Abstract
The primary objective of this study was to investigate whether the presence of comorbidities was associated with a lower health-related quality of life (HRQOL) in elderly patients with chronic myeloid leukemia (CML). A sample of 174 CML patients aged 60 years or above was analyzed. HRQOL was assessed with the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). A number of pre-selected sociodemographic and disease-related factors were considered as potential confounding factors for the association between comorbidity and HRQOL. Mean age of the 174 patients analyzed was 70 years (range 60–87 years) and 55 % were male. Overall, 111 patients (64 %) reported at least one comorbidity. Analysis stratified by age group category showed a greater proportion of patients with comorbidities in the older sub-group population (≥70 years) compared to younger patients (60 to 69 years). Differences in HRQOL outcomes between patients with no comorbidity at all and those with two or more comorbid conditions were at least twice the magnitude of a clinically meaningful difference in all the physical and mental health scales of the SF-36. In multivariate analysis, after adjusting for key confounding factors, the following scales were significantly lower in those with comorbidity: general health (p < 0.001), bodily pain (p < 0.001), physical functioning (p = 0.002), and vitality (p = 0.002). Assessing comorbidity in elderly patients with CML is important to facilitate identification of those most in need of HRQOL improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of tyrosine kinase inhibitors (TKIs) in the treatment for chronic myeloid leukemia (CML) has greatly changed the prognosis of patients. With the advent of first TKI, namely imatinib, also elderly patients, usually candidate to palliative approach, have been treated and cured [1]. A 5-year relative survival of elderly CML patients (≥65 years) was shown to be comparable to that of younger patients [2]. As recent data from large population-based registries report a median age at diagnosis of 56 years [3], it is reasonable to consider that many of the CML patients currently under treatment have even a greater median age. Nonetheless, elderly patients and those with comorbidity have not been adequately represented in several of the pivotal clinical efficacy trials conducted so far [4–6].
While in general, elderly people are more likely to report comorbid medical conditions, recent data also shows that patients with a cancer diagnosis report significantly more comorbid conditions than do their peers without a cancer diagnosis [7]. According to Feinstein’s definition [8], comorbidity is any distinct additional clinical entity pre-existent or occurring during the course of a primary disease.
The possibility to prescribe other two TKIs (i.e., nilotinib and dasatinib) as first-line treatment, in addition to imatinib, makes the selection of the most appropriate treatment for individual patients a challenge. Therefore, considering individual characteristics, including comorbidity, is critical [6, 9].
In the coming decades, the prevalence of elderly patients living with CML will substantially increase given the dramatic improvements in treatment outcomes for this disease [10, 11]. Understanding the relationships between comorbidity, treatment outcomes, and patient’s health-related quality of life (HRQOL) is thus essential to robustly inform clinical decision-making.
There is convincing evidence showing that elderly patients treated with imatinib are able to achieve similar rates of cytogenetic and molecular responses compared to younger patients, although with increased incidence of temporary discontinuation or dose reduction due to toxicity [12, 13]. While some data exists on the relationships between comorbidity and clinical outcomes in CML, to our knowledge, the impact of comorbidity on patient-reported HRQOL in elderly CML patients has not been investigated. Considering the lifelong nature of current TKI therapies, even low-grade side effects can significantly impair patients’ daily functioning and well-being [14]. Therefore, improving HRQOL has become an important goal of modern CML therapies [15]. We have previously shown that CML patients aged 60 years or older, who respond to imatinib therapy (i.e., at least in complete cytogenetic response), can expect to have a HRQOL profile similar to that of their peers in the general population [16].
The primary objective of this analysis was to investigate whether the presence and number of comorbidities is associated with lower physical and mental HRQOL aspects in this elderly (i.e., aged 60 or older) CML population. Secondary objectives were to assess prevalence of comorbidities and to evaluate whether these were associated with greater symptom severity.
Patients and methods
Study population
Patients were previously enrolled in a large multicentre CML survivorship (n = 422) study investigating long-term effects of first-line imatinib therapy. Patients’ HRQOL results, inclusion criteria, and study logistic have been previously reported [16]. Briefly, patients had to be on treatment for at least 3 years and at least in complete cytogenetic response (CCyR) at the time of study entry. Patients could be enrolled regardless of number and type of comorbidity. Patients with a secondary malignancy and having psychiatric conditions or major cognitive dysfunctions hampering a self-reported evaluation, at study entry, were excluded. The current analysis is based on patients aged 60 or older (n = 174) enrolled in this study [16].
