Abstract
Recent studies have reported the associations between programmed death-ligand 1 (PD-L1) or PD-L2/PD-1 pathways and pro-angiogenic genes including hypoxia-inducible factors (HIFs) and vascular endothelial growth factor (VEGF) in several malignancies. However, no study has examined the relationship or prognostic implication of PD-L1, PD-L2, PD-1, VEGF expression, and microvessel density (MVD) in classical Hodgkin lymphoma (cHL) patients. Diagnostic tissues from 109 patients with doxorubicin, bleomycin, vinblastine, and dacarbazine-treated cHL were evaluated retrospectively by immunohistochemical analysis for PD-L1, PD-L2, PD-1, VEGF expression, and for CD31 expression as a measure of MVD. There was a positive correlation between PD-L1 and VEGF expression (P = 0.008) and additionally between PD-L2 and VEGF expression (P = 0.001). The mean MVD in tumors positive for both PD-L1 and VEGF was significantly (P = 0.022) higher than the mean MVD in tumors negative for both markers. High PD-1 expression group had lower (P = 0.019) 5-year overall survival rate than low PD-1 expression group. Multivariate analysis revealed that PD-1 was an independent prognostic factor for cHL with significance (P = 0.026). However, PD-L1, PD-L2, and VEGF expression had no prognostic impact. Our data confirmed the positive correlations between PD-L1, VEGF, or MVD. Our findings provided evidence supporting new therapeutic approaches including combinations of anti-PD-L1/PD-1 and anti-VEGF therapy in addition to the current standard regimen for cHL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classical Hodgkin lymphoma (cHL) has excellent prognosis. Despite significant advances in modern treatment, a significant minority of patients still have refractory disease [1]. Biological predictive markers for patients who need intensive therapy can help oncologists tailor treatment for refractory cHL.
Programmed death 1 (PD-1) and its ligand PD-L1/PD-L2 have emerged as important factors in tumor evasion from anti-tumor immune response [2]. By overexpressing PD-L1 or PD-L2 ligands on cancer cell surface and binding PD-1 receptor—T, B, and NK cells, tumors can inhibit the activation of T cells, thus suppressing T cell attack and inducing tumor immune escape [2]. Furthermore, anti-PD-1 monoclonal antibodies (nivolumab and pembrolizumab) have shown substantial therapeutic activities with acceptable safety profiles in relapsed or refractory cHL [3, 4]. PD-1/PD-L1 and PD-L2 have been described as prognostic biomarkers in breast cancer [5], lung cancer [6], and colorectal cancer [7] including cHL [8,9,10].
Cancer expression of PD-L1 has been associated with deregulated oncogene signaling [11, 12], tumor hypoxia [13], and local inflammatory signals including Interferon gamma (IFN-γ) [14]. A recent study has revealed that hypoxia can induce T cell apoptosis by upregulating PD-L1 expression in human prostatic carcinoma cells [13]. PD-L1 expression is significantly correlated with angiogenic factors such as vascular endothelial growth factor (VEGF) in clear cell renal carcinoma [15, 16].
VEGF plays a critical role in physiologic and pathologic angiogeneses, including neoangiogenesis in malignant tumors [17, 18]. The prognostic value of VEGF has also been shown in several solid malignancies [19, 20]. Furthermore, VEGF-A produced in the tumor microenvironment induced PD-1 expression on CD8+ T cells in mouse models of colon cancer [21]. CD31 is a reliable marker for endothelial cells. Evaluation of CD31-stained vessels in tumors is a standard method to measure intra-tumoral microvessel density (MVD) [22, 23], a useful prognostic indicator in various malignant tumors [24, 25]. MVD might play an important role in the immunosuppressive microenvironment in cHL [26].
While associations between PD-L1 or PD-L2/PD-1 pathways and HIF/VEGF pathway have been observed in several malignancies [15, 16, 27], no study has examined the relationship or prognostic implication of PD-L1, PD-L2, PD-1, VEGF expression, and MVD in cHL patients. Therefore, the objective of this study is to retrospectively evaluate PD-L1, PD-L2, PD-1, VEGF expression, and MVD in cHL patients to determine the correlations between these markers and assess their prognostic significance.
