function have been extrapolated to the anti-leukemia field. Hematopoietic stem cell transplantation (HSCT) has demonstrated that donor T cells and natural killer (NK) cells are able to suppress and eliminate blasts [4], providing a theoretical foundation for cellular immunotherapy in AML. Here, we review the association of the immune system with AML and the progress of immunotherapy. Furthermore, active and passive immunity, results from recent trials, and developing immunotherapeutic strategies will also be explained in this review.
Association of the immune system with AML
Features of the immune system in AML
Interactions between immunotherapy and AML are today clearer than ever. However, the most dramatic data regarding AML cell susceptibility to immune attack derive from efforts at allogeneic hematopoietic stem cell transplantation (allo-HSCT) [4]. The benefits of HSCT in AML are that it represents an available treatment that improves overall survival and that it shows an immunotherapeutic approach that depends on the graft-versus-leukemia effect to eradicate leukemia cells [4].
The susceptibility of AML to immune attack is indicated by its immunological characteristics, making the disease an appropriate candidate for immunotherapy. In this regard, AML cells express both major histocompatibility complex (MHC) classes I and II, and they are therefore susceptible to T cell recognition and attack [5]. In addition, expression of co-stimulatory molecules CD80, CD86, and CD40 on AML blast cells is required for T cell stimulation [6], further indicating their susceptibility to T cells. Although there are many obvious characteristics of AML antigens, identifying an ideal target for AML remains challenging due to the clonal and genetic diversity of leukemia.
The mechanism of immune escape in AML
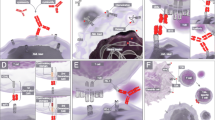
There is little doubt that therapeutic resistance and disease recurrence still occur in a considerable number of patients with AML, which may be partially explained by the escape of leukemic blasts from immune control. In Fig. 1, we illustrate the mechanisms of immune escape in AML. First, as shown in Fig. 1a, blockade of the immunosurveillance function of NK cells is caused by genetic features found in AML. The targeting of AML cells by NK cells depends on the balance between inhibitory and activating receptors and their ligands; AML blasts more strongly express inhibitory killer immunoglobulin-like receptor (KIR) ligands, suggesting a predisposition for AML to escape immune responses by reducing the cytotoxic potential of NK cells [7]. Natural killer group 2 member D (NKG2D) was initially discovered as an activating receptor expressed on NK cells, T cells, or NKT cells, which can promote tumor immune surveillance; however, based on further research, it is clear that tumor cells themselves, including AML blasts, also express NKG2D to activate oncogenic pathways by reducing NK cell-mediated immunosurveillance, leading to tumor cell growth [8, 9]. In addition, AML cells can shed ligands for NKG2D, which contributes to their escape from immune recognition [10]. Furthermore, studies have demonstrated that AML patients display surface-expressed and soluble glucocorticoid-induced tumor necrosis factor-related protein ligand (GITRL), which impairs NK cell effector functions and IFN-gamma production by inducing the release of tumor necrosis factor or interleukin-10 [11]. Regarding the mechanism, Salih et al. discovered that an Fc-engineered GITR-Fc-ADCC fusion protein can neutralize the inhibitory effects of GITR-GITRL on NK cells and target GITRL-expressing malignant cells [12]. Second, as shown in Fig. 1b, co-stimulatory molecules play an important role in the activation of T cells. Expression of negative co-stimulatory molecules, such as programmed cell death protein (PD-1) and its two ligands, PD-L1 and PD-L2, as well as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), is aberrantly upregulated in AML, which is involved in the incidence of relapse and the short survival of AML patients [13]. In addition, downregulation of positive co-stimulatory molecules for CD4+ and, in particular, CD8+ T cells has been shown to have a significant role in tumor cell escape from immune surveillance by limiting T cell activation [14, 15]. Third, one outstanding issue is the self-inhibitory activity of T cells in AML, which can be used to evade immune surveillance. Rifca et al. demonstrated that although T cells efficiently conjugated with blasts, a marked decrease in T cell immunologic synapse formation and recruitment of phosphotyrosine signaling molecules as well as altered actin cytoskeletal reorganization leads to T cell dysfunction in AML, which may have contributed to the failure of anti-leukemic immune responses [16, 17]. Also, Buggins et al. showed that the tumor cell supernatant (TSN) of AML cells blocked T cell mitogenesis and T helper cell 1 (Th1) cytokine production and prevented activated T cells from entering the cell cycle [18]. Moreover, severe T cell immunodeficiency in AML patients is associated with reduced levels of thymic emigrants and thymus atrophy [19, 20]. Finally, it is becoming evident that the bone marrow microenvironment protects and supports AML cells through an immunosuppressive milieu. The vascular niche regulates AML cell survival, cell cycling, and even resistance to cytotoxic chemotherapy via both paracrine secretion and adhesive contact with endothelial cells [21]. This hostile milieu consists of certain soluble factors secreted by AML cells, such as expressed signal transducer and activator of transcription 3, indoleamine 2,3-dioxygenase (IDO), and nitric oxide synthase (NOS), which impair the function of T and NK cells [22]. In this immune scenario, clinical immunotherapeutic trials have explored various cytokines, vaccines, and treatments to boost T cell immunity and increase the susceptibility of target cells as well as strategies to directly attack AML cells or overcome immune escape.
Mechanisms of immune escape in AML. a NK cells play an important role in the immune system, targeting and killing leukemia cells, and are regulated by the balance between inhibitory and activating signals. In AML, overexpressed inhibitory receptor KIR ligands can reduce the cytotoxicity of NK cells. Expression of NKG2D and GITRL on AML cells and shedding of NKG2D ligands from AML cells can also impair NK cell function. b Co-stimulatory molecules play an important role in the activation of T cells. Aberrantly upregulated negative co-stimulatory molecules such as PD-1/PD-L1 and CTLA-4 and downregulation of positive co-stimulatory molecules for T cell activation are involved in the incidence of relapse and the shorter survival of AML patients. c The reduced activity of T cells in AML can be used to evade immune surveillance through a decrease in T cell immunologic synapse formation, blocked mitogenesis, and thymic dysfunction. d The bone marrow microenvironment protects and supports AML cells through an immunosuppressive milieu generated by vascular niche and soluble factors secreted by AML cells
The evolution of immunotherapy for AML
The necessity of immunotherapy for AML
Despite advances in AML therapy in recent decades, survival has not improved substantially due to a high incidence of leukemic relapse, suggesting that new therapeutic approaches are needed [23]. In addition to chemotherapy and the emerging field of targeted therapy, immunotherapy for AML is an important and appealing area of research. The strong intrinsic interactions of myeloid cells with the immune system direct immunotherapeutic exploration with the purpose of targeting leukemic blasts. Besides, achievements in AML immunotherapy have suggested the possibility of improved outcomes. Induction chemotherapy for AML aims to achieve complete remission (CR), yet immunotherapy has generally been thought of as a means of sustaining remission and avoiding relapse. Although immunotherapy for AML is in its infancy, it is proceeding in multiple directions at an accelerating rate.
Active immunotherapy for AML
Active tumor immunotherapy consists of a tumor antigen that stimulates the immune system to produce a specific anti-tumor response, which requires identification and characterization of appropriate antigen structures. Along with the apparent outcomes of cancer vaccines for solid tumors, researchers have extrapolated the utility of cancer vaccines to hematologic malignancies in view of their immunological and clinical features. Currently, four types of vaccines are used in AML treatment as shown in Fig. 2c: autologous whole AML cells, peptide vaccines, dendritic cell (DC)-based vaccines, and DNA vaccines.
Immunotherapeutic approaches to AML. a Adoptive immunotherapy is a novel therapy that uses autologous or allogeneic immune molecules or cells with anti-tumor activity, such as LAK, TILs, CTLs, NK, CIK, and CAPRI, for infusion into patients to treat hematological malignancies, with many novel methods to improve efficacy. b Antigen-specific MoAb immunotherapy is regarded as a means to improve the outcomes of patients with AML. CD33 and CD123 are typical targets in MoAb therapy, and to improve anti-tumor activity, studies of antibody-based immunotherapies such as bispecific antibodies and radioimmunotherapy are ongoing. c In view of their immunological and clinical features, researchers have extrapolated the utility of cancer vaccines to hematologic malignancies. There are currently four types of vaccines that have been used in AML treatment: autologous whole AML cells, peptide vaccines, dendritic cell (DC)-based vaccines, and DNA vaccines. d CAR-T and TCR-T technologies both employ genetic engineering to improve the ability of T cell receptors to recognize and attack specific cancer cell antigens. Ongoing trials with novel designs, as well as previous results, show enhanced efficacy of CAR-T and TCR-T cell-based therapy in AML. e Regulatory signals of immune checkpoints are essential to maintain immune homeostasis and self-tolerance and prevent autoimmunity. However, negative immune regulatory factors have been implicated in immune escape mechanisms of cancer cells. The two common immune checkpoints are CTLA-4 and PD-1, and clinical studies have focused on targeting these checkpoints or the ligands PD-L1/PD-L2 to block immune checkpoints. Aiming to identify efficacy for extensive use in the clinic, there are many ongoing clinical trials about PD-1/PD-L1 and CTLA-4 inhibition in patients with AML
Autologous whole AML cells
A tumor cell vaccine is a vaccine that utilizes modified autologous whole tumor cells, which stimulate the body to produce anti-tumor responses. Given the presence of multiple known and unknown antigens on the surface of AML blasts, autologous whole AML cells appear to be useful for stimulating AML-specific immune responses that are directed against multiple antigens, providing a customized treatment vaccine for each individual. This approach can decrease the risk of the generation of tumor escape variants. In one of the pioneer vaccination trials using autologous whole AML cells, researchers analyzed the capacity of lymphocytes collected from patients pre and post vaccination to react to their own leukemia cells in vitro, revealing upregulation of the activity of peripheral blood effector cells against autologous leukemic cells after vaccination with whole AML cells [24]. However, several studies have noted the issues and disadvantages of vaccines, including adverse autoimmune events. Although researchers and clinicians have sought methods to improve the effects of cell vaccines, such as transferring cytokine or co-stimulatory into AML cells [25], mixing cells with interleukin-2 (IL-2), granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6 [26], and transfecting AML cells with Toll-like receptor 3 ligand polyinosinic:polycytidylic acid (poly(I:C)) [27], no explicit data have demonstrated the clinical effects of these approaches or the potency of whole AML cells, both of which require further evaluation in larger cohorts.
Peptide vaccines
A peptide vaccine is prepared via exogenous chemical synthesis technology based on the amino acid sequence of known or predicted epitopes of leukemia-associated antigens (LAAs) to induce a T cell response. Peptide vaccines developed from LAAs, such as Wilms’ tumor 1 (WT1) antigen, proteinase-3 (PR-3) peptide, preferentially expressed antigen of melanoma (PRAME), and receptor for hyaluronic acid-mediated motility (RHAMM), have been explored and/or are under clinical investigation for the treatment of AML [28].
