Abstract
Purpose
To determine the normal range of aortic and pulmonary artery diameters on chest CT, and to search a constant ratio when the diameters of thoracic vascular structures are compared with an internal reference.
Methods
Contrast-enhanced chest CT scans of 133 pediatric patients were retrospectively evaluated. Diameters of ascending and descending aorta, main pulmonary artery, right and left pulmonary arteries and a constant thoracic vertebra were measured. The mean ratios of thoracic vascular diameters to the diameter of the thoracic vertebra were calculated.
Results
There was a positive correlation between the age of the patients and vascular diameters. The mean ratios of vascular diameters to the diameter of thoracic vertebra, ranged from 1.1 for the ascending aorta to 0.70 for the right and left pulmonary arteries, were consistent.
Conclusions
Diameters of thoracic vascular structures increase with age. The consistent vertebral to vessel ratios can be useful in evaluation of chest CT of pediatric patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) is widely used in diagnosis of chest disorders not only in adults but also in pediatric patients [16]. CT is the major diagnostic modality in evaluation of mediastinal structures [19]. The development of new technologies, such as greater number of detector rows and submillimeter image thicknesses, has led to expanded CT applications such as CT angiography. In pediatric patients, multidetector–row CT (MDCT) and CT angiography have improved evaluation of the cardiovascular system, thus decrease the necessity of diagnostic conventional angiography [9, 19]. The number of MDCT examinations performed for assessment of congenital and postoperative cardiovascular diseases of pediatric patients has been increasing [9].
In radiology practice, when we evaluate vascular structures of pediatric patients on CT, we need to know normal diameters of the aorta and pulmonary arteries in order to determine some vascular pathology such as aortic aneurysms and pulmonary hypertension. Dilatation of pulmonary arteries is a reliable criterion in diagnosis of pulmonary hypertension [4, 10]. The number of cases with aortic dilatation or aneurysms in children with Takayasu arteritis, bicuspid aortic valve, Marfan syndrome, Turner syndrome, Larsen syndrome, Loey-Dietz syndrome and cutis laxa is increasing [2, 5, 6, 11, 15, 20, 22]. Several studies have measured the diameters of pulmonary arteries [3, 7] and both pulmonary arteries and thoracic aorta [14] in adults. There are studies which measured diameters of thoracic aorta by echocardiography in pediatric patients [17, 18]. However, to our knowledge, there is only one study which has been designed to determine the range of normal aortic diameters of pediatric patients on CT [8].
In this retrospective study, we aimed to determine the normal range of aortic and pulmonary artery diameters on chest CT scans, which were performed for chest disorders apart from cardiovascular diseases. We also searched whether there was a relationship between the diameters of thoracic vascular structures and the anteroposterior (AP) diameter of thoracic vertebral body.
Materials and methods
In a 15-month period, from June 2006 to October 2007, the chest CT examinations of 250 patients, age range from 1 month to 16 years, were evaluated retrospectively. Chest CT was performed with preliminary diagnoses of various chest pathologies, most of which were pneumonia with complications (Table 1). The patients who had abnormal cardiac physical examination findings, cardiovascular anomalies on echocardiography, chest diseases that can affect pulmonary flow and pressure, connective tissue diseases and skeletal pathologies were excluded from the study. Preliminary diagnoses and CT findings of the study group are listed in Tables 1 and 2.
Patients with congenital cardiac disease, Takayasu arteritis, bicuspid aortic valve, Marfan syndrome, Turner syndrome, Larsen syndrome, Loey-Dietz syndrome and cutis laxa are at risk for progressive aortic dilation and aneurysm [2, 11, 12, 20, 23]. Pulmonary artery aneurysms are rare; most patients with pulmonary artery dilatation have pulmonary hypertension. The causes of pulmonary hypertension in pediatric patents are respiratory distress syndrome, chronic lung disease, severe infection, congenital diaphragmatic hernia, congenital cardiac diseases, chronic hypoxia, cystic fibrosis, high altitude, interstitial disease, scoliosis, neuromuscular disease, airway obstruction and vasculitis and idiopathic [21]. Pulmonary artery aneurysms are generally associated with congenital heart disease. Secondary acquired lesions, such as bacterial endocarditis, septic emboli, syphilis, tuberculosis, cystic medial necrosis and Behçets’ syndrome are also associated with pulmonary artery aneurysms [1, 13]. Considering these pathologies, we excluded patients who had either clinical or CT findings associated with congenital cardiac disease, disseminated or chronic chest diseases, massive pleural effusion, empyema, connective tissue diseases and skeletal pathologies. Out of the 133 study subjects, 49 had normal chest CT. To our knowledge pathologies listed in Table 2, namely focal parenchymal consolidation, atelectasis, focal pulmonary contusion, minimal pleural effusion, mediastinal lymphadenopathy, nodule, solitary hydatic cyst have not been reported to cause aortic or pulmonary artery dilatation.