Ethic committees of participating centers approved the study and all patients provided written informed consent.
Health-related quality of life and symptom severity
The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) was used to assess HRQOL. It consists of 36 items yielding eight scales: physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems and mental health. Each scale provides a score ranging between 0 and 100 with higher scores indicating better outcomes [17].
Patient-reported symptoms were evaluated with a previously developed checklist for assessing the severity a core set of symptoms for CML patients [18]. The following symptoms were evaluated: abdominal discomfort, diarrhea, edema, fatigue, headache, muscular cramps, musculoskeletal pain, nausea, and skin problems. Each symptom was scored as: not at all, a little, quite a bit, and very much. To ease outcome interpretation in this report, and similarly to other symptom ratings included in well-validated questionnaires [19], raw scores were linearly transformed into a standardized scale ranging from 0 to 100 with higher scores indicating greater symptom severity.
Assessment of comorbidity
Patients themselves reported comorbidity at the time of HRQOL assessment by completing an adapted version of the Charlson Comorbidity Index [20]. The questionnaire addressed the following comorbidities: osteoarthritis/rheumatism, vascular pathology, diabetes, pulmonary problems, obesity, liver problems, renal pathology, myocardial infarction, ulcer, and other conditions. Also, a free text field was added to allow patients entering any other comorbidity.
Disease and sociodemographic variables analyzed
Key variables potentially contributing to explaining patient’s HRQOL were considered and these were selected based on clinical relevance for the purpose of this analysis. These were grouped into (1) disease-related and (2) sociodemographic factors. Disease-related factors included time since diagnosis, Sokal risk at diagnosis, and achievement of a complete molecular response. Sociodemographic factors included age, sex, education, and social support. This latter was included being a potential determinant of HRQOL in cancer survivors and being associated with survival in elderly cancer patients [21, 22]. This was assessed with the well-validated multidimensional scale of perceived social support (MSPSS) [23] that consists of 12 items that evaluate perceptions of social support from three main sources: friends, family members, and significant others. It also yields a total score (i.e., global social support) that was used for the purpose of this analysis.
Statistical analysis
Analyses were performed on 174 CML patients aged at least 60 years. As there is paucity of research regarding HRQOL and comorbidity in elderly CML patients, all SF-36 scales were investigated in this study as primary scales. Patients were classified in three groups by number of comorbidities and categorized as no comorbidity at all, one comorbidity, and two comorbidities or more. Differences were investigated among such groups in self-reported symptoms and SF-36 scales by Kruskal-Wallis test (α = 0.05). Also, multivariate analysis of covariance (ANCOVA) was used to assess differences in SF-36 scales by number of comorbidities, adjusting for other key variables selected on clinical grounds. Based on multivariate ANCOVA, the proportion of variance of SF-36 scales explained by presence of comorbidities was investigated using the total eta-square index. This index provides the specific contribution of each variable included in the model in explaining the outcome variance. For descriptive purposes, proportions of explained variance of variables other than presence of comorbidities were grouped into “disease-related” and “sociodemographic” variables. Clinical significance of differences in HRQOL outcomes was also evaluated in this study, defined as at least eight points on each SF-36 scale [24]. Statistical significance for SF-36 scales was Bonferroni corrected to account for multiple testing. All analyses were performed with SAS v.4 (SAS Institute, Inc., Cary, NC, USA) and SPSS v. 20.
Results
Mean age of the 174 patients analyzed was 70 years (range 60–87 years) and 55 % were male. Mean time between date of diagnosis and study participation was 5.5 years (SD 1.9). Seventy percent of patients were receiving a standard dose of imatinib of 400 mg/day at the time of survey. Sokal risk classification at diagnosis was low in 37 % of the patients, intermediate in 51 %, and high in 12 %. Overall, 111 patients (64 %) reported at least one comorbidity. Of the 202 comorbidities reported by these patients, these were classified as follows: osteoarthritis/rheumatism (30 % patients), vascular pathology (17 %), diabetes (10 %) and pulmonary problems (9 %), obesity (6 %), liver problems (6 %), renal pathology (5 %), myocardial infarction (4 %), ulcer (3 %), and other comorbid conditions (24 %). Sixty-three patients (36 %) did not report any comorbidity, 55 (32 %) reported only one comorbidity, and 56 (32 %) reported two or more comorbidities.