Materials and methods
Patients
This retrospective study reviewed histological and immunohistochemical data from 109 consecutive patients diagnosed with cHL at Asan Medical Center, Seoul, South Korea, between 1990 and 2012. All patients were ≥ 15 years of age at diagnosis. They had pathologically confirmed cHL. They had no previous treatment or history of malignancy. They had been treated with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) therapy regimens with or without radiation. Paraffin-embedded tumor tissues and follow-up data were available for all included patients.
The median follow-up time was 4.91 years (range, 0.2–17.3 years). Response criteria were based on standard guidelines. Routine follow-up imaging analyses were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and then annually (or whenever clinically indicated) thereafter. The present research was approved by the Institutional Review Board of Asan Medical Center. The requirement for the informed consents prior to the study was given a waiver by the institutional review board of Asan Medical Center.
Histopathological analysis and immunohistochemistry
All histological and immunophenotypic data of the 109 patients with cHL were reviewed by two pathologists (JH and YWK). According to the World Health Organization (WHO) criteria, these cases were subtyped as follows: nodular sclerosis (NS), lymphocyte-rich (LR), mixed cellularity (MC), lymphocyte-depleted (LD), or not otherwise specified type of cHL.
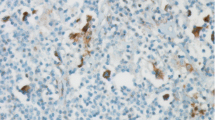
Representative tumor section paraffin blocks were arrayed with a tissue-arraying instrument. Each sample was arrayed in three 1-mm-diameter cores to minimize tissue loss and overcome tumor heterogeneity. Tissue microarray sections were arranged in a Benchmark XT automatic immunohistochemical staining device and detect the protein using OptiView DAB IHC Detection Kit (Ventana Medical Systems, Tucson, AZ, USA). Samples were incubated with antibodies specific for PD-L1 [monoclonal, clone (E1L3N) XP®; Cell Signaling Technology, Danvers, MA, USA], PD-L2 (monoclonal, clone 176611; R&D systems, Minneapolis, MN, USA), PD-1 (monoclonal, clone MRQ-22; Cell Marque, Rocklin, CA, USA), VEGF (monoclonal, G153-694, Pharmingen, NJ, USA), and CD31 (monoclonal, 1A10, Novo, Newcastle, UK). The staining results were scored based on staining intensity: 0 (no staining), 1 (faint staining = light yellow), 2 (moderate staining = yellow-brown), and 3 (strong staining = brown). Cases with a score of 2 or 3 were considered positive. In some fibroblast, endothelial cell, macrophages, and small lymphocyte, there was a weak positivity (score 1) of PD-L1 or PD-L2 antibodies.
To minimize the counting of staining in cells other than Hodgkin/Reed-Sternberg (HRS) cells, we only counted staining in cells that were morphologically compatible with HRS cells, avoiding fibroblast, endothelial cell, macrophages, and small lymphocyte, on the basis of their size, shape, and CD30 staining. We examined protein expression levels of PD-L1, PD-L2, PD-1, and VEGF in 5% steps. The cutoff values of PD-L1, PD-L2, PD-1, and VEGF showing most significant difference in overall survival (OS) were selected. A sample was considered as high PD-L1 or PD-L2 expression if the expression of these markers was detected in ≥ 20% of HRS cells. A sample was considered as high PD-1 expression if PD-1 expression was detected in ≥ 20% of peritumoral microenvironment. A sample was considered as high VEGF expression if 25% or more of the HRS cells showed reactivity to the VEGF antibody.
For microvessel counting, the area with the highest vascularization was selected at low magnification (×100). Counting was performed at high magnification (×400). Three hot spots were selected per case and quantified simultaneously by two pathologists. The final MVD for each case was expressed as the mean number of vessels counted in the three hot spots scored. Microvessel with a clearly defined lumen or with a well-defined linear vessel shape was selected for counting. Branching vessel structures were considered as a single vessel. In situ hybridization (ISH) analysis for EBV-encoded RNA-1 and RNA-2 (EBER) was performed and scored as described elsewhere [28].