The body weight and height of all individuals in the study group were within normal limits considering their ages. The study group included 133 patients, 91 boys and 42 girls. The patients were divided into five groups according to their age: Group 1, 0–12 months; Group 2, 13–36 months; Group 3, 37–72 months; Group 4, 73–120 months; Group 5, 121–192 months.
CT protocol
All studies were performed on a CT with 64 detectors (Philips Brilliance CT scanner, Philips Medical Systems, Cleveland, Ohio). None of the patients required sedation. A 20–24 gauge angiocatheter was introduced into antecubital vein of each patient. Administration rate was 1–3 ml/s and 300 mg/ml of 20–50 cc (1–2 ml/kg) nonionic iodine solution was used as contrast medium. Bolus tracking was installed at the descending aorta and after a 10–12 s delay; the region between the apex and the base of the lungs was scanned in 4–6 s. Variable dose parameters (range 30–60 mA and 80–120 kV) were selected considering the body weight of the child. The scan parameters were as follows: reconstruction interval 0.75, the pitch factor 0.67–1.72, the rotation time 0.5–0.75 s and the collimation 64 × 0.625. Evaluation was performed using a work station (Philips Extended Brilliance Workspace Philips Medical Systems, Best, The Netherlands) after image reconstruction of 3-mm thickness.
The measurements were performed on the workstation with the consensus of two radiologists. Taking into account that there was some difference in contrast bolus and vascular enhancement between patients, observers used a mediastinal window of variable settings (window level: 60–80; window width: 300–400) which demonstrated vascular structures clearly.
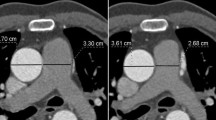
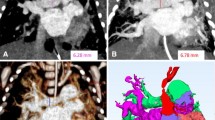
The largest transverse diameter perpendicular to the long axis of the main pulmonary artery (MPA) was measured at the level of pulmonary bifurcation. Distal to the bifurcation, the diameters of the right pulmonary artery (RPA) and the left pulmonary artery (LPA) were measured perpendicular to their long axis. The measurements were performed within proximal 2 cm distance from the lobar branching of the right and left pulmonary arteries (Fig. 1). The measurements of the aortic lumen were performed in the true axial plane perpendicular to the long axis of the vessel using oblique multiplanar reconstruction. The transverse diameters of ascending and descending aorta were measured at the level of pulmonary bifurcation (Figs. 2, 3). In order to facilitate evaluation of vascular structures in daily radiology practice, we tried to find out whether there was a constant value when the measurements of vascular diameters were compared with an internal reference. The thoracic (T) vertebra, which was at the level of pulmonary bifurcation, was used as the internal reference. The largest AP diameter of the T vertebra was measured (Fig. 4). The ratios obtained by division of each vessel diameter to the AP diameter of the T vertebra were calculated for each subject.
Statistics
The patients were divided into five groups according to their age. The distribution of patients in age groups is shown in Table 3. The aortic and pulmonary artery diameters were analyzed with respect to the age groups, sex and the T vertebral body size. Pearson’s correlation was used to evaluate the relationship between the age of the subjects and the measurements of thoracic vascular structures and the T vertebra. The measurements of the two genders were compared by Chi-square test. Analysis of variance (ANOVA) and post hoc Bonferroni were used to compare data obtained from the five age groups. The results were expressed as the mean ± standard deviation (SD) and considered significant when P was less than 0.05.
Results
When the diameters of AA, DA, MPA, RPA, LPA and AP diameter of the T vertebra were compared between genders, the difference was not statistically significant (P > 0.05); however, the differences between the age groups were statistically significant (P < 0.001). The mean diameters of the vessels and the T vertebra in age groups are displayed in Table 4.
There was a positive correlation with high coefficients (r) between the age of the patients and the diameters of AA, DA, MPA, RPA, LPA and the T vertebra (Table 5; Fig. 5).