Analysis stratified by age group category showed a greater proportion of patients with comorbidities in the older sub-group population (≥70 years) compared to younger patients (60 to 69 years). For example, within the group of patients with no comorbidity at all, 62 % were aged between 60 to 69 years while only 38 % were aged 70 years or more. Characteristics of patients stratified by number of comorbidities are reported in Table 1.
Univariate analysis of comorbidity and health-related quality of life and symptom burden
Patients with no comorbidity (Com0) had overall statistically significant better outcomes (i.e., higher scores) across all the physical and mental health scales of the SF-36 compared to those with either one comorbidity (Com1) or two or more comorbid conditions (Com≥2) (Fig. 1).
a, b Health-related quality of life profile of elderly CML patients by number of comorbidities. Mean outcomes for the eight scales of the SF-36 questionnaire by number of patients’ self-reported current comorbidities are shown. For each scale, higher scores denote better outcomes and lower scores worse outcomes. p value refers to the overall comparison of mean scores among patients with no, one, or at least two comorbidities, under the null hypothesis of no difference. Δ represents the positive score difference between patients with no comorbidities at all and those with either one or at least two comorbidities. Values reported in the figure were rounded. A single asterisk denotes that the score difference exceeds the minimally important difference (i.e., 8 points). Double asterisks indicate that the score difference equals to or exceeds twice the minimally important difference. Triple asterisks entail that the score difference equals to or exceeds three times the minimally important difference
Differences between groups Com0 and Com≥2 were at least twice the magnitude of a clinically meaningful difference for all the eight scales (physical and mental health) of the SF-36 questionnaire. The differences in bodily pain (Δ = 25.3 points) and role physical (Δ = 24.1 points) were at least three times the magnitude of a clinically meaningful difference. For physical functioning (Δ = 23), general health (Δ = 22.6), and role-emotional (Δ = 22.4), the differences between Com0 and Com≥2 were just below three times the magnitude of a clinically meaningful difference.
Exploratory analysis comparing HRQOL profile of our elderly population stratified by number of comorbidities with that of the general population (adjusted by age and gender) revealed that CML patients with no comorbidity had broadly even better outcomes than their peers in some domains (data not shown).
Symptom severity by number of comorbidities is depicted in Fig. 2. Statistically significant differences were found in symptom mean scores by comorbidity for muscular cramps (p = 0.045), musculoskeletal pain (p = 0.003), edema (p = 0.008), and fatigue (p < 0.001).
Self-reported symptom severity of elderly CML patients by number of comorbidities. For each symptom, higher scores denote higher symptom severity while lower scores indicate lower symptom severity. Values reported in the figure were rounded. p value refers to the overall comparison of mean scores among patients with no, one, or at least two comorbidities, under the null hypothesis of no difference
Multivariate analysis of the association of comorbidity and health-related quality of life
In a multivariate ANCOVA, we adjusted the comparison of the three comorbidity groups with regard to the SF-36 domains for important sociodemographic and disease-related patient characteristics. In this analysis, four out of eight SF-36 scales (physical functioning, bodily pain, general health, and vitality) remained significantly different among comorbidity groups even after Bonferroni correction for multiple testing. Details for this analysis are given in Tables 2 and 3.
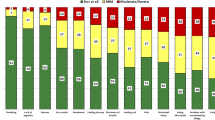
To investigate the relative importance of comorbidity in comparison with disease-related and sociodemographic factors, we calculated proportions of explained variance in terms of total eta-square index (Fig. 3). For each scale, we report proportions of variance which are uniquely explained by either presence of comorbidities, disease-related, or sociodemographic variables. We found that for general health (15 % explained variance) and bodily pain (12 % explained variance), the presence of comorbidities explained substantially more variance than disease-related or sociodemographic variables.
