Abstract
Purpose
Measure out of the standard interval in the aorta diameter is a clue for aortic aneurysm or hypoplasia. Pediatric studies focusing specifically on the normal diameter of the abdominal aorta (AA) were limited in the literature. Therefore, the main goal of this work was to determine changes in the effective diameter of AA in healthy children aged 1–18 years for diagnosis of vascular diseases.
Methods
This retrospective work focused on abdominopelvic computed tomography views of 180 children (sex: 90 males / 90 females, average age: 9.50 ± 5.20 years) without any abdominopelvic disease to measure diameters of AA, common iliac artery (CIA), external iliac artery (EIA), and first lumbar vertebra (L1).
Results
Vessel and vertebra diameters increased in pediatric subjects between 1 and 18 years (p < 0.001). Considering pediatric age periods, vessel diameters increased steadily, but L1 diameter showed an irregular growth pattern between age periods. All parameters were greater in males than females (p < 0.05), except from effective diameters of AA over the coeliac trunk (p = 0.084) and over the renal artery (p = 0.051). The ratios of diameters of vessels to L1 increased depending on ages between 1 and 18 years. Considering pediatric age periods, the ratios increased from infancy period to postpubescent period in irregular pattern; however, the ratios for right and left CIA, and AA over the aortic bifurcation did not alter after late childhood period. All ratios for males were similar to females (p > 0.05).
Conclusion
Our age-specific ratios may be beneficial for surgeons and radiologists for the diagnosis of vascular disorders such as aortic aneurysm.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On the posterior abdominal wall, the abdominal aorta (AA) lies slightly to the left of the midline due to the position of the inferior vena cava [19]. It is found between the 12th thoracic vertebra (level of aortic hiatus on the diaphragm) and 4th lumbar vertebra (level of aortic bifurcation) [19]. At the inferior level, it bifurcates into the common iliac arteries (CIA) [19]. In front of the sacroiliac joint, each CIA gives off its terminal branches: the external (EIA) and internal iliac arteries [19]. AA is a vital structure for human development and growth, as AA-related pathologies (e.g., vascular tumors, thrombotic occlusion, middle aortic syndrome, dissections, aneurysms, and hypoplasia) may pose risks up to 90% mortality [15]. A precondition for determining vascular diameter abnormalities such as dilation or stenosis is to firstly define normal [3, 4, 10]. In this context, some authors propose further investigations focused on morphometric features of AA, CIA and EIA to create a normal range for these arteries’ diameters in different populations (e.g., children), considering the limited studies [3, 10,11,12, 14, 15].
Acquired and congenital disorders with or without originating from the heart in children may manifest with changes in AA, CIA or EIA diameter such as dilation or stenosis [3, 10, 15]. The importance of having information about the diameters of these vessels in children may be listed as follows: (a) assessing renal hypertension, (b) tracing hydration status, (c) assessing and giving chase renal transplant, and (d) diagnosing vascular anomalies such as vasculitis, stenosis or aneurysm [1, 7, 15, 18, 20]. Using different imaging tools (e.g., magnetic resonance imaging: MRI, computed tomography: CT, or ultrasound: US), increasing anatomical information (e.g., novel morphometric datasets on different populations), and determining exact standards for diagnosing vascular disorders allow clinicians to decrease morbidity and/or mortality rates [3, 5, 14, 15]. Nevertheless, most of the data reported by previous works is based on information from adult subjects [3, 10, 15]. In this context, there is a need to establish normal standard diameters for AA, CIA and EIA in pediatric populations for better enlightening the anatomy of aorta. Therefore, the main goal of this work was to determine changes in the effective diameter of AA in healthy children aged 1–18 years for diagnosis of vascular diseases.
Materials and methods
Patient selection
The Clinical Research Ethics Committee approved our retrospective multidetector CT study (dated: 13.05.2020, no: 2020/383). After ethical confirmation, patients’ folders (including information such as CT images, treatments, diagnoses, sex, age, hospital admission/discharge dates, and complaints) were reviewed retrospectively. 180 children (sex: 90 males / 90 females, average age: 9.50 ± 5.20 years, range: 1–18 years) without any abdominopelvic disease were included in our study.
Inclusion and exclusion criteria
Inclusion and exclusion criteria of the study were presented in Table 1. Contrast-enhanced abdominopelvic CT views of 180 children admitted to the hospital owing to abdominal pain or trauma were included in the study. Patients with history of surgeries and diseases (splenectomy, cirrhosis etc.), potentially affecting vascular diameter were excluded. Some patients had CT images in their folders taken at different times, in which case a single image was used.