WT1 gene is overexpressed in > 90% of patients with AML and associated with adverse prognosis risk[29]. So WT1 is a promising and attractive target for immunotherapy. Several studies have already suggested that WT1 peptide vaccination may be effective in inducing a functional immune response associated with clinical improvement [30]. And WT1 peptide vaccine can control the minimal residual disease (MRD) to prevent recurrence in AML patients [31].
Proteinase-1 (PR1) is a 9-amino acid HLA-A*0201 (HLA-A2)-restricted peptide derived from PR3 that evokes myeloid leukemia-specific cytotoxic T lymphocyte (CTL) responses that selectively kill leukemic CD34+ cells in vitro, which suggests that CTLs specific for PR1 (PR1-CTL) can be used for AML patients [32]. Molldrem et al. have shown that adoptive transfer of PR1-CTL can reduce AML cells in NOD/SCID mice, and these authors developed a T cell receptor (TCR)-like anti-PR1/HLA-A2 antibody, 8F4, that can mediate lysis of AML cells in vitro [33, 34]. Based on preclinical results, TCR-like CAR with specificity for the PR1/HLA-A2 epitope has been transduced into adult human peripheral blood or umbilical cord blood T cells to produce h8F4-CAR-T cells, which can rapidly and efficiently kill AML in vitro. This may be a feasible and effective therapy for AML patients in the future [35]. In a phase I/II trial comprising 66 HLA-A2+ patients, including 42 AML patients receiving PR1 peptide vaccinations, 12 (24%) of 53 evaluable patients with active disease exhibited objective clinical responses [36].
The PRAME gene, which has been the focus of much attention in recent years, is highly expressed in acute leukemia; this gene is an ideal target antigen for AML immunotherapy and a useful marker for detecting minimal residual disease (MRD) in patients with leukemia [37]. For example, Concetta et al. developed a high-avidity CTL specific for the PRAME-derived peptide, generated from normal donors and from subjects with PRAME+ disease by using professional and artificial antigen-presenting cells (APCs) loaded with a PRAME peptide library, which can target leukemic blasts and leukemic progenitor cells without affecting normal hematopoietic precursors [38]. Based on these results, the PRAME-specific CTL can be further studied in preclinical and clinical trials with regard to the efficacy in AML patients and clinical outcomes.
RHAMM is an immunogenic antigen that plays a fundamental role in cell growth, differentiation, and motility [39]; it is overexpressed in AML, providing a theoretical foundation for the development of RHAMM vaccines for AML [40]. In a phase I clinical trial, 10 patients received a RHAMM-R3 peptide vaccine, a highly immunogenic CD8+ T cell epitope peptide derived from RHAMM, and three of six patients with myeloid disorders (1/3 AML, 2/3 myelodysplastic syndrome (MDS)) achieved a clinical response [41]. In addition, Stefani et al. explored TCR-transgenic lymphocytes specific for the impact of RHAMM on HMMR+ solid tumors and AML cells in vivo, reporting attack of residual leukemia and HLA-A2+ hematopoietic stem cells after HLA-A2-mismatched stem cell transplantation (SCT) [42]. Moreover, the above antigens are well suited for AML immunotherapy due to their expression in AML. These antigens may also represent suitable simultaneous targets of a polyvalent vaccine, such as WT1-PR1 [43], which may be an important focus of upcoming studies.
Dendritic cell (DC)-based vaccines
DCs are professional antigen-presenting cells (APCs) and known as the “sentinels” of the immune system because of their ability to provide all the signals required for antigen-specific T cell activation to induce efficient anti-microbial or anti-tumor responses [44]. Accordingly, DC-based vaccines have received significant interest over the past decade and have occupied a foremost position in this research area. Broadly, DC-based vaccines can be divided into two categories. The first is DC vaccines, which are prepared in vitro using tumor antigens to stimulate DCs that can recognize and engulf antigens. Reinfusion of these DCs can then induce anti-tumor immunity. An advantage of this vaccine is that it is easy to prepare; a shortcoming is its lack of specificity, which may induce autoimmune diseases [45]. The second category is transfected DC-based vaccines. Within the context of the co-stimulatory machinery delivered to DCs, a variety of strategies for loading tumor antigens onto DCs have been evaluated in clinical studies, including individual peptides, proteins, DNA/RNA-encoding tumor-associated antigens, apoptotic bodies derived from tumor cells, or whole tumor cell antigens [46, 47]. For instance, a WT1-targeted DC cell vaccine has been explored, as WT1 is a known immunotherapeutic target due to its role in leukemogenesis and relapse prediction, as mentioned above. Zwi N Berneman and colleagues conducted a phase I/II trial of the DC-WT1 vaccine in 10 AML patients who achieved complete remission (CR) or partial remission (PR) after chemotherapy with a high risk of relapse. Two patients in the PR group were able to reach CR, and these two patients as well as three other patients in the CR group had been induced into molecular remission after DC-WT1 vaccination [48]. Subsequently, the clinical outcomes of DC-WT1 vaccination in 66 cancer patients, including 30 AML patients, were reported: 8 of 23 patients with AML had increased WT1 transcript levels, an assessment of residual disease, and achieved a clinical and molecular response, and 8 of 30 AML patients had not relapsed by the time of publication [49]. Vaccines such as DC-WT1 have strong immunogenicity and high specificity, though the preparation methods have not yet been optimized and require further study. Although the technique has resulted in new treatments for patients, further studies are needed to address several issues such as improving DC vaccine design, including controlling DC maturation, and optimizing the route of vaccination and the dose and schedule of application, and to suppress the negative immune regulatory mechanisms that hamper the development of effective vaccine-induced immunity.
DNA vaccines and genetic adjuvants for vaccines
DNA vaccines are bacterial plasmids constructed to express an encoded protein following in vivo administration and subsequent cell transcription and translation after uptake. Such protein antigens can stimulate immune responses. Some studies have shown that DNA vaccination with a PML-RARA cDNA can induce protective immunity against acute promyelocytic leukemia (APL) in mouse models [50], suggesting that therapeutic DNA vaccines may be suitable for treating this disease when relevant tumor-specific antigens are identified. However, most ongoing clinical trials have reported unimpressive immune responses to the majority of clinically tested DNA vaccines, emphasizing many different effects between animal models and patients [51, 52]. Further optimization using adjuvant delivery systems or prime-boost-based schedules may boost the efficiency of DNA vaccines. Genetic adjuvants are typically cytokine genes, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, IL-7, IL-12, CpG-ODN, and ubiquitin, that provide general immune stimulation and increase the immunogenicity of vaccines [53]. Many efforts have been made to develop genetic adjuvants that increase the immunogenicity of vaccines and activate the immune system, but there are limited data concerning the clinical efficacy of vaccine-based approaches in AML.
The above-described vaccines have recently been reported as potentially promising methods to prolong disease-free survival in patients with AML after induction therapy. However, successfully combining various vaccines with induction chemotherapy and determining the optimal timing and schedule of these therapies during the course of AML represent major challenges in the use of vaccines.
Passive immunotherapy for AML
Passive immunization is an approach by which the body obtains specific immunity via passive acceptance of antibodies or sensitized lymphocytes. It is distinct from active immunization and is characterized by a rapid effect without the incubation period; once the antibodies or sensitized lymphocytes enter the body, the host will immediately obtain immunity. Thus far, several passive strategies have been studied, including monoclonal antibody (MoAb) therapies, adoptive immunotherapies, chimeric antibody receptor-engineered T cells (CAR-T) and TCR-engineered T cells (TCR-T), and checkpoint inhibitors.
Monoclonal antibody therapy
Hematopoietic cells express surface antigens that are critical for normal immune responses and immunotherapies. In 1975, Kohler and Milstein developed a technique for producing antigen-specific MoAbs that represents a cornerstone for the feasibility of MoAbs to treat tumors [54]. Overall, antigen-specific MoAb immunotherapy is regarded as a means to improve the outcomes of patients with AML as shown in Fig. 2b.
Anti-CD33 antibodies
CD33 is a myeloid differentiation antigen displayed on AML blasts in most patients, and this antigen is a target for antibody-based therapies [55]. The unconjugated antibody lintuzumab (SGN-33, HuM195), a humanized immunoglobulin IgG1 with a high affinity for CD33, has been extensively tested in humans. Unfortunately, the response in clinical trials was not promising [56, 57]. To increase the potency of the antibody without invoking non-specific cytotoxicity, the α-particle-emitting radionuclide bismuth-213 has been conjugated to lintuzumab, which can induce remission in some patients with AML [58]. Conjugated antibodies are a long-pursued strategy to improve the efficacy of MoAbs and are created by linking antibodies to an effector molecule, such as a small molecule drug, a radionuclide, or a toxic protein from a plant or bacterium, in an effort to combine the specificity and selectivity of the antibody with the anti-tumor activity of the effector molecule. In clinical trials, the CD33 antibody-drug conjugate gemtuzumab ozogamicin (GO), which employs a calicheamicin derivative to induce DNA strand breaks, apoptosis, and cell death [59], provided the most prominent benefit for AML patients [60]. Despite these positive results, GO was withdrawn from most commercial markets in 2010 because it showed no benefit and increased toxicity in a phase III trial [61]. However, some researchers thought that this regulation was to some extent premature [62], thus supporting further investigations of GO in combination with other methods in a larger patient population [63]. Additionally, CD33-based immunotoxins have sparked strong interest due to favorable outcomes in different cohorts of patients treated with GO. Advancements in the technology of conjugation and linkers to the cytotoxic drug have resulted in the production of SGN-CD33A, a humanized CD33 antibody with engineered cysteines conjugated to synthetic DNA cross-linking a pyrrolobenzodiazepine dimer via a protease cleavable linker. According to preclinical tests in AML models, the anti-leukemic activity of SGN-CD33A was more potent than that of GO [64]. An interim analysis of an ongoing phase I trial of SGN-CD33A in patients with CD33-positive AML who either had relapsed/refractory AML or had declined intensive therapy demonstrated that 8 of 17 patients (47%) treated at 40 mcg/kg experienced clearance of marrow blasts, with 5 (29%) achieving either CR or complete remission with incomplete marrow recovery (Cri) [65]. IMGN779 is another CD33-targeting antibody-drug conjugate comprising a humanized anti-CD33 antibody conjugated to DGN462, a novel DNA-alkylating agent, via a cleavable disulfide linker [66]. It showed potent in vitro activity against primary patient AML cells isolated from peripheral blood or bone marrow samples and contributed to enhanced anti-leukemia potency in a preclinical AML model when combined with the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib [67]. In addition, HUM-195/rGel is an immunotoxin created by conjugating rGel with HUM-195 via an N-succinimidyl-3-(2-pyridyl-dithio)-propionate linkage; in a phase I study, 4 of 22 evaluable patients with relapsed or refractory myeloid malignancies received HUM-195/rGel which had > 50% reductions of peripheral blood blasts, but no complete or partial remission was achieved, which suggested that HUM-195/rGel may play a potential role in anti-CD33 antibodies but needed further researches [68].