Statistical analysis
OS was defined as the interval between the date of diagnosis and the date of death from any cause. Follow-up of living patients (with or without events) was censored at their last follow-up date. Event-free survival (EFS) was defined as the interval between the date of diagnosis and the date of disease progression, relapse, or death from any cause. Cumulative OS and EFS were analyzed by the Kaplan-Meier method. Comparisons were performed with log-rank testing. Multivariate prognostic analyses were performed on OS and EFS with Cox proportional hazard regression model using the enter method. Categorical variables were compared using chi-square test. Continuous variables were compared using independent t test. All statistical analyses were performed using SPSS statistical software program (version 18.0; SPSS, Chicago, IL). All P values are two-sided associations. Statistical significance was considered when P value was less than 0.05.
Results
Patient characteristics
The clinical characteristics of the 109 patients included in this study are summarized in Table 1. Patient age ranged from 15 to 77 years (median, 36 years). Forty-two patients experienced relapse, disease progression, or death (20 patients died). Median OS and EFS were not reached. The estimated 5-year OS and EFS were 82.7 and 58.4%, respectively.
Correlations of PD-L1, PD-L2, PD-1, and VEGF expression with MVD
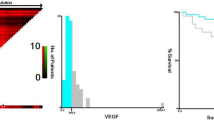
PD-L1, PD-L2, PD-1, and VEGF were expressed in 75.2, 11.9, 12.7, and 33% of the specimens, respectively (Fig. 1). A statistically significant (P = 0.008) positive correlation between PD-L1 expression and VEGF expression was observed (Table 2). Similarly, PD-L2 expression had statistically significant positive correlation with VEGF expression (P < 0.001, Table 2). However, the correlation between PD-1 and VEGF expression (P = 0.534) was not statistically significant. PD-L1 and PD-L2 expression levels were not significantly correlated with PD-1 expression either (P = 0.448 and P = 0.626, respectively).
When MVD was evaluated as a continuous variable, it was positively correlated with VEGF expression (P = 0.004). The mean MVD value of PD-L1-positive tumors was slightly higher than that of PD-L1-negative tumors, although the difference between the two was not statistically significant (15.07 ± 6.61 vs. 13.37 ± 7.26, P = 0.26). MVD was not significantly associated with PD-L2 (P = 0.587) or PD-1 (P = 0.613). However, PD-L1 and PD-L2 were significantly associated with VEGF. We performed correlation analyses among PD-L1, PD-L2, VEGF, and MVD. The mean MVD in tumors positive for both PD-L1 and VEGF was significantly higher than the mean MVD in tumors negative for both markers (17.85 ± 7.42 vs. 13.33 ± 6.89, P = 0.022, Fig. 2a). However, patients with tumors positive or negative for both PD-L2 and VEGF markers showed similar MVD (P = 0.301, Fig. 2b).
Neither PD-L1-positive expression nor PD-L1-negative expression was associated with clinical variables. The PD-L2-positive group had more patients who were younger (100 vs. 53%, P = 0.021). PD-1 positivity was not associated with clinical variables wither. The VEGF-positive group had more patients who had NS histologic type (83 vs. 57%, P = 0.031) and EBER positivity (53 vs. 31%, P = 0.038).
Prognostic significance of PD-L1, PD-L2, PD-1, and VEGF expression
PD-L1 expression was not significantly associated with EFS or OS (P = 0.477 and P = 0.215, respectively). PD-L2 expression was not significantly correlated with EFS or OS (P = 0.676 and P = 0.309, respectively). High PD-1 expression group had lower 5-year OS rate (59.3 vs. 85.5%, P = 0.019; Fig. 3a) than PD-1-negative group. However, EFS rate was not significantly (P = 0.443) associated with PD-1 positivity (Fig. 3b). VEGF-positive and VEGF-negative patients also showed similar EFS and OS rates (P = 0.636 and P = 0.427, respectively).
By univariate analysis, IPS (≥ 3) and high PD-1 expression were significant factors associated with OS (P = 0.021 and P = 0.026, respectively, Table 3). By multivariate analysis, high PD-1 expression and high-risk IPS (≥ 3) remained as independent prognostic markers for OS (PD-1: hazard ratio = 3.000, P = 0.026; and IPS (≥ 3): hazard ratio = 3915, P = 0.006, Table 3).