CT protocol
Abdominopelvic CT views were obtained using a 64-row multidetector CT scanner (Aquillion 64, matrix: 512 × 512, FOV: 240 mm, pixel size: 0.46 mm, 0.5 mm thick slices, 230 mA, 120 kV, 0.3 mm interval, Toshiba Medical Systems, Tokyo, Japan). By using intravenous contrast agent, children underwent a routine CT protocol (hepatic phase). Taking into account ALARA (as low as reasonably achievable) guidelines, milliampere-seconds (range between 20 and 150 mAs) and peak kilovoltage (120 kVp for children over 23 kg; 100 kVp for children between 9 and 23 kg; and 80 kVp for children under 9 kg) values suitable to child’s age were determined for CT scan. The raw data from the multidetector CT scanner was reformatted in several planes for creating three-dimensional multiplanar reconstruction on a work station (Vitrea 2). All assessments were performed by the same radiologist (B.T.) with 15 years of experience.
Morphometric parameters
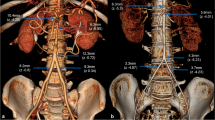
Explanations and abbreviations of the parameters were given in Table 2. Diameters of AA, CIA and EIA were measured in axial planes vertical to their long axes by the use of oblique multiplanar reconstruction (Figs. 1, 2, 3). Transverse and/or antero-posterior diameters were measured by following from one outside wall to the other outside wall of vessels. Since it is difficult to measure between the inner walls of the vessels, especially in young children under 2 years of age, we measured the distance between the outer walls of the vessels to create a standardization. The transverse and antero-posterior diameters of AA were measured at three levels one cm superior to the coeliac trunk, renal artery, and aortic bifurcation. Then, the mean value of these two measurements was used to calculate the effective diameter of AA separately for three levels. Since it was difficult to measure the transverse and antero-posterior diameters of CIA and EIA, especially in young children, effective diameter calculations did not perform in these arteries. That’s why, the longest diameters of the right and left CIA and EIA were separately measured at two levels, proximal and distal. On the other hand, the transverse diameter of the body of the first lumbar vertebra (L1) was measured to use as a standard anatomical indicator, since we did not find always the body surface area of patients, especially in infants. In this context, the ratio of vessel-vertebral diameters was determined to use as a standard indicator.
The photographs show measurement diameters (blue lines) and levels (red lines). a AAD-CT on axial plane, b AAD-RA on axial plane, c the level of AA over the coeliac trunk, and the level of AA over the renal artery on sagittal plane, and d the level of AA over the coeliac trunk, and the level of AA over the renal artery on coronal plane (color figure online)
The photographs show measurement diameters (blue lines) and levels (red lines). a RCIA-DL and LCIA-DL on axial plane, b REIA-PL and LEIA-PL on axial plane, c REIA-DL and LEIA-DL on axial plane, and d the level of distal CIA, the level of proximal EIA, and the level of distal EIA on sagittal plane (color figure online)
Statistical analysis
The normality control of the dataset involving AA, CIA, EIA and L1 diameters was checked with Shapiro–Wilk. Considering the examination of Goodway et al. [9], the child samples were divided into five groups; (a) infancy group aged from birth to 2 years, (b) early childhood group aged from 3 to 5 years, (c) late childhood group aged from 6 to 9 years, (d) prepubescent group aged from 10 to 13 years, and (e) postpubescent group aged from 14 to 18 years. Alterations in the parameters and ratios relative to child ages (from one to 18 years) and child age groups (from infancy to postpubescence) were examined with One-way ANOVA (post-hoc Bonferroni test). Male–female (the independent test) and right–left (the paired test) comparisons was carried out with the student’s t-test. Statistical differences between measurements belonging to AA were evaluated with ANOVA with repeated measures. Correlations between the parameters were examined with the Pearson correlation coefficient test. Through the simple linear regression test, regression equations and scatter plots of the parameters were obtained. For statistical analysis, the “p < 0.05” was accepted as threshold.
Results
Numerical data belonging to AA, CIA, EIA and L1 diameters were presented as average data ± standard deviations in Tables 3, 4, 5.