Anti-CD123
CD123 is another important AML-associated antigen predominantly expressed on myeloid cells and on a subpopulation of B-lymphocytes [69]. Elevated expression of CD123 in AML is involved in higher blast counts at diagnosis and a lower CR rate with a poorer prognosis, which contributes to CD123 being a target for antibody-derived therapies [70]. The first anti-CD123 antibody, CSL360, was a recombinant, chimeric immunoglobulin G1 against CD123 that prevented IL-3 binding to its receptor in vitro; however, it did not exhibit efficacy against relapsed, refractory, or high-risk AML in a clinical study, with only one CR achieved among 26 patients [71]. Another anti-CD123 antibody, CSL362, was a fully humanized anti-CD123 monoclonal antibody engineered with greater antibody-dependent cell-mediated cytotoxicity due to its high affinity for NK cell CD16. In a phase I study of CSL362 involving 25 patients with CD123+ AML in CR but at high risk for early relapse, the antibody showed potent efficacy against AML and maintained CR: among 20 patients evaluable for a response, 10 maintained CR with a median duration of 34+ weeks from the start of CR, and CR was still present at the last follow-up. Of six evaluable patients with MRD positivity at baseline, three converted to negative [72]. Following encouraging results in a phase I trial of AML and MDS patients and in the latest preclinical studies of AML cells or an AML patient-derived xenograft model, the anti-CD123 antibody SL-401, a recombinant fusion protein consisting of truncated diphtheria toxin and a human IL-3 ligand, is currently in a phase II clinical study of consolidation therapy for high-risk AML patients in first CR [73].
Radioimmunotherapy
Another method is radioimmunotherapy, in which MoAbs are conjugated with radioisotopes, including beta-emitters (131iodine, 90yttrium) and alpha-emitters (213bismuth, 225actinium), to deliver radiation directly to malignant cells. These radioisotopes can be used to enhance the anti-tumor effect of MoAbs, such as the abovementioned bismuth-213-lintuzumab. In addition, β-particles, such as 131I [74] and 90Y [75], have been conjugated with anti-CD33 MoAbs; 131I has also been combined with anti-CD45 antibodies [76], both of which have been studied in patients with AML. Early studies about 131I-labeled anti-CD33 or anti-CD45 as a part of the preparative regimen showed feasibility and tolerable toxicity for HSCT in patients with refractory or high-risk AML, but efficacy needs to be proven in phase II studies [77, 78]. To enhance efficacy and reduce toxicity, some studies have evaluated the high energy emitted by radionuclides or short-range alpha particles, such as 213Bi and 225Ac. In a phase I trial, CR was not achieved, but patients with relapsed or refractory AML who received 213Bi-lintuzumab showed decreases in leukemic blasts in circulation or in bone marrow [58, 79]. Furthermore, in a clinical study, 79% of 14 evaluated patients displayed bone marrow reduction among older patients with untreated AML-administered 225Ac-labeled anti-CD33 and low-dose cytarabine, and 28% CR was observed among the 18 patients [80]. Given the myeloablative potential of 211At, 211At-anti-CD45 may serve as a beneficial adjunct to HSCT in the treatment of AML and may provide the possibility of new treatments for these patients [81].
Bispecific antibodies
An emerging immunotherapy in AML involves monoclonal antibodies designed to improve anti-tumor activity through T cell engagement. The designed antibodies have been called bispecific, with dual affinities for a tumor cell antigen and an antigen on an immune effector cell, and consist of the minimal binding domains of the two different antibodies on one (e.g., bispecific T cell-engager (BiTE) antibodies) or two (e.g., dual-affinity retargeting (DART) antibodies) polypeptide chains. The first-in-class BiTE antibody, anti-CD19/CD3 blinatumomab, showed exciting clinical efficacy in the treatment of relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL) [82]. Based on antigen expression in AML, attention has primarily been paid to CD3-directed agents targeting CD33 or CD123. AMG 330 is a novel CD33/CD3 BiTE antibody designed to activate T cells against CD33-expressing human AML cells [83]. In an in vitro study, AMG 330 contributed to potent cytolysis depending on the level of cell-surface CD33 expression against human AML cells [84]. The DART antibody is a class of bispecific antibodies that consists of heavy- and light-chain variable domains of two antigen-binding specificities linked to two independent polypeptide chains [85]. CD3/CD123 DART(also referred to as MGD006) can result in dose-dependent killing of AML cells and primary AML blasts in vitro and in vivo, providing a theoretical basis for further study [86]. As a consequence, bispecific antibodies warrant further study for treatment of AML patients in high medical need for effective therapies. In addition, ongoing preclinical and clinical efforts are paying increasing attention to improving existing immunotherapy methods and exploring novel therapeutics.
Adoptive immunotherapy
Adoptive immunotherapy is a novel method in which autologous or allogeneic immune molecules or cells with anti-tumor activity are directly infused into patients, which is anticipated to be a promising approach for treating hematological malignancies. There are several types of adoptive immunotherapies as shown in Fig. 2a, including lymphokine-activated killer (LAK) cells, tumor-infiltrating lymphocytes (TILs), CTLs, NK cells, cytokine-induced killer (CIK) cells, CAPRI cells, and CARs.
LAK cells, TILs, and CTLs
The earliest adoptive immunotherapy was performed in the 1980s with LAK cells in the presence of high levels of IL-2 to kill tumor cells [87, 88]. LAK cells are leukocytes produced by culturing autologous peripheral blood mononuclear cells with IL-2. Additionally, LAK cell infiltrates completely disappear in murine models after stopping IL-2 administration [89]. However, high-dose IL-2 toxicity can cause side effects, such as hypotension, weight gain, oliguria, elevation of bilirubin and creatinine levels, and even treatment-related death; these effects and the intrinsic low anti-tumor effects of LAK cells have limited the use of LAK cell therapy [88, 90]. Newer LAK cell-based therapies require time and replicative experiments before they can be used safely and substantially in the treatment of patients with cancer. Furthermore, it is reported that TILs composed of different lymphocyte subtypes have more apparent lethality and specificity for tumor cells than LAK cells. Regardless, the disadvantages of TILs, including the complicated culture process, difficulty in obtaining these cells, and their incomplete removal of tumor cells have limited their application in the clinic [91]. However, there are almost no reports on the use of TILs for hematological tumors due to the difficulty in obtaining these cells. Interestingly, a recent study reported the identification of marrow-infiltrating lymphocytes obtained from the marrow microenvironment in myeloma that likely play the same role as TILs [92]. These cells have been applied with positive outcomes in the treatment of multiple myeloma patients [93], suggesting that TIL adoptive immunotherapy may be extended to hematological malignancies if the correct TILs are found. To overcome the limitations of LAK cells or TILs, researchers have focused on combining lymphocytes and targeted antigens in vitro to generate powerful CTLs. As previously stated, myeloid leukemia cells express LAAs, such as WT1 antigen, PR3 peptide, PRAME, and RHAMM. In view of this, CTLs can be produced and utilized to treat patients with AML. In addition, many studies have reported that CTL therapy can increase relapse-free survival for AML patients, especially following allogeneic SCT [94, 95]. As myeloid leukemia cells express many types of LAAs, a single LAA cannot achieve the desired results. Thus, CTLs that are stimulated by multi-tumor antigens have caught the attention of researchers as candidates for adoptive immunotherapy for AML transplant patients [96].
NK, CIK, and CAPRI cells
As previously described, one of the mechanisms of immune escape in AML is the reduction in the cytotoxic potential of NK cells due to stronger inhibitory KIR ligands expressed on AML blasts. NK cells account for 10–15% of the peripheral blood lymphocytes originating from CD34 hematopoietic progenitor cells, which indicates their important role in the immune system; these cells can target and kill leukemia cells without T cell assistance or prior sensitization via recognizing “loss of self” HLAs invoked by malignant transformation [97]. NK cells have an anti-leukemic effect, preventing relapse, reducing graft-versus-host disease (GVHD), and eradicating leukemia cells during haploidentical HSCT [98, 99]. The efficacy of NK cells in anti-leukemia is determined by the balance of inhibitory and activating signals. The anti-tumor effect of NK cells in AML is decreased due to overexpression of inhibitory receptors, downregulation of activating receptors, and production of immunosuppressive soluble ligands by leukemic blasts [100, 101]. Therefore, many studies have focused on autogenous or allogeneic NK cell adoptive immunotherapy using amplification in vitro to obtain many high-purity NK cells and then transfuse them into patients. In 2004, Koehl et al. demonstrated the safety and feasibility of IL-2-stimulated NK cell immunotherapy in patients with persistent leukemia blasts [102]. And a prospective phase II study primarily aimed at evaluating the effectiveness of immunotherapy with NK cells was performed in 16 patients (8 with AML), but the results were not as expected. The study demonstrated that NK cells are safe and that their effects may depend on the optimal dose and timing of this therapy, which will require additional work [103]. Moreover, researchers have used NK cells with external factors, such as a chimeric receptor with NKG2D, or co-culture with K562-mb15-41BBL cells to increase responses [104, 105]. Recently, CARs have been demonstrated to induce more powerful cytotoxicity and redirect NK cell specificity toward tumor cells. Regardless, some questions remain that require further study [106].