Discussion
This is the first study that reports the correlation of PD-L1, PD-L2, and PD-1 expression with VEGF expression, and MVD in patient-matched histological sections of cHL. There was a positive correlation between VEGF expression and PD-L1 or PD-L2 expression. The mean MVD value of PD-L1-positive tumors was slightly higher than that of PD-L1-negative tumors, although the difference in MVD value between the two was not statistically significant. The MVD was significantly higher in tumors positive for both PD-L1 and VEGF than the MVD in those negative for both markers. However, PD-L1 was not associated with clinical outcomes.
The HIF pathway is currently considered as a master regulator of angiogenesis [29]. HIF-1α and HIF-2α regulate the expression of pro-angiogenic genes including VEGF, GLUT1, Ang-1, Ang-2, and Tie-2 [29,30,31]. HIF-1α regulates VEGF expression and its receptor VEGF-R2, thereby promoting autocrine VEGF signaling loop that can stimulate the proliferation and migration of endothelial cell and tube formation [32]. HIF-1α also significantly increases the expression of PD-L1 in macrophages, dendritic cells, and tumor cells [33]. HIF-2α overexpression increases PD-L1 mRNA and protein levels in clear cell renal carcinoma [30]. In clear cell renal carcinoma, PD-L1 expression is associated with VEGF expression [15, 16]. In our analysis, PD-L1-positive cHL was significantly correlated with HIF signaling pathway including VEGF and MVD. This interesting finding was similar to that of previous studies [15, 16, 30, 33].
Previous study revealed that VEGF-A produced in the tumor microenvironment enhanced PD-1 expression on CD8+ T cells in mouse models of colon cancer [21]. In our study, VEGF expression on HRS cells was not correlated with PD-1 expression. Although we additionally evaluated VEGF-A expression in the tumor microenvironment of our cases, there was no correlation between VEGF-A expression in the tumor microenvironment and PD-1 expression. The role of VEGF expression in the tumor microenvironment will have to be clarified in further studies.
To interpret PD-L1, PD-L2, PD-1, and VEGF immunostaining, we used a measure of percent positivity and staining intensity. The cutoff values of PD-L1, PD-L2, PD-1, and VEGF showing most significant difference in OS were selected. PD-1 expression was correlated with OS alone; however, other parameters were not associated with OS. In the pioneering study by Muenst et al., patients were classified into two subgroups based on the prognostic discrimination power of PD-1-positive cells per square millimeter. They also used the OS to set cutoff [8]. Paydas et al. also used a measure of percent positivity and staining intensity. But they do not have a description for cutoff of immunostaining value [9]. Recent study by Roemer performed double staining of PD-L1 and PAX5 and they also measured percent positivity and staining intensity [34]. A modified H-score was generated by multiplying the percentage of malignant cells with positive staining and average intensity of HRS cells. They did not produce cutoffs of immunostaining value because they did not perform a survival analysis using H-score. It is difficult to set cutoff according to immunohistochemistry because of interobserver or intraobserver variability and technical problem. Therefore, to use PD-L1, PD-L2, PD-1, and VEGF as a biomarker, a multicenter study is required to test a specific antibody with a standardized technique.
In our study, only 11.9 and 12.7% of samples were described as PD-L2 and PD-1 positive, respectively, while PD-L1 positivity was documented in 75.2% of the samples.
However, previous studies reported that positive rates of PD-L1 and PD-L2 are similar [3, 34]. Although there is a difference in scoring method and cutoff between studies, the difference in expression rate between PD-L1 and PD-L2 in our study is significant. We used different antibodies from the previous two studies in PD-L1 and PD-L2 immunostaining. We used commercially available antibodies clone (E1L3N) XP® and clone 176611 for PD-L1 and PD-L2 immunostaining, respectively. These antibodies have been widely studied in solid tumors [15, 35,36,37]. Since the solid tumor and hematologic malignancy are different entities, sensitivity of the antibody to the antigen may be different in the two diseases. It may be that the PD-L2 antibody used itself has a lower sensitivity than PD-L1. The positive rate of PD-1 expression was similar to previous experiments using the same antibody [9].