Explained variance (%) of health-related quality of life of elderly CML patients. Mean outcomes for the eight scales of the SF-36 questionnaire are shown. For each scale, the figure shows the percentage of variance (total eta-squared) explained in the multivariate ANCOVA model (as reported in Tables 2 and 3), by presence of comorbidities, sociodemographic factors (age, sex, education, and social support), and disease-related variables (Sokal risk, complete molecular response, time since diagnosis). Values reported in the figure were rounded. A single asterisk signifies that the explained variance is less than 1 %
Discussion
We have found that some two thirds of CML patients aged 60 years or above reported at least one comorbidity and that the presence of this was associated with important impairments in both physical and mental health domains. This negative association with HRQOL outcomes was particularly remarkable in patients who reported two or more comorbid conditions.
In a recent large population-based registry study, it was found that 55 % of the 2904 CML patients analyzed had at least one comorbidity. Although this percentage is slightly lower than that found in our study (i.e., 64 %), it should be noted that in this registry, also patients younger than 60 years were represented.
Saussele and colleagues [25] recently showed that comorbidity at diagnosis was an independent prognostic factor for shorter survival in imatinib-treated CML patients regardless of age. They concluded that the presence of comorbidity is more important than the disease itself in determining the survival of patients. Our findings complement this remarkable data [25] by showing that comorbidity also heavily influence HRQOL of elderly patients. Notably, in our analysis of variance, the presence of comorbidities was by far the most important contributor in determining the subjective general health status scale of the SF-36.
Considering the paucity of research on this topic, it is difficult to compare our findings with other similar CML studies. However, our results are broadly consistent with the few studies conducted in patients with other hematologic malignancies (mainly Hodgkin’s and non-Hodgkin’s lymphoma patients) indicating the negative impact of comorbidities on patients’ HRQOL [26–28].
In our previous study [29] analyzing the whole sample, we found that fatigue was independently associated with all HRQOL physical and mental health domains of the SF-36. In this analysis, specifically investigating the role of comorbidity on HRQOL in the elderly sub-population (i.e., with a substantial higher number of comorbid conditions), we found that comorbidity was associated with both HRQOL and fatigue. Taken together, both findings might suggest that, in elderly patients, comorbidity could possibly impair HRQOL by raising patients’ fatigue levels. Whether the presence of comorbidities can determine an increased symptom severity, which can in turn negatively affect more general aspects of HRQOL, should be investigated in future prospective studies. The identification of a causal model on the pathways of how comorbidity could influence the various aspects of HRQOL, would be important to inform the development of future intervention studies in elderly patients.
Our findings have two important implications. From a clinical practice perspective, our results suggest that elderly patients with comorbidities should be more closely monitored due to their poorer HRQOL outcomes. They might represent a specific population who can benefit the most from supportive care programs. Another implication is for better designing and interpreting future HRQOL research studies of elderly CML patients. Our results indicate that comorbidity should be considered as a key variable to adjust for, in order to increase robustness of study results and outcome interpretation. Therefore, routinely collecting and quantifying comorbid conditions possibly using well-validated indices in clinical practice and research is strongly recommended.
Our study has limitations. First, given the cross-sectional design, we cannot draw conclusions on causal relationships between comorbidity and HRQOL or specific symptoms. Also, given the self-reported nature of comorbidity data collection, we cannot rule out the possibility of underreporting of comorbid conditions. For example, as major cognitive dysfunction or psychiatric problems were an exclusion criterion of the original study, this might have contributed to the underreporting of psychological-related comorbidities.
These limitations notwithstanding, our study also has strengths. Our sample was homogenous with regard to therapy received, thus ruling out the potential confounding effect of type and schedule of therapy when analyzing the association between comorbidity and HRQOL. Also, we accounted for the role of social support, which might be particularly important in determining HRQOL of elderly people. Finally, our results have been generated in an observational setting thus most likely to be generalizable to CML patients seen in daily practice.
In conclusion, the presence of comorbidity in elderly CML patients is associated with diminished physical and mental health well-being. Therefore, assessing comorbidity in elderly patients is important to facilitate identification of those most in need of HRQOL improvements. It is likely that assessing comorbidities is also equally important in younger patients and this should be examined in future studies.