CIK cells are lymphocytes that are activated by the sequential addition of cytokines in vitro under well-defined culture conditions; these cells are thought to be better candidates for adoptive immunotherapy than LAK cells due to their higher cytotoxicity and proliferative ability [91]. CIK cell cultures mostly consist of three lymphocyte subsets at maturity: CD3−CD56+, CD3+CD56−, and CD3+CD56+. Among these, the CD3+CD56+ subset exhibits a more efficient ability to kill AML targets [107]. Therefore, CIK cells are CD3+CD56+ double-positive T cells that exert cytotoxicity toward diverse tumor cells, especially hematological malignancies, which is attributable to both NK cell-like and T cell-like activities [108]. The cytotoxicity of CIK cells is non-MHC-restricted and mainly depends on interaction between NKG2D molecules on CIK cells and MHC class I-related chain (MIC) A/B and UL-16-binding protein 1–4 overexpression on tumor cells [109]. Preclinical mouse model studies and clinical trials have reported that CIK cells can target leukemic cells with low odds of inducing GVHD and do not affect normal cells [110, 111]. In addition, a case reported that a female patient with relapsed AML-M5a after allogeneic peripheral blood stem cell transplantation was infused with CIK cells expanded from recipient peripheral mononuclear cells with full donor chimerism and achieved complete cytogenetic remission [112]. This approach may resolve the problem of donor unavailability, but larger clinical studies are still needed to determine whether this can be a reliable and alternative origin of CIK cells. In addition, the expanded scope of many preclinical studies on CIK cells is to improve the efficacy and specificity of treatment through methods such as co-culture of CIK cells with DC cells [113, 114], enhancing the cytotoxicity of CIK cells by bispecific antibodies to tumor targets [115], co-expansion of CIK cells and gamma delta (γδ) T cells for adoptive cellular immunotherapy applications, such as CAR-T cell therapy [116], or blockade of inhibitory receptors on CIK cells by immune checkpoint inhibitors [117]. Such research results using CIK cells may be translated into clinical therapeutics for the maximum benefit of AML patients.
To enhance immune responses and avoid side effects, CAPRI cells use mature patient monocytes with stored expression of cancer information in vitro to activate T lymphocytes into cytotoxic effectors to destroy cancer cells. Unlike non-MHC-restricted CIK cells, CAPRI cells consist of NK cells, NK-like T cells (NKT cells), DC cells, CD4+ T cells, and CD8+ cytotoxic T lymphocytes; however, CD4+ T and CD8+ T cells account for 80% of the cytotoxic activity of CAPRI cells. CAPRI cells can induce upregulated expression of MHC class I and class II on cancer cells, which can lead to efficient MHC-restricted cancer cell destruction [118]. Although CAPRI cell therapy is to some extent superior to other adoptive cell therapies and some results of clinical cancer case series in breast cancer and lung cancer have been promising [119], no extensive clinical trials or studies on the treatment of hematological tumors have been reported.
Chimeric antibody receptor-engineered T cells (CAR-T) and TCR-engineered T cells (TCR-T)
Both CAR-T and TCR-T technologies, as shown in Fig. 2d, use genetic engineering to improve the ability of T cell receptors to recognize and attack specific cancer cell antigens. TCR-engineered T cells derive from patient lymphocytes transfected with a viral vector that carries TCR genes and has the ability to recognize antigens specifically derived from a tumor-reactive T cell clone. Clinical trials have shown promising results of TCR-T cell treatment in many forms of malignancy, including melanoma, synovial cell sarcomas, and myeloma [120]. However, application of TCR-T cells for AML may be limited by their immunological characteristics [121]. For example, TCR-T cells recognize a specific antigen at the base of MHC but not the antigen itself; downregulation of HLA molecules may be a classic AML blast escape mechanism, resulting in relatively low TCR-binding affinity to AML cells.
Compared with TCR-T cells, CAR-T cells can overcome MHC restriction and thus result in unprecedented responses in hematological malignancies. Third-generation CAR-T cells primarily consist of an antigen-binding region (a single chain antibody Fv), transmembrane region (co-stimulatory or co-receptor signals), and signal transduction region (immune-receptor tyrosine-based activation motif). Due to continuous reformation and improvement, these third-generation CAR-T cells were created with the idea that T cell-mediated anti-tumor immunity requires at least two signals from a T cell receptor and co-stimulatory molecules, which can enhance CAR-T cell survival, expansion, and activity. The first clinical application of CAR-T cells was employed in B cell malignancies, with promising results. Anti-CD19 CAR-T cells (CART-19) have been reported to achieve CR in over 90% of patients with B-ALL [122, 123]. However, for the establishment of CAR-T cells for treating AML, the putative targets of anti-AML CAR-T cells must be expressed only on leukemic hematopoietic cells to prevent normal myeloid cells from being destroyed. Using an anti-CD33 single-chain variable fragment, CD33 has been developed into CART-33 for treatment of patients with AML. Wang et al. reported one AML patient treated with CART-33 cells who exhibited a transient marked reduction in blasts in the bone marrow, but these cells gradually increased again, and the patient relapsed at 9 weeks after cell infusion [124]. In addition, Kenderian et al. demonstrated that CART-33 cells can eradicate leukemia and prolong survival in AML xenografts, but application is limited by its unacceptable toxicity to myeloid progenitors. To avoid long-term myelosuppression, a transiently expressed anti-CART-33 messenger RNA (mRNA) was then designed [125].
Antigen expression is heterogeneous on leukemia blasts, and TCR-T and CAR-T cells have been found to be an attractive option for the design of cell-mediated immunotherapy for targeting LAAs, such as PRAME and WT-1. By using immune-deficient NSG mice engrafted with U266 cells, Tsvetalina et al. have demonstrated that PRAME TCR-T cells are more capable of tumor control compared to non-transduced control T cells (P = 0.01) and have shown that rimiducid activation and expansion of inducible MyD88/CD40 PRAME TCR-T cell can notably increase anti-tumor capacity compared to T cells expressing the PRAME TCR only (P = 0.005) [126]. Stauss et al. reported that T cells from patients with leukemia engineered to express WT1-TCR can eliminate autologous leukemia blasts in NOD/SCID mice [127]. In addition, WT1-specific TCR-T cells have a potential effect on AML and MDS, though antigen-specific TCR-gene infusion may cause autoimmune disease because of TCR mispairing between introduced and endogenous TCR chains. To avoid this, Shiku et al. established a first-in-man trial of a retroviral vector system, MS3-WT1-siTCR, which was derived from DNA encoding WT1235-243/HLA*A24:02-specific TCR chains and small interfering RNAs (siRNAs) for endogenous TCR genes to eliminate TCR mispairing in AML and MDS patients, and they demonstrated that adoptive transfer of WT1-siTCR/T cells is feasible and safe for anti-leukemia reactivity [128, 129]. These authors also developed WT#213 CAR, whereby the CAR consists of the isolated scFv antibody clone WT#213 able to recognize the WT1 p235-243 peptide; the results suggested that WT#213 CAR is a safe and effective immunotherapy for AML [130]. Zwi et al. established another strategy to combine WT1-TCR and Dicer-substrate small interfering RNA (DsiRNA), showing that DsiRNA electroporation can enhance WT1-TCR expression, which can increase the killing activity of WT1-specific CD8+ T cells [131]. Other preclinical trials of various types of CAR-T cell therapies in AML have been published, such as CD123, CD44v6, and LeY antigen-directed CAR-T [132,133,134]. While Shiku et al. also have studied on human telomerase reverse transcriptase (hTERT) for treatment of adult T cell leukemia, these hTERT-specific TCR-T cells showed antitumor activity both in vitro and in vivo, demonstrating that hTERT is a promising therapeutic target [135]. Another group found that new-generation DCs transfected with mRNA from hTERT, survivin, and autologous tumor can evoke specific immune responses and prolong survival in different types of cancer, including AML [136]. hTERT is considered to be a promising antigen for AML in future applications. In addition, Hans J. Stauss reported that TCR-T cell therapy may be an effective option for patients who show no response to CAR-T cell treatment. T cell engineering with TCR and CAR can also be complementary to some extent [137]. Although there have been no dramatic results to date, the results from these ongoing trials and accumulating evidence will demonstrate the efficacy of CAR-T and TCR-T cell-based therapy in AML.
Checkpoint inhibitors
For healthy humans, the regulatory signals of immune checkpoints are essential to maintain immune homeostasis and self-tolerance and to prevent autoimmunity. However, negative immune regulatory factors have been implicated in immune escape mechanisms of cancer cells. Two common immune checkpoints are CTLA-4 and PD-1 as shown in Fig. 2e, and clinical studies have focused on targeting these checkpoints or ligand PD-L1/PD-L2 to block immune checkpoints; such drugs are called checkpoint inhibitors [138].
CTLA-4
CTLA-4 is an inhibitory receptor expressed on T cells that suppresses activation of effector T cells by competing with CD28, a co-stimulatory molecule on T cells, for binding to ligands CD80 and CD86 [138]. Anti-CTLA-4 antibodies were the first immune checkpoints to be targeted for cancer immunotherapy; in clinical trials, patients with metastatic melanoma were treated with ipilimumab, a CTLA-4 inhibitor, which led to increased survival [139]. Due to the promising responses achieved in other cancers, researchers have proposed that CTLA-4 blockade can also be beneficial for patients with hematologic malignancies. Furthermore, studies have shown that AML cells express CD80 and CD86 for engagement with CTLA-4, suppressing activation of T cells [140]. Several preclinical and clinical trials have reported that CTLA-4 blockade can establish an anti-leukemic effect without GVHD after allogeneic HSCT [141] and restore anti-tumor reactivity for patients with relapse; durable responses were observed [142]. Although the efficacy of CTLA-4 inhibition appears obvious, the response was too small to clearly affirm the effect in AML. Nonetheless, many clinical trials on CTLA-4 inhibition in AML patients to determine the efficacy are ongoing [143].
PD-1/PD-L1
PD-1 is an immune checkpoint that suppresses the activity of T cells after antigen activation, acting as a brake of the immune response [144]. Increased expression of its ligands (PD-L1/PD-L2) on human AML blasts has been linked to resistance to treatment; thus, targeting of the PD-1/PD-L1 pathway may be another alternative treatment for AML [145, 146]. In a phase I study of PD-1 inhibition for patients with hematologic malignancies using CT-011, including eight patients with AML, a minimal response was observed in one AML patient, with a decrease in blast percentage from 50 to 5% [147]. Another study reported that patients with MDS or AML highly expressed PD-L1, PD-L2, PD-1, and CTLA-4, which has been connected with the pathogenesis and resistance mechanisms; thus, blockade of immune checkpoints is a potential therapy in MDS and AML [13]. Overall, PD-1/PD-L1 blockade can produce possible clinical benefits in selected therapies for AML. There are many ongoing clinical trials on PD-1/PD-L1 inhibition in patients with AML aiming to identify efficacy for extensive use in the clinic [143].
Additional immune checkpoints, such as LAG-3 (lymphocyte activation gene-3) and TIM-3 (T cell immunoglobulin and mucin domain-containing protein 3), have been evaluated in preclinical trials for AML. For example, LAG-3 inhibition has enhanced the effector function of immunotherapy in murine models of leukemia [148]. TIM-3 is often co-expressed with PD-1, and dual blockade of TIM-3 and PD-L1 reduced the tumor burden and prolonged the survival of mice with advanced AML [149]. According to the achievement, checkpoint inhibitor therapies can produce desirable clinical outcomes, but require further preclinical studies and clinical trials to identify the function for patients with AML.