The vessel to vertebra ratios that obtained when diameters of AA, DA, MPA, RPA, LPA divided into the AP diameter of the T vertebra were also compared with ANOVA. The differences were not statistically significant (P > 0.05) among the five groups for each vessel. The results are shown in Table 6. The ratios were proved to be a constant value for each vascular structure with standard deviations in the 10–20% range. These mean ratios for each vessels of all groups are as follows: 1.1 ± 0.18 for AA; 1.0 ± 0.20 for MPA; 0.70 ± 0.10 for RPA and LPA; and 0.83 ± 0.1 for DA.
Discussion
One of the most important contributions of MDCT in pediatric patients is expanded cardiovascular CT applications. The limitations of CT angiography in pediatric patients, such as motion artifacts and the smaller amount of contrast material used, have been overcome with the invention of MDCT and diagnostic images have been obtained. Thus, MDCT is becoming one of the first-line studies to diagnose the cardiovascular pathologies of infants and children [9]. The normal diameters of main cardiovascular structures should be known to determine dilatation or stenosis. However, to our knowledge there is no study with MDCT on normal thoracic vascular diameters of pediatric individuals in the English literature. Therefore, in this study we tried to determine mean diameters of the normal thoracic vascular structures in pediatric patients according to their age and gender. Since the mean diameters change according to the age groups, we searched for a constant value using the AP diameter of the T vertebra as an internal reference. The ratios obtained by division of the diameter of each vascular structure to the AP diameter of the T vertebra remained constant for each vessel in our study group. Since the difference of these ratios was not statistically significant between genders and age groups, we postulate that these constant ratios can be useful in evaluation of vascular structures and predilection of normal diameters in pediatric patients regardless of age and gender. To our knowledge, this is the first study to determine constant ratios to evaluate diameters of thoracic vascular structures in pediatric patients.
In a study by Edwards et al. [7], it was postulated that the diameters of the pulmonary arteries did not change with age. However, most of the patients in their study group, ages ranged from 11 to 90, were adults with only few children all of whom were over age 10 [7]. Therefore, we believe that the results of the study by Edwards et al. cannot be regarded valid for pediatric age group. Our study, of which the study group included only pediatric patients, showed that there was a positive correlation with age and diameters of MPA, RPA and LPA. In addition, we also found that the diameters of AA and DA and T vertebra showed a positive correlation with age.
In the literature search we could not find studies concerning variations of pulmonary arteries. During the study we did not encounter any variations on pulmonary bifurcation. Thus, there was no effect on variations on pulmonary bifurcation in regards with our measurements.
The study performed by Bozlar et al. [3] showed that in adults the diameter of LPA was significantly larger that the diameter of RPA. Although the same measurement technique was applied, our study revealed that the difference between the diameters of LPA and RPA was not statistically significant. Our study also showed that the ratios of diameters of LPA and RPA to the AP diameter of the T vertebra were constant (0.70) regardless of age and gender in pediatric age.
Bozlar et al. [3] also showed that the diameters of MPA and RPA were not significantly different in men and women, whereas the diameters of LPA were significantly larger in men when compared to the diameters of LPA of women. However, the study by Edwards et al. and our study showed that the diameters of pulmonary arteries did not significantly differ when the means of the two genders were compared.
In this study we measured the diameters of ascending and descending aorta as well as the diameters of pulmonary arteries in pediatric patients. To our knowledge the study by Fitzgerald et al. [8] is the only study to evaluate thoracic aorta in pediatric patients. They measured the diameters of thoracic aorta and the width of the thoracic vertebra at the level of pulmonary bifurcation. They showed that both the diameters of thoracic aorta and vertebral body width had a linear relationship to age. Neither mean diameters of thoracic aorta with respect to age groups, nor the ratio of the diameters of thoracic aorta to the width of the vertebra were calculated in their study. Our study revealed that there was a positive correlation between the diameters of thoracic aorta and the AP diameter of the T vertebra. In addition, we found that the ratio of the diameters of thoracic aorta to the AP diameters of the T vertebra did not significantly differ when the age groups were compared. These constant values can be useful in evaluation of thoracic vascular structures to determine whether the diameters are within normal limits. More studies with larger study groups are necessary to confirm these findings.
An important limitation of this study is that all individuals in the study group were patients most of whom with chest diseases. However, we excluded patients with clinical and CT findings which might affect the vascular structures.