All parameters increased depending on ages between 1 and 18 years (p < 0.001) (Table 3). Considering child age periods, the parameters increased linearly, except from L1TD. This diameter was similar in early and late childhood periods (p = 0.999), and also in prepubescent and postpubescent periods (p = 0.901), and thus showed an irregular growth pattern between age periods (Table 4). The parameters in males were greater than that in females (p < 0.05), apart from AAD-CT (p = 0.084) and AAD-RA (p = 0.051) (Table 5). Strong positive correlations were found between the parameters (Table 6).
The regression equations for L1TD (y = 24.644 + 0.732 × years, p < 0.001), AAD-CT (y = 7.297 + 0.542 × years, p < 0.001), AAD-RA (y = 6.467 + 0.513 × years, p < 0.001), AAD-AB (y = 5.432 + 0.481 × years, p < 0.001), RCIA-PL (y = 3.775 + 0.322 × years, p < 0.001), LCIA-PL (y = 3.786 + 0.317 × years, p < 0.001), RCIA-DL (y = 3.655 + 0.319 × years, p < 0.001), LCIA-DL (y = 3.636 + 0.318 × years, p < 0.001), REIA-PL (y = 3.070 + 0.273 × years, p < 0.001), LEIA-PL (y = 3.040 + 0.276 × years, p < 0.001), REIA-DL (y = 2.930 + 0.273 × years, p < 0.001), and LEIA-DL (y = 2.912 + 0.275 × years, p < 0.001) were calculated.
The ratios of vessel-vertebral diameters increased steadily depending on ages between 1 and 18 years (Table 7) (Figs. 4 and 5). Considering child age groups, the ratios increased with age between infancy and postpubescent periods in irregular pattern; however, AAD-AB/L1TD, RCIA-PL/L1TD, and LCIA-PL/L1TD did not alter after late childhood period (Table 8). The ratios of vessel-vertebral diameters for males were similar to females (p > 0.05) (Table 9).
The order of the diameter measurements of AA from larger to smaller was found as AAD-CT (12.45 ± 3.10 mm) > AAD-RA (11.34 ± 2.99 mm) > AAD-AB (10.01 ± 2.81 mm) (p < 0.001). RCIA-PL (6.83 ± 1.93 mm) was found greater than LCIA-PL (6.80 ± 1.91 mm) (p = 0.026), while RCIA-DL (6.69 ± 1.91 mm) was similar to LCIA-DL (6.66 ± 1.92 mm) (p = 0.072). Moreover, the proximal values of CIA were larger than its distal values (p < 0.001). No statistically significant difference was found between the REIA-PL (5.66 ± 1.65 mm) and LEIA-PL (5.67 ± 1.66 mm) (p = 0.892), or the REIA-DL (5.53 ± 1.64 mm) and LEIA-DL (5.53 ± 1.65 mm) (p = 0.926). The proximal values of EIA were larger than its distal values (p < 0.001).
Discussion
The present work: first, presented regression equations for AA, CIA, EIA and L1 to estimate their age-specific diameters. Second, the ratio of vessel-vertebral diameters may be used as an indicator of vessel stenosis or dilation to create a standard in the diagnosis of pediatric vascular disorders. Third, most of the parameters were greater in males than females, but vessel-vertebral diameters’ ratios in males were similar to females; therefore, the ratios may be used as a reliable indicator of arterial dilation or stenosis regardless of sex.
The aorta (via its parts and branches) distributes oxygenated blood to the body, and thus it plays a significant functional act in the circulatory system [3]. Changes in its elasticity affect the peripheral circulation, the blood flow in heart and brain, and the function of the left ventricle [6, 13]. Alterations in its size and distensibility may be determined via US, MRI and multidetector CT [15, 17]. In this work, we used multidetector CT views of children due to the following reasons: (a) it provides to monitor the vascular morphology, (b) it decreases the need for sedation in children, (c) it decreases the requirement for diagnostic interventional methods, and (d) it is easy and fast to apply [4, 16]. The first condition to understand changes in vascular morphology on imaging tools such as multidetector CT is to know what is normal [4]. For example, knowing the normal range of the aortic diameter may be useful to diagnose aortic aneurysm in patients with Marfan syndrome, or diffuse aortic stenosis in patients with Williams syndrome [10]. In children, acquired and congenital disorders with or without originating from the heart may manifest with changes in AA, CIA or EIA diameter such as dilation or stenosis [3, 10, 15]. In this context, our calculated linear functions may be helpful to estimate age-specific normal diameters of these vessels in pediatric subjects.