Summary
Although chemotherapy can lead to remission in AML patients, this disease has a high probability of relapse. The current methods to prevent relapse consist of consolidation chemotherapy, immunotherapy, and autologous or allogeneic SCT. In addition, the elderly, who account for a large proportion of AML patients, cannot tolerate stem cell transplants or large doses of chemotherapy. Therefore, immunotherapy is a promising alternative.
AML is a complex and heterogeneous disease characterized by diverse genetic landscape. The Cancer Genome Atlas Research Network compiles the genomes of 200 clinically annotated adult cases of de novo AML by using whole-genome sequencing (50 cases) or whole-exome sequencing (150 cases), with the average number of coding mutations per patient being 13 genes; 23 genes were considered to be a significant mutation, and another 237 genes were mutated in two or more samples [150]. In addition to mutation, gene interaction can also affect leukemogenesis and prognosis [151]; thus, it is difficult to identify a perfect and differential antigen to target. Moreover, as antigen expression is heterogeneous on leukemia blasts and targets are also expressed on healthy cells, CAR-T cells may not have promising outcomes due to the limited antigen information and the need to balance efficacy and safety [152]. To improve efficacy, third-generation CAR-T cells were created with the idea that T cell-mediated anti-tumor immunity requires the presence of T cell receptor and co-stimulatory molecules, which can enhance CAR-T cell survival, expansion, and activity. Researchers have studied dual-targeting CAR-T cells for two LAAs, which were shown to prevent antigen escape mechanisms without increasing cytotoxicity [153, 154]. Based on results to date, such approaches may be promising for the clinic, with beneficial outcomes after further preclinical and clinical studies. PD-1 blockade also appears to be an ideal partner for CAR-T therapy to enhance the efficacy of CAR-T cells [155]. And due to the complex heterogeneity of AML, every patient may have different associated molecular and chromosomal aberrations, requiring individualized treatment based on risk factors and biomarkers. Accordingly, the individualized combination of different immune approaches along with chemotherapy and autologous or allogeneic SCT to treat AML to achieve the highest efficacy may be a promising future trend, which will improve overall outcomes for patients. Although, the design and application of optimal therapeutic strategies during the course of AML also face many challenges, achievements, and information regarding the mechanisms involved in AML relapse and resistance may lead to novel treatments for AML.
References
Wiseman DH, Greystoke BF, Somervaille TC (2014) The variety of leukemic stem cells in myeloid malignancy. Oncogene 33(24):3091–3098. https://doi.org/10.1038/onc.2013.269
Pleyer L, Stauder R, Burgstaller S, Schreder M, Tinchon C, Pfeilstocker M, Steinkirchner S, Melchardt T, Mitrovic M, Girschikofsky M, Lang A, Krippl P, Sliwa T, Egle A, Linkesch W, Voskova D, Angermann H, Greil R (2013) Azacitidine in patients with WHO-defined AML—results of 155 patients from the Austrian Azacitidine Registry of the AGMT-Study Group. J Hematol Oncol 6:32. https://doi.org/10.1186/1756-8722-6-32
Yates JW, Wallace HJ Jr, Ellison RR, Holland JF (1973) Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep 57(4):485–488
Barrett AJ (2008) Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol 142(6):877–888. https://doi.org/10.1111/j.1365-2141.2008.07260.x
Whiteway A, Corbett T, Anderson R, Macdonald I, Prentice HG (2003) Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol 120(3):442–451
Cheng K, Wong SC, Linn YC, Ho LP, Chng WJ, Schwarz H (2014) CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells. Br J Haematol 165(1):134–144. https://doi.org/10.1111/bjh.12732
Shen M, Linn YC, Ren EC (2016) KIR-HLA profiling shows presence of higher frequencies of strong inhibitory KIR-ligands among prognostically poor risk AML patients. Immunogenetics 68(2):133–144. https://doi.org/10.1007/s00251-015-0888-4
Tang M, Acheampong DO, Wang Y, Xie W, Wang M, Zhang J (2016) Tumoral NKG2D alters cell cycle of acute myeloid leukemic cells and reduces NK cell-mediated immune surveillance. Immunol Res 64(3):754–764. https://doi.org/10.1007/s12026-015-8769-3
Benitez AC, Dai Z, Mann HH, Reeves RS, Margineantu DH, Gooley TA, Groh V, Spies T (2011) Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. Proc Natl Acad Sci U S A 108(10):4081–4086. https://doi.org/10.1073/pnas.1018603108
Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL (2012) Natural killer cell immune escape in acute myeloid leukemia. Leukemia 26(9):2019–2026. https://doi.org/10.1038/leu.2012.87
Baessler T, Krusch M, Schmiedel BJ, Kloss M, Baltz KM, Wacker A, Schmetzer HM, Salih HR (2009) Glucocorticoid-induced tumor necrosis factor receptor-related protein ligand subverts immunosurveillance of acute myeloid leukemia in humans. Cancer Res 69(3):1037–1045. https://doi.org/10.1158/0008-5472.can-08-2650
Steinbacher J, Schmiedel BJ, Werner A, Nuebling T, Buechele C, Grosse-Hovest L, Kanz L, Salih HR (2012) Bimodal induction of NK cell reactivity against acute myeloid (AML) and chronic lymphoid leukemia (CLL) by Fc-engineered GITR-Fc fusion proteins [abstract]. Blood 120(21):2143
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, Garcia-Manero G (2014) Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 28(6):1280–1288. https://doi.org/10.1038/leu.2013.355
Salih HR, Schmetzer HM, Burke C, Starling GC, Dunn R, Pelka-Fleischer R, Nuessler V, Kiener PA (2001) Soluble CD137 (4-1BB) ligand is released following leukocyte activation and is found in sera of patients with hematological malignancies. J Immunol 167(7):4059–4066
Scholl N, Loibl J, Kremser A, Liepert A, Grabrucker C, Salih HR, Kolb HJ, Schmetzer HM (2009) The role of soluble and cell-surface expressed 4-1BB ligand in patients with malignant hemopoietic disorders. Leuk Lymphoma 50(3):427–436. https://doi.org/10.1080/10428190802709453
Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, Lee AM, Lister TA, Gribben JG (2009) Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood 114(18):3909–3916. https://doi.org/10.1182/blood-2009-02-206946
Khaznadar Z, Henry G, Setterblad N, Agaugue S, Raffoux E, Boissel N, Dombret H, Toubert A, Dulphy N (2014) Acute myeloid leukemia impairs natural killer cells through the formation of a deficient cytotoxic immunological synapse. Eur J Immunol 44(10):3068–3080. https://doi.org/10.1002/eji.201444500
Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, Hirst WJ (2001) Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol 167(10):6021–6030
Li Y, Yin Q, Yang L, Chen S, Geng S, Wu X, Zhong L, Schmidt CA, Przybylski GK (2009) Reduced levels of recent thymic emigrants in acute myeloid leukemia patients. Cancer Immunol Immunother: CII 58(7):1047–1055. https://doi.org/10.1007/s00262-008-0621-3
Driss V, Quesnel B, Brinster C (2015) Monocyte chemoattractant protein 1 (MCP-1/CCL2) contributes to thymus atrophy in acute myeloid leukemia. Eur J Immunol 45(2):396–406. https://doi.org/10.1002/eji.201444736
Cogle CR, Bosse RC, Brewer T, Migdady Y, Shirzad R, Kampen KR, Saki N (2016) Acute myeloid leukemia in the vascular niche. Cancer Lett 380(2):552–560. https://doi.org/10.1016/j.canlet.2015.05.007
Ishii K, Barrett AJ (2016) Novel immunotherapeutic approaches for the treatment of acute leukemia (myeloid and lymphoblastic). Ther Adv Hematol 7(1):17–39. https://doi.org/10.1177/2040620715616544
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, Vellenga E, De Greef GE, Jacky E, Van der Lelie J, Boogaerts MA, Lowenberg B (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23(9):1969–1978. https://doi.org/10.1200/jco.2005.06.027
Powles RL, Balchin LA, Fairley GH, Alexander P (1971) Recognition of leukaemia cells as foreign before and after autoimmunization. Br Med J 1(5747):486–489
Van Tendeloo VF, Van Broeckhoven C, Berneman ZN (2001) Gene-based cancer vaccines: an ex vivo approach. Leukemia 15(4):545–558
Zhang WG, Liu SH, Cao XM, Cheng YX, Ma XR, Yang Y, Wang YL (2005) A phase-I clinical trial of active immunotherapy for acute leukemia using inactivated autologous leukemia cells mixed with IL-2, GM-CSF, and IL-6. Leuk Res 29(1):3–9. https://doi.org/10.1016/j.leukres.2004.04.015
Smits EL, Ponsaerts P, Van de Velde AL, Van Driessche A, Cools N, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, Van Tendeloo VF (2007) Proinflammatory response of human leukemic cells to dsRNA transfection linked to activation of dendritic cells. Leukemia 21(8):1691–1699. https://doi.org/10.1038/sj.leu.2404763
Greiner J, Dohner H, Schmitt M (2006) Cancer vaccines for patients with acute myeloid leukemia—definition of leukemia-associated antigens and current clinical protocols targeting these antigens. Haematologica 91(12):1653–1661
Kwon M, Martinez-Laperche C, Infante M, Carretero F, Balsalobre P, Serrano D, Gayoso J, Perez-Corral A, Anguita J, Diez-Martin JL, Buno I (2012) Evaluation of minimal residual disease by real-time quantitative PCR of Wilms’ tumor 1 expression in patients with acute myelogenous leukemia after allogeneic stem cell transplantation: correlation with flow cytometry and chimerism. Biol Blood Marrow Transplant 18(8):1235–1242. https://doi.org/10.1016/j.bbmt.2012.01.012
Tsuboi A, Oka Y, Kyo T, Katayama Y, Elisseeva OA, Kawakami M, Nishida S, Morimoto S, Murao A, Nakajima H, Hosen N, Oji Y, Sugiyama H (2012) Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease. Leukemia 26(6):1410–1413. https://doi.org/10.1038/leu.2011.343
Liu H, Zha Y, Malnassy G, Fulton N, Green M, Park J-H, Nakamura Y, Larson RA, Salazar AM, Odenike O, Gajewski T, Stock W (2016) WT1 peptide vaccine is able to induce WT1-specifc immune response with TCR clonal enrichment to control minimal residual disease in patients with myeloid leukemia [abstract]. Blood 128(22):3984
Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ (1996) Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood 88(7):2450–2457
Ma Q, Wang C, Jones D, Quintanilla KE, Li D, Wang Y, Wieder ED, Clise-Dwyer K, Alatrash G, Mj Y, Munsell MF, Lu S, Qazilbash MH, Molldrem JJ (2010) Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy 12(8):1056–1062. https://doi.org/10.3109/14653249.2010.506506
Sergeeva A, He H, Ruisaard K, St John L, Alatrash G, Clise-Dwyer K, Li D, Patenia R, Hong R, Sukhumalchandra P, You MJ, Gagea M, Ma Q, Molldrem JJ (2016) Activity of 8F4, a T-cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia 30(7):1475–1484. https://doi.org/10.1038/leu.2016.57
Ma Q, Garber HR, Lu S, He H, Tallis E, Ding X, Sergeeva A, Wood MS, Dotti G, Salvado B, Ruisaard K, Clise-Dwyer K, John LS, Rezvani K, Alatrash G, Shpall EJ, Molldrem JJ (2016) A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy 18(8):985–994. https://doi.org/10.1016/j.jcyt.2016.05.001
Qazilbash MH, Wieder E, Thall PF, Wang X, Rios R, Lu S, Kanodia S, Ruisaard KE, Giralt SA, Estey EH, Cortes J, Komanduri KV, Clise-Dwyer K, Alatrash G, Ma Q, Champlin RE, Molldrem JJ (2017) PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia 31(3):697–704. https://doi.org/10.1038/leu.2016.254
Ding K, Wang XM, Fu R, Ruan EB, Liu H, Shao ZH (2012) PRAME gene expression in acute leukemia and its clinical significance. Cancer Biol Med 9(1):73–76. https://doi.org/10.3969/j.issn.2095-3941.2012.01.013
Quintarelli C, Dotti G, Hasan ST, De Angelis B, Hoyos V, Errichiello S, Mims M, Luciano L, Shafer J, Leen AM, Heslop HE, Rooney CM, Pane F, Brenner MK, Savoldo B (2011) High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 117(12):3353–3362. https://doi.org/10.1182/blood-2010-08-300376
Sherman L, Sleeman J, Herrlich P, Ponta H (1994) Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6(5):726–733
Greiner J, Ringhoffer M, Taniguchi M, Li L, Schmitt A, Shiku H, Dohner H, Schmitt M (2004) mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer 108(5):704–711. https://doi.org/10.1002/ijc.11623
Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F, Yu Y, Gotz M, Heyduk M, Ritter G, Speiser DE, Gnjatic S, Guillaume P, Ringhoffer M, Schlenk RF, Liebisch P, Bunjes D, Shiku H, Dohner H, Greiner J (2008) RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 111(3):1357–1365. https://doi.org/10.1182/blood-2007-07-099366
Spranger S, Jeremias I, Wilde S, Leisegang M, Starck L, Mosetter B, Uckert W, Heemskerk MH, Schendel DJ, Frankenberger B (2012) TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood 119(15):3440–3449. https://doi.org/10.1182/blood-2011-06-357939
Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, Jafarpour B, Boss C, Barrett AJ (2008) Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 111(1):236–242. https://doi.org/10.1182/blood-2007-08-108241
Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN (2007) Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 82(6):1365–1374. https://doi.org/10.1189/jlb.0307166
Meidenbauer N, Andreesen R, Mackensen A (2001) Dendritic cells for specific cancer immunotherapy. Biol Chem 382(4):507–520. https://doi.org/10.1515/bc.2001.065
Subklewe M, Geiger C, Lichtenegger FS, Javorovic M, Kvalheim G, Schendel DJ, Bigalke I (2014) New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol Immunother: CII 63(10):1093–1103. https://doi.org/10.1007/s00262-014-1600-5
Pyzer AR, Avigan DE, Rosenblatt J (2014) Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum Vaccin Immunother 10(11):3125–3131. https://doi.org/10.4161/21645515.2014.982993
Van Tendeloo VF, Van de Velde A, Van Driessche A, Cools N, Anguille S, Ladell K, Gostick E, Vermeulen K, Pieters K, Nijs G, Stein B, Smits EL, Schroyens WA, Gadisseur AP, Vrelust I, Jorens PG, Goossens H, de Vries IJ, Price DA, Oji Y, Oka Y, Sugiyama H, Berneman ZN (2010) Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 107(31):13824–13829. https://doi.org/10.1073/pnas.1008051107
Berneman ZN, Velde ALVd, Willemen Y, Anguille S, Saevels K, Germonpré P, Huizing MT, Peeters M, Snoeckx A, Parizel P, Tendeloo VFV, Lion E, Nijs G, Stein B, Vermeulen K, Maes M-B, Malfait R, Vrelust I, Verlinden A, Gadisseur AP, Schroyens WA, Lammens M, Smits EL (2014) Vaccination with WT1 mRNA-electroporated dendritic cells: report of clinical outcome in 66 cancer patients [abstract]. Blood 124(21):310
Padua RA, Larghero J, Robin M, le Pogam C, Schlageter MH, Muszlak S, Fric J, West R, Rousselot P, Phan TH, Mudde L, Teisserenc H, Carpentier AF, Kogan S, Degos L, Pla M, Bishop JM, Stevenson F, Charron D, Chomienne C (2003) PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nat Med 9(11):1413–1417. https://doi.org/10.1038/nm949
Tagawa ST, Lee P, Snively J, Boswell W, Ounpraseuth S, Lee S, Hickingbottom B, Smith J, Johnson D, Weber JS (2003) Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer 98(1):144–154. https://doi.org/10.1002/cncr.11462
Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, Rajapaksa R, Caspar CB, Van Beckhoven A, Levy R (2002) Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res 62(20):5845–5852
Lin C, Li Y (2013) The role of peptide and DNA vaccines in myeloid leukemia immunotherapy. Cancer Cell Int 13(1):13. https://doi.org/10.1186/1475-2867-13-13
Kohler G, Milstein C (2005) Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol 174(5):2453–2455
Cowan AJ, Laszlo GS, Estey EH, Walter RB (2013) Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front Biosci (Landmark Ed) 18:1311–1334
Feldman E, Kalaycio M, Weiner G, Frankel S, Schulman P, Schwartzberg L, Jurcic J, Velez-Garcia E, Seiter K, Scheinberg D, Levitt D, Wedel N (2003) Treatment of relapsed or refractory acute myeloid leukemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia 17(2):314–318. https://doi.org/10.1038/sj.leu.2402803
Caron PC, Dumont L, Scheinberg DA (1998) Supersaturating infusional humanized anti-CD33 monoclonal antibody HuM195 in myelogenous leukemia. Clin Cancer Res 4(6):1421–1428
Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS, Heaney ML, Chanel S, Morgenstern A, Sgouros G, Larson SM, Scheinberg DA, Jurcic JG (2010) Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 16(21):5303–5311. https://doi.org/10.1158/1078-0432.ccr-10-0382
Damle NK, Frost P (2003) Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol 3(4):386–390
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, Yin JA, Hunter A, Goldstone AH, Wheatley K (2011) Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 29(4):369–377. https://doi.org/10.1200/jco.2010.31.4310
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR (2013) A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 121(24):4854–4860. https://doi.org/10.1182/blood-2013-01-466706
Ravandi F, Estey EH, Appelbaum FR, Lo-Coco F, Schiffer CA, Larson RA, Burnett AK, Kantarjian HM (2012) Gemtuzumab ozogamicin: time to resurrect? J Clin Oncol 30(32):3921–3923. https://doi.org/10.1200/jco.2012.43.0132
Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, Annino L, Venditti A, Voso MT, Mazzone C, Magro D, De Fabritiis P, Muus P, Alimena G, Mancini M, Hagemeijer A, Paoloni F, Vignetti M, Fazi P, Meert L, Ramadan SM, Willemze R, de Witte T, Baron F (2016) Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol 34(9):972–979. https://doi.org/10.1200/jco.2015.64.0060
Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, Stone I, Ryan MC, Sussman D, Lyon RP, Zeng W, Harrington KH, Klussman K, Westendorf L, Meyer D, Bernstein ID, Senter PD, Benjamin DR, Drachman JG, McEarchern JA (2013) SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 122(8):1455–1463. https://doi.org/10.1182/blood-2013-03-491506
Stein EM (2014) Interim analysis of a phase 1 trial of SGN-CD33A in patients with CD33-positive acute myeloid leukemia (AML). Blood Abstract 124:623
Krystal WM, Walker R, Fishkin N, Audette C, Kovtun Y, Romanelli A (2015) IMGN779, a CD33-targeted antibody-drug conjugate (ADC) with a novel DNA-alkylating effector molecule, induces DNA damage, cell cycle arrest, and apoptosis in AML cells [abstract]. Blood 126(23):1366
Portwood S, Puchalski RA, Walker RM, Wang ES (2016) Combining IMGN779, a novel anti-CD33 antibody-drug conjugate (ADC), with the PARP inhibitor, olaparib, results in enhanced anti-tumor activity in preclinical acute myeloid leukemia (AML) models [abstract]. Blood 128(22):1645
Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S, Freireich EJ, Kadia T, Garcia-Manero G, Kantarjian H, Cortes JE (2013) Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica 98(2):217–221. https://doi.org/10.3324/haematol.2012.071092
Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, Brunet S, Sierra J (2001) Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica 86(12):1261–1269
Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, Latagliata R, Mariani G, Rossini A, Battistini A, Lo-Coco F, Peschle C (2002) Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 100(8):2980–2988. https://doi.org/10.1182/blood-2002-03-0852
He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, Marlton P, McLachlan AJ, Kerridge I, Bradstock KF, Kennedy G, Boyd AW, Yeadon TM, Lopez AF, Ramshaw HS, Iland H, Bamford S, Barnden M, DeWitte M, Basser R, Roberts AW (2015) A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma 56(5):1406–1415. https://doi.org/10.3109/10428194.2014.956316
Smith BD, Roboz GJ, Walter RB, Altman JK, Ferguson A, Curcio TJ, Orlowski KF, Garrett L, Busfield SJ, Barnden M, Sedgmen B, Ghosh S, Hosback S, Davis R, Dyson A, Dasen S, DeWitte M, Bensen-Kennedy DM, Roberts AW (2014) First-in man, phase 1 study of CSL362 (anti-IL3Rα/anti-CD123 monoclonal antibody) in patients with CD123+ acute myeloid leukemia (AML) in CR at high risk for early relapse [abstract]. Blood 124(21):120
Sweet KL, Pemmaraju N, Lane AA, Stein AS, Vasu S, Blum W, Rizzieri DA, Wang ES, Rowinsky EK, Szarek M, Brooks CL, Disalvatore S, Liu D, Duvic M, Schwartz JD, Konopleva M (2015) Lead-in stage results of a pivotal trial of SL-401, an interleukin-3 receptor (IL-3R) targeting biologic, in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) or acute myeloid leukemia (AML) [abstract]. Blood 126(23):3795