References
Breccia M, Tiribelli M, Alimena G (2012) Tyrosine kinase inhibitors for elderly chronic myeloid leukemia patients: a systematic review of efficacy and safety data. Crit Rev Oncol Hematol 84:93–100
Proetel U, Pletsch N, Lauseker M et al (2014) Older patients with chronic myeloid leukemia (≥65 years) profit more from higher imatinib doses than younger patients: a subanalysis of the randomized CML-Study IV. Ann Hematol 93(7):1167–76
Hoffmann VS, Baccarani M, Hasford J et al (2015) The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European countries. Leukemia 29:1336–43
Rohrbacher M, Berger U, Hochhaus A et al (2009) Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia 23:602–4
Harrison SJ, Johnson PR, Holyoake TL (2004) The Scotland Leukaemia Registry audit of incidence, diagnosis and clinical management of new patients with chronic myeloid leukaemia in 1999 and 2000. Scott Med J 49:87–90
Gugliotta G, Castagnetti F, Fogli M et al (2013) Impact of comorbidities on the treatment of chronic myeloid leukemia with tyrosine-kinase inhibitors. Expert Rev Hematol 6:563–74
Smith AW, Reeve BB, Bellizzi KM et al (2008) Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev 29:41–56
Feinstein AR (1970) The pre-therapeutic classification of co-morbidity in chronic disease. J Cron Dis 23:455–468
Hughes T, White D (2013) Which TKI? An embarrassment of riches for chronic myeloid leukemia patients. ASH Educ Book 1:168–175
O’Brien SG, Guilhot F, Larson RA et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994–1004
Bjorkholm M, Ohm L, Eloranta S et al (2011) Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol 29:2514–20
Cortes J, Talpaz M, O’Brien S et al (2003) Effects of age on prognosis with imatinib mesylate therapy for patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Cancer 98:1105–13
Gugliotta G, Castagnetti F, Palandri F et al (2011) Frontline imatinib treatment of chronic myeloid leukemia: no impact of age on outcome, a survey by the GIMEMA CML Working Party. Blood 117:5591–9
Pinilla-Ibarz J, Cortes J, Mauro MJ (2011) Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: definitions and clinical implications. Cancer 117:688–97
Hochhaus A (2011) Educational session: managing chronic myeloid leukemia as a chronic disease. Hematol Am Soc Hematol Educ Program 2011:128–35
Efficace F, Baccarani M, Breccia M et al (2011) Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 118:4554–60
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–83
Efficace F, Breccia M, Baccarani M et al (2010) Development and feasibility of a patient-reported symptom checklist for chronic myeloid leukemia patients [abstract]. Haematologica 95(S2):189, Abstract 0465
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–83
Parker PA, Baile WF, de Moor C et al (2003) Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology 12:183–93
Rottenberg Y, Litwin H, Manor O et al (2014) Prediagnostic self-assessed health and extent of social networks predict survival in older individuals with cancer: a population based cohort study. J Geriatr Oncol 5:400–7
Zimet GD, Powell SS, Farley GK et al (1990) Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess 55:610–7
Sloan J, Symonds T, Vargas-Chanes D, Fridley B (2003) Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J 37:23–31
Saussele S, Krauss MP, Hehlmann R et al (2015) Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 126:42–9
Vissers PA, Thong MS, Pouwer F et al (2013) The impact of comorbidity on health-related quality of life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv 7:602–13
Smith SK, Mayer DK, Zimmerman S et al (2013) Quality of life among long-term survivors of non-Hodgkin lymphoma: a follow-up study. J Clin Oncol 31:272–9
Korszun A, Sarker SJ, Chowdhury K et al (2014) Psychosocial factors associated with impact of cancer in longterm haematological cancer survivors. Br J Haematol 164:790–803
Efficace F, Baccarani M, Breccia M et al (2013) Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 27:1511–9
Acknowledgments
We are deeply grateful to all patients who participated in this study. We also thank Alessandro Perreca (from GIMEMA Data Center) for his assistance with data management and administrative support throughout the study period.
Authors’ contributions
FE, MB, MBA, and GR did the conception and design of this study. FC and FE completed the statistical analysis. FE, FC, GR, and MB carried out manuscript writing. All authors did the collection and assembly of data and the final approval of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethic committees of participating centers approved the study. All patients provided written informed consent.
Funding
This study was funded by the “Associazione Italiana contro le Leucemie, Linfomi e Mieloma (AIL)”.
The work of Johannes M. Giesinger has been funded by a grant from the Austrian Science Fund (FWF J3353).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Efficace, F., Rosti, G., Breccia, M. et al. The impact of comorbidity on health-related quality of life in elderly patients with chronic myeloid leukemia. Ann Hematol 95, 211–219 (2016). https://doi.org/10.1007/s00277-015-2541-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2541-6