In the present study, PD-1 was associated with OS rates. However, PD-L1 or PD-L2 was not associated with OS rates. Muenst et al. [8] have found that PD-1 has a negative prognostic impact on cHL [8]. Paydas et al. [9] have reported that patients with co-expression of PD-1 and PD-L1 have inferior OS rates, whereas expression of PD-1 or PD-L1 is not associated with OS. In our series, cases with co-expression of PD-1 and PD-L1 were not associated with clinical outcomes. Recent analysis has revealed highly significant associations between amplification of PD-L1/PD-L2 and advanced stage disease at presentation and inferior progression-free survival in cHL treated with the Stanford V regimen [34]. Although Roemer et al. [34] reported a correlation between PD-L1 protein expression and amplification of PD-L1, they did not perform prognostic analysis for PD-L1 protein expression. The reason behind the discrepancy between the results of this study and the result of Roemer et al. [34] remains unclear. Further studies are needed to determine the precise prognostic impact of PD-L1, PD-L2, and PD-1 expression on cHL patients.
Bevacizumab, an anti-VEGF monoclonal antibody, has been extensively used in solid malignancies [38,39,40]. Therapeutic effects of bevacizumab have been investigated against xenografts of human HL and in five patients with refractory/relapsed HL. A significant delay in the growth of HL tumors has been observed in the xenografts model, while the combination of bevacizumab and gemcitabine has resulted in partial or complete remission in three of five patients [41]. Since bevacizumab as monotherapy seems to have minimal responses on relapsed/refractory cHL patients, combination therapy with nivolumab or pembrolizumab may yield greater antitumor activity than monotherapy. Combination of nivolumab and bevacizumab has shown an acceptable toxicity profile and achieved partial response in patients with advanced non-small cell lung cancer [42]. Combination of pembrolizumab and anti-VEGF tyrosine kinase inhibitor axitinib has also shown partial responses or disease stabilization in metastatic renal cell carcinoma [43].
EBV is categorized as group 1 human carcinogen by the International Agency for Research on Cancer in cHL based on its association with lymphoid and epithelial malignancies [44]. It has been shown that EBV latent membrane protein-1 (LMP-1) can increase VEGF production and some other angiogenesis factors such as HIF-1α and interleukin-8 (IL-8) [45, 46]. Wakisaka et al. [4] have reported that transfection of an LMP1 expression plasmid into an EBV-negative nasopharyngeal epithelial cell line can induce the synthesis of HIF-1 alpha and VEGF protein. LMP-1 induced expression of IL-8, which may contribute in part to angiogenesis in nasopharyngeal carcinoma [46]. Our study also found a positive correlation between EBER and VEGF expression.
Limitations of this study include the retrospective design and relatively small sample size. The tissue microarray design could not reflect whole tumor because of heterogenous distribution of immunohistochemical stainings.
In summary, our study revealed that PD-L1 expression is associated with the expression of pro-angiogenic genes, including VEGF. Furthermore, patients with co-expression of PD-L1 and VEGF showed significantly higher MVD than both negative patients. PD-L1 and VEGF expression had no prognostic impact on cHL. PD-1 expression alone was associated with OS rates. Our findings provide evidence supporting new therapeutic approaches, including combinations of anti-PD-L1/PD-1 or anti-VEGF therapy in addition to the current ABVD regimen.