Schwartz MA, Lovett DR, Redner A, Finn RD, Graham MC, Divgi CR, Dantis L, Gee TS, Andreeff M, Old LJ et al (1993) Dose-escalation trial of M195 labeled with iodine 131 for cytoreduction and marrow ablation in relapsed or refractory myeloid leukemias. J Clin Oncol 11(2):294–303
Jurcic JG (2012) What happened to anti-CD33 therapy for acute myeloid leukemia? Curr Hematol Malig Rep 7(1):65–73. https://doi.org/10.1007/s11899-011-0103-0
Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM, Matthews DC, Deeg HJ, Gopal AK, Martin PJ, Storb RF, Press OW, Appelbaum FR (2009) Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 114(27):5444–5453. https://doi.org/10.1182/blood-2009-03-213298
Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, Ruffner K, Nemecek E, Sickle E, Durack L, Carreras J, Horowitz MM, Press OW, Gopal AK, Martin PJ, Bernstein ID, Matthews DC (2006) 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood 107(5):2184–2191. https://doi.org/10.1182/blood-2005-06-2317
Burke JM, Caron PC, Papadopoulos EB, Divgi CR, Sgouros G, Panageas KS, Finn RD, Larson SM, O'Reilly RJ, Scheinberg DA, Jurcic JG (2003) Cytoreduction with iodine-131-anti-CD33 antibodies before bone marrow transplantation for advanced myeloid leukemias. Bone Marrow Transplant 32(6):549–556. https://doi.org/10.1038/sj.bmt.1704201
Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, Ballangrud AM, Hamacher KA, Ma D, Humm JL, Brechbiel MW, Molinet R, Scheinberg DA (2002) Targeted alpha particle immunotherapy for myeloid leukemia. Blood 100(4):1233–1239
Jurcic JG, Levy MY, Park JH, Ravandi F, Perl AE, Pagel JM, Smith BD, Estey EH, Kantarjian H, Cicic D, Scheinberg DA (2016) Phase I trial of targeted alpha-particle therapy with actinium-225 (225Ac)-lintuzumab and low-dose cytarabine (LDAC) in patients age 60 or older with untreated acute myeloid leukemia (AML) [abstract]. Blood 128(22):4050
Orozco JJ, Back T, Kenoyer A, Balkin ER, Hamlin DK, Wilbur DS, Fisher DR, Frayo SL, Hylarides MD, Green DJ, Gopal AK, Press OW, Pagel JM (2013) Anti-CD45 radioimmunotherapy using (211)At with bone marrow transplantation prolongs survival in a disseminated murine leukemia model. Blood 121(18):3759–3767. https://doi.org/10.1182/blood-2012-11-467035
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Kohne-Volland R, Bruggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele H, Riethmuller G, Hoelzer D, Zugmaier G, Bargou RC (2011) Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29(18):2493–2498. https://doi.org/10.1200/jco.2010.32.7270
Krupka C, Kufer P, Kischel R, Zugmaier G, Bogeholz J, Kohnke T, Lichtenegger FS, Schneider S, Metzeler KH, Fiegl M, Spiekermann K, Baeuerle PA, Hiddemann W, Riethmuller G, Subklewe M (2014) CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 123(3):356–365. https://doi.org/10.1182/blood-2013-08-523548
Laszlo GS, Gudgeon CJ, Harrington KH, Dell'Aringa J, Newhall KJ, Means GD, Sinclair AM, Kischel R, Frankel SR, Walter RB (2014) Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 123(4):554–561. https://doi.org/10.1182/blood-2013-09-527044
Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, Zhang W, Tuaillon N, Rainey J, Barat B, Yang Y, Jin L, Ciccarone V, Moore PA, Koenig S, Bonvini E (2010) Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 399(3):436–449. https://doi.org/10.1016/j.jmb.2010.04.001
Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, Gao F, Eades WC, Bonvini E, Chichili GR, Moore PA, Johnson S, Collins L, DiPersio JF (2016) Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood 127(1):122–131. https://doi.org/10.1182/blood-2014-05-575704
Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA et al (1993) Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 85(8):622–632
Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT et al (1987) A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 316(15):889–897. https://doi.org/10.1056/nejm198704093161501
Ettinghausen SE, Lipford EH 3rd, Mule JJ, Rosenberg SA (1985) Recombinant interleukin 2 stimulates in vivo proliferation of adoptively transferred lymphokine-activated killer (LAK) cells. J Immunol 135(5):3623–3635
Thatcher N, Dazzi H, Johnson RJ, Russell S, Ghosh AK, Moore M, Chadwick G, Craig RD (1989) Recombinant interleukin-2 (rIL-2) given intrasplenically and intravenously for advanced malignant melanoma. A phase I and II study. Br J Cancer 60(5):770–774
Lu PH, Negrin RS (1994) A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 153(4):1687–1696
Borrello I, Noonan KA (2016) Marrow-infiltrating lymphocytes—role in biology and cancer therapy. Front Immunol 7:112. https://doi.org/10.3389/fimmu.2016.00112
Noonan KA, Huff CA, Davis J, Lemas MV, Fiorino S, Bitzan J, Ferguson A, Emerling A, Luznik L, Matsui W, Powell J, Fuchs E, Rosner GL, Epstein C, Rudraraju L, Ambinder RF, Jones RJ, Pardoll D, Borrello I (2015) Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med 7(288):288ra278. https://doi.org/10.1126/scitranslmed.aaa7014
Kapp M, Stevanovic S, Fick K, Tan SM, Loeffler J, Opitz A, Tonn T, Stuhler G, Einsele H, Grigoleit GU (2009) CD8+ T-cell responses to tumor-associated antigens correlate with superior relapse-free survival after allo-SCT. Bone Marrow Transplant 43(5):399–410. https://doi.org/10.1038/bmt.2008.426
Amir AL, van der Steen DM, van Loenen MM, Hagedoorn RS, de Boer R, Kester MD, de Ru AH, Lugthart GJ, van Kooten C, Hiemstra PS, Jedema I, Griffioen M, van Veelen PA, Falkenburg JH, Heemskerk MH (2011) PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res 17(17):5615–5625. https://doi.org/10.1158/1078-0432.ccr-11-1066
Mohamed YS, Bashawri LA, Vatte C, Abu-Rish EY, Cyrus C, Khalaf WS, Browning MJ (2016) The in vitro generation of multi-tumor antigen-specific cytotoxic T cell clones: candidates for leukemia adoptive immunotherapy following allogeneic stem cell transplantation. Mol Immunol 77:79–88. https://doi.org/10.1016/j.molimm.2016.07.012
Leung W (2014) Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res 20(13):3390–3400. https://doi.org/10.1158/1078-0432.ccr-13-1766
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (New York, NY) 295(5562):2097–2100. https://doi.org/10.1126/science.1068440
Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Jang YJ, Kang M, Yeom YI, Lee JL, Kim DY, Lee YS, Kang YA, Jeon M, Seol M, Lee JH, Lee JH, Kim HJ, Yun SC, Lee KH (2014) Donor-derived natural killer cells infused after human leukocyte antigen-haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant 20(5):696–704. https://doi.org/10.1016/j.bbmt.2014.01.031
Dulphy N, Chretien AS, Khaznadar Z, Fauriat C, Nanbakhsh A, Caignard A, Chouaib S, Olive D, Toubert A (2016) Underground adaptation to a hostile environment: acute myeloid leukemia vs. natural killer cells. Front Immunol 7:94. https://doi.org/10.3389/fimmu.2016.00094
Sandoval-Borrego D, Moreno-Lafont MC, Vazquez-Sanchez EA, Gutierrez-Hoya A, Lopez-Santiago R, Montiel-Cervantes LA, Ramirez-Saldana M, Vela-Ojeda J (2016) Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch Med Res 47(1):55–64. https://doi.org/10.1016/j.arcmed.2016.02.001
Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, Seidl C, Seifried E, Klingebiel T, Schwabe D (2004) IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis 33(3):261–266. https://doi.org/10.1016/j.bcmd.2004.08.013
Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P, Tichelli A, Schwabe D, Koehl U (2013) Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant 48(3):433–438. https://doi.org/10.1038/bmt.2012.162
Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D (2013) A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 73(6):1777–1786. https://doi.org/10.1158/0008-5472.can-12-3558
Kamiya T, Chang YH, Campana D (2016) Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res 4(7):574–581. https://doi.org/10.1158/2326-6066.cir-15-0229
Hermanson DL, Kaufman DS (2015) Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol 6:195. https://doi.org/10.3389/fimmu.2015.00195
Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM (2009) Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology 126(3):423–435. https://doi.org/10.1111/j.1365-2567.2008.02910.x
Introna M, Golay J, Rambaldi A (2013) Cytokine induced killer (CIK) cells for the treatment of haematological neoplasms. Immunol Lett 155(1-2):27–30. https://doi.org/10.1016/j.imlet.2013.09.017
Sangiolo D (2011) Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer 2:363–368
Rettinger E, Meyer V, Kreyenberg H, Volk A, Kuci S, Willasch A, Koscielniak E, Fulda S, Wels WS, Boenig H, Klingebiel T, Bader P (2012) Cytotoxic capacity of IL-15-stimulated cytokine-induced killer cells against human acute myeloid leukemia and rhabdomyosarcoma in humanized preclinical mouse models. Front Oncol 2:32. https://doi.org/10.3389/fonc.2012.00032
Linn YC, Niam M, Chu S, Choong A, Yong HX, Heng KK, Hwang W, Loh Y, Goh YT, Suck G, Chan M, Koh M (2012) The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant 47(7):957–966. https://doi.org/10.1038/bmt.2011.202
Zhong ZD, Luo Y, Zou P, Zheng JE, Yao JX, Huang SA, Zhou DF, You Y (2012) Infusions of recipient-derived cytokine-induced killer cells of donor origin eradicated residual disease in a relapsed leukemia patient after allo-hematopoietic stem cell transplantation. Chin Med J 125(9):1669–1671
Marten A, Renoth S, von Lilienfeld-Toal M, Buttgereit P, Schakowski F, Glasmacher A, Sauerbruch T, Schmidt-Wolf IG (2001) Enhanced lytic activity of cytokine-induced killer cells against multiple myeloma cells after co-culture with idiotype-pulsed dendritic cells. Haematologica 86(10):1029–1037
Cheng XY, Li JL (2015) Biological activity of cytotoxic dendritic cells cocultured with cytokine-induced killer cells and their effect on acute leukemia cells. Genet Mol Res: GMR 14(4):13208–13214. https://doi.org/10.4238/2015.October.26.17
Chan JK, Hamilton CA, Cheung MK, Karimi M, Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, Lum LG, Negrin RS (2006) Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res 12(6):1859–1867. https://doi.org/10.1158/1078-0432.ccr-05-2019