In comparison with the normal diameter, an enlargement of the aortic diameter at least 1.5 times may indicate aortic aneurysm [5, 20]. If an aneurysm is to be diagnosed using this cutoff, the normal range of the aortic diameter must be known [15]. However, the aortic diameter is affected by race, diet, body height, age, sex, body shape, and disorders [11]. For this reason, some authors used anthropometric measurements (e.g., the thoracic or lumbar vertebrae, or the body surface area) to create a standard for determining changes in vessel diameters [2, 3, 10, 12]. For evaluating the relation of vessel dimensions to the body size, Akturk and Gunes [3] utilized L1’s largest transverse diameter as the dorsal cut-off to trace of L1’s body. Geraghty and Boone [8] stated that this vertebra might be a useful anatomical indicator, due to the following reasons: (a) L1 was present in almost all abdominal CT images, (b) L1 was easy to locate on CT images, (c) Variations in L1’s orientation had a minimal effect on the diameter and area outcomes, and (d) Results of initial studies conducted on calculation of organ volumes, via cross-sectional imaging, showed that normalization of data according to L1-based indices accounted for body habitus. Similar to the study of Aktürk and Gunes [3], we used L1’s transverse diameter (at the largest level) as an anatomical indicator. Strong positive correlations were observed between the transverse diameter of L1 and the vessels’ diameters. The ratios of vessel-vertebral diameters increased depending on ages between 1 and 18 years. All ratios for males were similar to females. Aktürk and Gunes [3] stated that before 133 months of age (the period when L1 diameter was similar in both gender), the ratio of proximal aorta (the effective diameter of AA superior to the celiac axis) – L1 (its largest transverse diameter) was 0.41, covering 90% of study population; thus, ratio over 0.4 might indicate AA dilatation. Moreover, they calculated the ratio for distal aorta (the effective diameter of AA superior to the aortic bifurcation) to L1 between 0.28 and 0.36 in children over the age of 3 [3]. In this study, the ratio was found as 0.39 (range: 0.30–0.48) for the proximal AA, and as 0.31 (range: 0.23–0.38) for the distal AA. We think that a ratio outside these ranges may be used to determine abnormal diameters as a sign of arterial dilation or stenosis.
Investigations focused on diameters of AA, CIA, and EIA with multidetector CT scans is very limited. Aktürk and Gunes [3] measured effective diameters of proximal and distal AA, and right and left CIA, and the transverse diameters of L1 on CT images in 142 children aged 0–17 years (with age subgroups: 12–36 months, 37–84 months, 85–132 months, 133–180 months, 181–204 months). They observed no significant difference in the parameters relative to sex, but that on subgroup evaluation, L1 or CIA diameter started to vary relative to age after 133 months [3]. Hegde et al. [10] conducted on effective diameters of 110 AA and 88 thoracic aortae with CT, and found that males had larger aortic diameters compared to females (p < 0.05). Munk et al. [14] measured diameters of CIA and the infrarenal AA in 176 children aged 1–16 years using US, and found that male vessels were larger than female vessels (p < 0.001). In this work, we found that most of the parameters in males were greater than that in females (p < 0.05). However, vessel-vertebral diameters’ ratios in males were similar to females. In our opinion, the ratios may be used as a reliable indicator of arterial dilation or stenosis regardless of sex.
Conclusion
Age-specific ratios calculated in this study may be beneficial for surgeons and radiologists for the diagnosis of vascular disorders such as aortic aneurysm.
Data availability
Available with the authors on request.