References
Quddus F, Armitage JO (2009) Salvage therapy for Hodgkin’s lymphoma. Cancer J 15(2):161–163. https://doi.org/10.1097/PPO.0b013e3181a1438a
Weber J (2010) Immune checkpoint proteins: a new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37(5):430–439. https://doi.org/10.1053/j.seminoncol.2010.09.005
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372(4):311–319. https://doi.org/10.1056/NEJMoa1411087
Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose S, Moskowitz CH (2016) Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. https://doi.org/10.1200/jco.2016.67.3467
Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD (2014) Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 146(1):15–24. https://doi.org/10.1007/s10549-014-2988-5
Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, Li H, Luo X, Ye T, Sun Y, Chen H (2014) Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. OncoTargets Ther 7:567–573. https://doi.org/10.2147/ott.s59959
Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer (Oxford, England: 1990) 49(9):2233–2242. https://doi.org/10.1016/j.ejca.2013.02.015
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40(12):1715–1722. https://doi.org/10.1016/j.humpath.2009.03.025
Paydas S, Bagir E, Seydaoglu G, Ercolak V, Ergin M (2015) Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. https://doi.org/10.1007/s00277-015-2403-2
Koh YW, Jeon YK, Yoon DH, Suh C, Huh J (2016) Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol 37(6):7507–7514. https://doi.org/10.1007/s13277-015-4622-5
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13(1):84–88. https://doi.org/10.1038/nm1517
Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, Ohmori K, Uchiyama T (2009) B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci 100(11):2093–2100. https://doi.org/10.1111/j.1349-7006.2009.01302.x
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74(3):665–674. https://doi.org/10.1158/0008-5472.can-13-0992
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra137. https://doi.org/10.1126/scitranslmed.3003689
Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H (2016) Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol 23(2):694–702. https://doi.org/10.1245/s10434-015-4903-7
Kammerer-Jacquet SF, Crouzet L, Brunot A, Dagher J, Pladys A, Edeline J, Laguerre B, Peyronnet B, Mathieu R, Verhoest G, Patard JJ, Lespagnol A, Mosser J, Denis M, Messai Y, Gad-Lapiteau S, Chouaib S, Belaud-Rotureau MA, Bensalah K, Rioux-Leclercq N (2016) Independent association of PD-L1 expression with noninactivated VHL clear cell renal cell carcinoma—a finding with therapeutic potential. Int J Cancer. https://doi.org/10.1002/ijc.30429
Folkman J, Merler E, Abernathy C, Williams G (1971) Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133(2):275–288
Ferrara N (2000) VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 11(6):617–624
Komatsu H, Oishi T, Itamochi H, Shimada M, Sato S, Chikumi J, Sato S, Nonaka M, Sawada M, Wakahara M, Umekita Y, Harada T (2017) Serum vascular endothelial growth factor-A as a prognostic biomarker for epithelial ovarian cancer. Int J Gynecol Cancer. https://doi.org/10.1097/igc.0000000000001027
Park DJ, Seo AN, Yoon C, Ku GY, Coit DG, Strong VE, Suh YS, Lee HS, Yang HK, Kim HH, Yoon SS (2015) Serum VEGF-A and tumor vessel VEGFR-2 levels predict survival in Caucasian but not Asian patients undergoing resection for gastric adenocarcinoma. Ann Surg Oncol 22(Suppl 3):S1508–S1515. https://doi.org/10.1245/s10434-015-4790-y
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M (2015) VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212(2):139–148. https://doi.org/10.1084/jem.20140559
Koh YW, Park CS, Yoon DH, Suh C, Huh J (2014) CD163 expression was associated with angiogenesis and shortened survival in patients with uniformly treated classical Hodgkin lymphoma. PLoS One 9(1):e87066. https://doi.org/10.1371/journal.pone.0087066
Koh YW, Park C, Yoon DH, Suh C, Huh J (2013) Prognostic significance of COX-2 expression and correlation with Bcl-2 and VEGF expression, microvessel density, and clinical variables in classical Hodgkin lymphoma. Am J Surg Pathol 37(8):1242–1251. https://doi.org/10.1097/PAS.0b013e31828b6ad3
Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H (2001) Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res 7(11):3410–3415
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J (1993) Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 143(2):401–409