Du SH, Li Z, Chen C, Tan WK, Chi Z, Kwang TW (2016) Co-expansion of cytokine-induced killer cells and Vgamma9Vdelta2 T cells for CAR T-cell therapy. 11 (9):e0161820. https://doi.org/10.1371/journal.pone.0161820
Poh SL, Linn YC (2016) Immune checkpoint inhibitors enhance cytotoxicity of cytokine-induced killer cells against human myeloid leukaemic blasts. 65 (5):525-536. https://doi.org/10.1007/s00262-016-1815-8
Laumbacher B, Gu S, Wank R (2012) Activated monocytes prime naive T cells against autologous cancer: vigorous cancer destruction in vitro and in vivo. Scand J Immunol 75(3):314–328. https://doi.org/10.1111/j.1365-3083.2011.02652.x
Wank R, Song X, Gu S, Laumbacher B (2014) Benefits of a continuous therapy for cancer patients with a novel adoptive cell therapy by cascade priming (CAPRI). Immunotherapy 6(3):269–282. https://doi.org/10.2217/imt.14.6
Ikeda H (2016) T-cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells. Int Immunol 28(7):349–353. https://doi.org/10.1093/intimm/dxw022
Geiger TL, Rubnitz JE (2015) New approaches for the immunotherapy of acute myeloid leukemia. Discov Med 19(105):275–284
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6(224):224ra225. https://doi.org/10.1126/scitranslmed.3008226
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD (2015) Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther 23(1):184–191. https://doi.org/10.1038/mt.2014.164
Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, June CH, Gill S (2015) CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 29(8):1637–1647. https://doi.org/10.1038/leu.2015.52
Hoang T, Foster A, Crisostomo J, Lu A, Moseley A, Heemskerk MHM, Slawin KM, Spencer DM (2015) Inducible MyD88/CD40 enhances proliferation and survival of PRAME-specific TCR-engineered T cells and increases anti-tumor effects in myeloma [abstract]. Blood 126(23):1886
Xue SA, Gao L, Thomas S, Hart DP, Xue JZ, Gillmore R, Voss RH, Morris E, Stauss HJ (2010) Development of a Wilms’ tumor antigen-specific T-cell receptor for clinical trials: engineered patient’s T cells can eliminate autologous leukemia blasts in NOD/SCID mice. Haematologica 95(1):126–134. https://doi.org/10.3324/haematol.2009.006486
Tawara I, Masuya M, Kageyama S, Nishida T, Terakura S, Murata M, Fujiwara H, Akatsuka Y, Ikeda H, Miyahara Y, Tomura D, Nukaya I, Takesako K, Emi N, Yasukawa M, Katayama N, Shiku H (2015) Adoptive transfer of WT1-specific TCR gene-transduced lymphocytes in patients with myelodysplastic syndrome and acute myeloid leukemia [abstract]. Blood 126(23):97
Tanimoto K, Fujiwara H, Tawara I, Masuya M, Kageyama S, Nishida T, Murata M, Terakura S, Akatsuka Y, Ikeda H, Miyahara Y, Nukaya I, Takesako K, Emi N, Katayama N, Shiku H, Yasukawa M (2016) Phase 1 clinical trial of adoptive immunotherapy for acute myelogenous leukemia and myelodysplastic syndrome, using gene-modified autologous lymphocytes expressing WT1-specific T-cell receptor [abstract]. Blood 128(22):1653
Ikeda H, Akahori Y, Yoneyama M, Orito Y, Miyahara Y, Amaishi Y, Okamoto S, Mineno J, Takesako K, Fujiwara H, Yasukawa M, Shiku H (2015) Immunotherapy with chimeric antigen receptor targeting intracellular WT1 gene product complexed with HLA-a*24:02 molecule [abstract]. Blood 126(23):4292
Campillo-Davo D, Fujiki F, Bergh JMJVd, Smits EL, Sugiyama H, Tendeloo VFIV, Berneman ZN (2016) Electroporation of Dicer-substrate siRNA duplexes targeting endogenous TCR enhance tumor killing activity of Wilms’ tumor 1 (WT1)-specific TCR-redirected cytotoxic T cells [abstract]. Blood 128(22):813
Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, Chen K, Shin M, Wall DM, Honemann D, Gambell P, Westerman DA, Haurat J, Westwood JA, Scott AM, Kravets L, Dickinson M, Trapani JA, Smyth MJ, Darcy PK, Kershaw MH, Prince HM (2013) Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther 21(11):2122–2129. https://doi.org/10.1038/mt.2013.154
Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang WC, Bretzlaff W, Chang B, Jonnalagadda M, Starr R, Ostberg JR, Jensen MC, Bhatia R, Forman SJ (2013) T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 122(18):3138–3148. https://doi.org/10.1182/blood-2012-12-474056
Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, Marcatti M, Saudemont A, Bordignon C, Savoldo B, Ciceri F, Naldini L, Dotti G, Bonini C, Bondanza A (2013) CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 122(20):3461–3472. https://doi.org/10.1182/blood-2013-04-493361
Miyazaki Y, Fujiwara H, Asai H, Ochi F, Ochi T, Azuma T, Ishida T, Okamoto S, Mineno J, Kuzushima K, Shiku H, Yasukawa M (2013) Development of a novel redirected T-cell-based adoptive immunotherapy targeting human telomerase reverse transcriptase for adult T-cell leukemia. Blood 121(24):4894–4901. https://doi.org/10.1182/blood-2012-11-465971
Bigalke I, Honnashagen K, Lundby M, Kasten J, Inderberg EMS, Skoge L, Saboe-Larssen S, Schendel DJ, Kvalheim G (2014) Vaccination with a new generation of fast dendritic cells transfected with mRNA from hTERT, survivin and autologous tumor mount strong immune responses and prolong survival [abstract]. Blood 124(21):2440
Stauss HJ (2017) Turn to TCRs when CARs fail. Oncotarget 8(8):12538–12539. https://doi.org/10.18632/oncotarget.15088
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. https://doi.org/10.1056/NEJMoa1003466
LaBelle JL, Hanke CA, Blazar BR, Truitt RL (2002) Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7-1+, but not B7-2+, murine myelogenous leukemia. Blood 99(6):2146–2153
Fevery S, Billiau AD, Sprangers B, Rutgeerts O, Lenaerts C, Goebels J, Landuyt W, Kasran A, Boon L, Sagaert X, De Wolf-Peeters C, Waer M, Vandenberghe P (2007) CTLA-4 blockade in murine bone marrow chimeras induces a host-derived antileukemic effect without graft-versus-host disease. Leukemia 21(7):1451–1459. https://doi.org/10.1038/sj.leu.2404720
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, Lukez AP, Avigan D, Chen YB, McSweeney P, LeBoeuf NR, Rooney MS, Bowden M, Zhou CW, Granter SR, Hornick JL, Rodig SJ, Hirakawa M, Severgnini M, Hodi FS, CJ W, Ho VT, Cutler C, Koreth J, Alyea EP, Antin JH, Armand P, Streicher H, Ball ED, Ritz J, Bashey A, Soiffer RJ (2016) Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 375(2):143–153. https://doi.org/10.1056/NEJMoa1601202
Masarova L, Kantarjian H, Garcia-Mannero G, Ravandi F, Sharma P, Daver N (2017) Harnessing the immune system against leukemia: monoclonal antibodies and checkpoint strategies for AML. Adv Exp Med Biol 995:73–95. https://doi.org/10.1007/978-3-319-53156-4_4
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Kronig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, Blank CU (2014) Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol 92(3):195–203. https://doi.org/10.1111/ejh.12228
Sehgal A, Whiteside TL, Boyiadzis M (2015) Programmed death-1 checkpoint blockade in acute myeloid leukemia. Expert Opin Biol Ther 15(8):1191–1203. https://doi.org/10.1517/14712598.2015.1051028
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14(10):3044–3051. https://doi.org/10.1158/1078-0432.ccr-07-4079
Berrien-Elliott MM, Jackson SR, Meyer JM, Rouskey CJ, Nguyen TL, Yagita H, Greenberg PD, DiPaolo RJ, Teague RM (2013) Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer Res 73(2):605–616. https://doi.org/10.1158/0008-5472.can-12-2179
Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR (2011) Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 117(17):4501–4510. https://doi.org/10.1182/blood-2010-10-310425
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr, Laird PW, Baty JD, Fulton LL, Fulton R, Heath SE, Kalicki-Veizer J, Kandoth C, Klco JM, Koboldt DC, Kanchi KL, Kulkarni S, Lamprecht TL, Larson DE, Lin L, Lu C, McLellan MD, McMichael JF, Payton J, Schmidt H, Spencer DH, Tomasson MH, Wallis JW, Wartman LD, Watson MA, Welch J, Wendl MC, Ally A, Balasundaram M, Birol I, Butterfield Y, Chiu R, Chu A, Chuah E, Chun HJ, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Jones S, Karsan A, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Mungall K, Parker J, Pleasance E, Plettner P, Schein J, Stoll D, Swanson L, Tam A, Thiessen N, Varhol R, Wye N, Zhao Y, Gabriel S, Getz G, Sougnez C, Zou L, Leiserson MD, Vandin F, HT W, Applebaum F, Baylin SB, Akbani R, Broom BM, Chen K, Motter TC, Nguyen K, Weinstein JN, Zhang N, Ferguson ML, Adams C, Black A, Bowen J, Gastier-Foster J, Grossman T, Lichtenberg T, Wise L, Davidsen T, Demchok JA, Shaw KR, Sheth M, Sofia HJ, Yang L, Downing JR, Eley G (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368(22):2059–2074. https://doi.org/10.1056/NEJMoa1301689
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Rotiroti MC, Arcangeli S, Casucci M, Perriello V, Bondanza A, Biondi A, Tettamanti S, Biagi E (2016) Acute myeloid leukemia (AML) targeting by CAR T cells: bridging the gap from preclinical modeling to human studies. Hum Gene Ther. https://doi.org/10.1089/hum.2016.092
Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY (2016) T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res 4(6):498–508. https://doi.org/10.1158/2326-6066.cir-15-0231
Arndt C, Feldmann A, Koristka S, Cartellieri M, Bonin Mv, Ehninger A, Bornhäuser M, Ehninger G, Bachmann MP (2015) Improved killing of AML blasts by dual-targeting of CD123 and CD33 via Unitarg a novel antibody-based modular T cell retargeting system [abstract]. Blood 126(23):2565
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ (2017) PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129(8):1039–1041. https://doi.org/10.1182/blood-2016-09-738245
Acknowledgements
This work was supported by the Municipal Science and Technology Bureau of Nanjing under Grant (YKK13116).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yang, D., Zhang, X., Zhang, X. et al. The progress and current status of immunotherapy in acute myeloid leukemia. Ann Hematol 96, 1965–1982 (2017). https://doi.org/10.1007/s00277-017-3148-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3148-x