References
Adewumi AA, Braimoh KT, Adesiyun OAM, Ololu-Zubair HT, Idowu BM (2019) Correlation of sonographic inferior vena cava and aorta diameter ratio with dehydration in Nigerian children. Niger J Clin Pract 22:950–956. https://doi.org/10.4103/njcp.njcp_591_18
Akay HO, Ozmen CA, Bayrak AH, Senturk S, Katar S, Nazaroglu H, Taskesen M (2009) Diameters of normal thoracic vascular structures in pediatric patients. Surg Radiol Anat 31:801–807. https://doi.org/10.1007/s00276-009-0525-8
Akturk Y, Gunes SO (2018) Normal abdominal aorta diameter in infants, children and adolescents. Pediatr Int 60:455–460. https://doi.org/10.1111/ped.13542
Bayindir P, Bayraktaroglu S, Ceylan N, Savas R, Alper HH (2016) Multidetector computed tomographic assessment of the normal diameters for the thoracic aorta and pulmonary arteries in infants and children. Acta Radiol 57:1261–1267. https://doi.org/10.1177/0284185115622074
Bhatt S, Ghazale H, Dogra VS (2007) Sonographic evaluation of the abdominal aorta. Ultrasound Clin 2:437–453. https://doi.org/10.1016/j.cult.2007.06.001
Bogren HG, Mohiaddin RH, Klipstein RK, Firmin DN, Underwood RS, Rees SR, Longmore DB (1989) The function of the aorta in ischemic heart disease: a magnetic resonance and angiographic study of aortic compliance and blood flow patterns. Am Heart J 118:234–247. https://doi.org/10.1016/0002-8703(89)90181-6
Feng Y, Tang X, Liu M, Zhou J, Zhao X, Li Q (2017) Clinical study of children with Takayasu arteritis: a retrospective study from a single center in China. Pediatr Rheumatol Online J 15:29. https://doi.org/10.1186/s12969-017-0164-2
Geraghty EM, Boone JM (2003) Determination of height, weight, body mass index, and body surface area with a single abdominal CT image. Radiology 228:857–863. https://doi.org/10.1148/radiol.2283020095
Goodway JD, Ozmun JC, Gallahue DL (2019) Understanding motor development: infants, children, adolescents, adults. Jones & Bartlett Learning, Burlington
Hegde SV, Lensing SY, Greenberg SB (2015) Determining the normal aorta size in children. Radiology 274:859–865. https://doi.org/10.1148/radiol.14140500
Hu J, Zheng ZF, Zhou XT, Liu YZ, Sun ZM, Zhen YS, Gao BL (2022) Normal diameters of abdominal aorta and common iliac artery in middle-aged and elderly Chinese Han people based on CTA. Med (Baltimore) 101:e30026. https://doi.org/10.1097/MD.0000000000030026
Kaiser T, Kellenberger CJ, Albisetti M, Bergsträsser E, Valsangiacomo Buechel ER (2008) Normal values for aortic diameters in children and adolescents–assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson 10:56. https://doi.org/10.1186/1532-429X-10-56
Kelly RP, Tunin R, Kass DA (1992) Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71:490–502. https://doi.org/10.1161/01.res.71.3.490
Munk A, Darge K, Wiesel M, Troeger J (2002) Diameter of the infrarenal aorta and the iliac arteries in children: ultrasound measurements. Transplantation 73:631–635. https://doi.org/10.1097/00007890-200202270-00028
Oluwole OO (2023) Reference values for paediatric abdominal aorta on B-mode ultrasound - an outer-to-outer methodology. Pediatr Radiol 53:875–884. https://doi.org/10.1007/s00247-022-05568-4
Pappas JN, Donnelly LF, Frush DP (2000) Reduced frequency of sedation of young children using new multi-slice helical CT. Radiology 215:897–899. https://doi.org/10.1148/radiology.215.3.r00jn34897
Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, Thanassoulis G, Isselbacher EM, Hoffmann U, O’Donnell CJ (2013) Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol 111:1510–1516. https://doi.org/10.1016/j.amjcard.2013.01.306
Schnabel A, Hedrich CM (2019) Childhood vasculitis. Front Pediatr 6:421. https://doi.org/10.3389/fped.2018.00421
Standring S, Borley NR, Collins P, Crossman AR, Gatzoulis MA, Healy JC (2008) Gray’s anatomy: the anatomical basis of clinical practice, 40th edn. Elsevier, London
Zucker EJ (2018) Syndromes with aortic involvement: pictorial review. Cardiovasc Diagn Ther 8:S71–S81. https://doi.org/10.21037/cdt.2017.09.14
Acknowledgements
We thank to Dr. Orhan Beger for his contributions to the article, especially due to statistical analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: BB, BT; methodology: BB, BT; data collection: BT; analysis and interpretation of results: BB, BT; writing original draft: BB, BT; critical revision of the manuscript: BB, BT; approval of the final version of the manuscript: all authors: BB, BT.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The Clinical Research Ethics Committee of Mersin University approved this retrospective work ethically (confirmation time: 13.05.2020, no: 2020/383).
Informed consent
Approval from the Institutional Review Board was obtained and in keeping with the policies for a retrospective review, informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beger, B., Ten, B. Effective diameter of the abdominal aorta in children. Surg Radiol Anat 46, 1201–1211 (2024). https://doi.org/10.1007/s00276-024-03385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-024-03385-y