Korkolopoulou P, Thymara I, Kavantzas N, Vassilakopoulos TP, Angelopoulou MK, Kokoris SI, Dimitriadou EM, Siakantaris MP, Anargyrou K, Panayiotidis P, Tsenga A, Androulaki A, Doussis-Anagnostopoulou IA, Patsouris E, Pangalis GA (2005) Angiogenesis in Hodgkin’s lymphoma: a morphometric approach in 286 patients with prognostic implications. Leukemia 19(6):894–900. https://doi.org/10.1038/sj.leu.2403690
Ruf M, Moch H, Schraml P (2016) PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer 139(2):396–403. https://doi.org/10.1002/ijc.30077
Huh J, Cho K, Heo DS, Kim JE, Kim CW (1999) Detection of Epstein-Barr virus in Korean peripheral T-cell lymphoma. Am J Hematol 60(3):205–214
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis: good and evil. Genes & cancer 2(12):1117–1133. https://doi.org/10.1177/1947601911423654
Messai Y, Gad S, Noman MZ, Le Teuff G, Couve S, Janji B, Kammerer SF, Rioux-Leclerc N, Hasmim M, Ferlicot S, Baud V, Mejean A, Mole DR, Richard S, Eggermont AM, Albiges L, Mami-Chouaib F, Escudier B, Chouaib S (2015) Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von Hippel-Lindau gene mutation status. Eur Urol. https://doi.org/10.1016/j.eururo.2015.11.029
Li QQ, Sun YP, Ruan CP, Xu XY, Ge JH, He J, Xu ZD, Wang Q, Gao WC (2011) Cellular prion protein promotes glucose uptake through the Fyn-HIF-2alpha-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci 102(2):400–406. https://doi.org/10.1111/j.1349-7006.2010.01811.x
Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS (2004) Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6(5):485–495. https://doi.org/10.1016/j.ccr.2004.09.026
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211(5):781–790. https://doi.org/10.1084/jem.20131916
Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, Armand P, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Rodig SJ, Shipp MA (2016) PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34(23):2690–2697. https://doi.org/10.1200/jco.2016.66.4482
Kim S, Koh J, Kwon D, Keam B, Go H, Kim YA, Jeon YK, Chung DH (2017) Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer (Oxford, England: 1990) 75:141–149. https://doi.org/10.1016/j.ejca.2017.01.004
Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim JH, Jeon YK, Lee JS, Kwon SK, Hah JH, Kwon TK, Kim DW, Wu HG, Sung MW, Heo DS (2016) PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget 7(13):15901–15914. 10.18632/oncotarget.7431
Kim S, Kim MY, Koh J, Go H, Lee DS, Jeon YK, Chung DH (2015) Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer (Oxford, England: 1990) 51(17):2698–2707. https://doi.org/10.1016/j.ejca.2015.08.013
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349(5):427–434
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Reiners KS, Gossmann A, von Strandmann EP, Boll B, Engert A, Borchmann P (2009) Effects of the anti-VEGF monoclonal antibody bevacizumab in a preclinical model and in patients with refractory and multiple relapsed Hodgkin lymphoma. J Immunother (Hagerstown, Md: 1997) 32(5):508–512. https://doi.org/10.1097/CJI.0b013e3181a25daf
Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Hozumi H, Tamura T (2016) Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol. https://doi.org/10.1093/annonc/mdw416
Di Lorenzo G, De Placido S, Pagliuca M, Ferro M, Lucarelli G, Rossetti S, Bosso D, Puglia L, Pignataro P, Ascione I, De Cobelli O, Caraglia M, Aieta M, Terracciano D, Facchini G, Buonerba C, Sonpavde G (2016) The evolving role of monoclonal antibodies in the treatment of patients with advanced renal cell carcinoma: a systematic review. Expert Opin Biol Ther 16(11):1387–1401. https://doi.org/10.1080/14712598.2016.1216964
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens—part B: biological agents. Lancet Oncol 10(4):321–322
Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS (2004) Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol 24(12):5223–5234. https://doi.org/10.1128/mcb.24.12.5223-5234.2004
Yoshizaki T, Horikawa T, Qing-Chun R, Wakisaka N, Takeshita H, Sheen TS, Lee SY, Sato H, Furukawa M (2001) Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin Cancer Res 7(7):1946–1951
Acknowledgments
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present research was approved by the Institutional Review Board of Asan Medical Center. The requirement for the informed consents prior to the study was given a waiver by the institutional review board of Asan Medical Center.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Koh, Y.W., Han, JH., Yoon, D.H. et al. PD-L1 expression correlates with VEGF and microvessel density in patients with uniformly treated classical Hodgkin lymphoma. Ann Hematol 96, 1883–1890 (2017). https://doi.org/10.1007/s00277-017-3115-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3115-6