In conclusion, we calculated the mean diameters of thoracic vascular structures of pediatric patients in five age groups. This study revealed that the mean diameters of thoracic vascular structures and AP diameter of the T vertebra increased with age. Presence of a constant value for each vessel independent of age will help us make distinction between normal and abnormal thoracic aorta and pulmonary arteries in pediatric age.
References
Bartter T, Irwin RS, Nash G (1988) Aneurysms of the pulmonary arteries. Chest 94:1065–1075
Baspinar O, Kilinc M, Balat A, Celkan MA, Coskun Y (2005) Long tortuous aorta in a child with Larsen syndrome. Can J Cardiol 21(3):299–301
Bozlar U, Ors F, Deniz O, Uzun M, Gumus S, Ugurel MS, Yazar F, Tayfun C (2007) Pulmonary artery diameters measured by multidetector-row computed tomography in healthy adults. Acta Radiol 24:1–6
Chaudry G, MacDonald C, Adatia I, Gundogan M, Manson D (2007) CT of the chest in the evaluation of idiopathic pulmonary arterial hypertension in children. Pediatr Radiol 37:345–350
Civilibal M, Sever L, Numan F, Altun G, Ocak S, Candan C, Kasapcopur O, Caliskan S, Cantasdemir M, Arisoy N (2008) Dissection of the abdominal aorta in a child with Takayasu’s arteritis. Acta Radiol 49:101–114
Dean JC (2007) Marfan syndrome: clinical diagnosis and management. Eur J Hum Genet 15:724–733
Edwards PD, Bull RK, Coulden R (1998) CT measurement of main pulmonary artery diameter. Br J Radiol 71:1018–1020
Fitzgerald SW, Donaldson JS, Poznanski AK (1987) Paediatric thoracic aorta: normal measurements determined with CT. Radiology 165:667–669
Frush DP, Herlong JR (2005) Paediatric thoracic CT angiography. Pediatr Radiol 35:11–25
Grubstein A, Benjaminov O, Dayan DB, Shitrit D, Cohen M, Kramer MR (2008) Computed tomography angiography in pulmonary hypertension. Isr Med Assoc J 10:117–120
Johnson PT, Chen JK, Loeys BL, Dietz HC, Fishman EK (2007) Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol 189:W29–W35
Isselbacher EM (2005) Thoracic and abdominal aortic aneurysms. Circulation 111:816–828
Lopez-Candales A, Kleiger RE, Aleman-Gomez J, Kouchoukos NT, Botney MD (1995) Pulmonary artery aneurysm: review and case report. Clin Cardiol 18:738–740
Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao YL, Budoff MJ (2008) Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults impact of age and gender. Acad Radiol 15(7):827–834
Matura LA, Ho VB, Rosing DR, Bondy CA (2007) Aortic dilatation and dissection in Turner syndrome. Circulation 116:1663–1670
Paterson A, Frush DP (2007) Dose reduction in paediatric MDCT: general principles. Clin Radiol 62:507–517
Poutanen T, Tikanoja T, Sairanen H, Jokinen E (2003) Normal aortic dimensions and flow in 168 children and young adults. Clin Physiol Funct Imaging 23:224–229
Sheil ML, Jenkins O, Sholler GF (1995) Echocardiographic assessment of aortic root dimensions in normal children based on measurement of a new ratio of aortic size independent of growth. Am J Cardiol 75:711–715
Siegel MJ (2003) Multiplanar and three-dimensional multi-detector row CT of thoracic vessels and airways in the paediatric population. Radiology 229:641–650
Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z (2006) Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet 43:255–258
Tulloh RMR (2005) Congenital heart disease in relation to pulmonary hypertension in paediatric practice. Paediatr Respir Rev 6:174–180
Warren AE, Boyd ML, O’Connell C, Dodds L (2006) Dilatation of the ascending aorta in paediatric patients with bicuspid aortic valve: frequency, rate of progression and risk factors. Heart 92:1496–1500
Yetman AT, Graham T (2009) The dilated aorta in patients with congenital cardiac defects. J Am Coll Cardiol 53:461–467
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akay, H.O., Ozmen, C.A., Bayrak, A.H. et al. Diameters of normal thoracic vascular structures in pediatric patients. Surg Radiol Anat 31, 801–807 (2009). https://doi.org/10.1007/s00276-009-0525-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-009-0525-8