Abstract
Purpose
Renal arterial anatomy has a great clinical importance during surgical and endovascular procedures. However, comprehensive data on renal arterial variations in the Bulgarian population has not yet been provided. The aim of this study was to conduct a detailed research about the normal anatomy and variations of the renal arteries in the Bulgarian population.
Methods
Five hundred sixty-one patients underwent contrast-enhanced multidetector computed tomography scans for the period 2016–2021. The images were retrospectively reviewed. Number, branching pattern, origin level and course of the renal arteries were noted. Data were categorized on the basis of laterality, gender and symmetry.
Results
Only 46.3% of the patients exhibited normal renal arterial anatomy. Variations were observed in 301 patients (53.7%). The most common variant was the presence of accessory renal arteries (ARA), discovered in 41.2% of the subjects. There was no significant difference based on gender and laterality (p > 0.05). Hilar ARA (72.6%) were significantly more common than polar ARA (p < 0.001). The most common origin location of the main renal arteries and ARA was the aorta, followed by the common iliac arteries. Early division was observed in 21.7% of the patients, significantly more common on the right. Precaval course was found in 0.5% of the right main renal arteries and in 30% of ARA and the difference was significant (p < 0.001).
Conclusion
These results show novel insight into the prevalence of renal arterial variations in the Bulgarian population. Anatomic renal vasculature variants are common therefore awareness is crucial for the success of surgical and interventional procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The traditional concept for the renal arterial blood supply describes one renal artery originating from the abdominal aorta at the level of L1–L2 vertebrae and entering the kidney at the hilum where it divides into presegmental and segmental branches [25]. However, kidneys are well known to show a diverse spectrum of variations in their vascular system. The variations in the renal arterial vasculature are attributed to the failure of one or several of the multiple renal arteries present during the embryologic development of the kidney to regress [1].
Renal vasculature variants have long captivated the interest of anatomists. Knowledge of these variations is essential in the modern era of medicine with the advances of minimally invasive surgery, renal transplantation and endovascular procedures. Therefore, careful evaluation is crucial before initiating treatment. Multidetector computed tomography angiography (MDCTA) is non-invasive, quick, widely available and less expensive compared to magnetic resonance angiography and replaces digital subtraction angiography. A retrospective comparative study of 2,144 living kidney donors found that the sensitivity, specificity and diagnostic accuracy of CTA to detect renal arterial variations is 100%, 97.48% and 97.9%, respectively, compared to intraoperative findings [23].
Renal arterial vascular variations have been studied among diverse population groups. There are great discrepancies in the literature with respect to the prevalence of renal artery variants with frequencies ranging between 4 and 61.5% [12, 21]. The differences vary among nationalities and within the same population groups [4, 20]. However, data on their prevalence in the Bulgarian population is currently lacking. Therefore, the purpose of this study was to conduct a comprehensive analysis of normal renal arterial vasculature and the prevalence of different anatomic variations.
Material and methods
This is a retrospective study with an initial cohort consisting of 610 patients who underwent CTA of the abdomen for various indications for the period January 2016–December 2021 in Prof. Dr. Alexander Chirkov University Hospital in Sofia, Bulgaria. The hospital is a major referral center for cardio-vascular diseases in Bulgaria, and therefore, the results should serve as an adequate representation of the vasculature variations in the general Bulgarian population. Informed consent and ethical approval were waived by local ethics due to the retrospective nature of the study.
The CT protocol included multiphase imaging or angiography phase only based on the study requirements. The scans were performed on a 320-slice Aquilion One CT scanner (Cannon Medical Systems). The scanning parameters were as follows: tube voltage: 100–120 kV, automatic milliamperage setting, slice thickness: 0.5 mm, tube rotation time 0.5 s. A volume of 90-110 ml non-ionic iodine contrast medium was administered with an automatic injector in an antecubital vein at a rate of 4–4.5 mL/s, followed by 30 ml saline. Bolus tracking technique was employed to determine the scan start time. Region of interest was placed in the descending thoracic aorta. Image acquisition in the arterial phase began after a 10–15 s delay when the enhancement at the region of interest reached 180 HU. The image analysis was performed on dedicated Vitrea workstations (Cannon Medical Systems). Interpretation included primary axial images followed by multiplanar (MPR), maximum intensity projection (MIP) and volume rendering (VR) reconstruction techniques.
The following exclusion criteria were applied to the initial cohort: (1) surgical interventions, or pathologic conditions that distorted the normal renal vascular anatomy, (2) CT studies done in other hospitals, and (3) studies with technically insufficient quality of the arterial phase scans. In addition, duplicate studies of patients with multiple exams were also excluded. Finally, after excluding the aforementioned groups, the remaining 561 patients were included in the study.
Classification
Normal anatomy was present when each kidney was supplied by one renal artery, originating from the aorta and dividing into segmental branches at the hilum. In the case of multiple renal arteries, the vessel with the biggest diameter was considered as main and the rest as “accessory.” Accessory renal arteries were further categorized on the basis of their point of entry into the kidney. Arteries with an entry point in the hilum were classified as “hilar.” Arteries entering the kidney parenchyma at the superior and inferior pole were described as superior and inferior polar arteries, respectively. To evaluate the level of origin of main renal arteries and ARA from the aorta, the place of each artery`s ostium was noted according to the vertebral body or intervertebral disk level. In case an artery originated from another vessel, the specific origin artery was reported. Any vessel that branched within 2 cm from the origin was classified as having “early division.” Data were categorized based on laterality, gender and symmetry. The final criterion of classification was based on the retro- or precaval course observed in both the right main renal artery and ARA.
Statistical analysis
The occurrence of each of the previously classified variables was recorded in a Microsoft Excel spreadsheet. Descriptive and comparative statistical analyses were performed on the data using SPSS, Version 20.0 software pack. The mean, standard deviation, minimum, maximum and percentage occurrence of the above categories was recorded. Comparisons between variations on the basis of laterality, gender and symmetry were carried out using chi-square test. Statistical significance was reached when p < 0.05.
Results
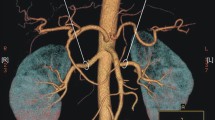
The mean age of the patients was 63.7 ± 16.2 years, range 19–90 years and the majority were male (58.6%). Only 260 patients (46.3%) had one renal artery with hilar bifurcation on both sides (Fig. 1). Anatomic variations in renal arterial vasculature were found in the remaining 301 patients (53.7%). The most common variation was the presence of ARA, discovered in 41.2% of the subjects (n = 231). Findings for laterality revealed that 24.2% (n = 136) of the right-sided kidneys and 25.5% (n = 143) of the left-sided kidneys were supplied by more than one renal artery. Statistical significance between these rates was not established (p = 0.684). The distribution in the number of ARA on both sides is presented on Table 1. Bilateral ARA were discovered in 8.55% of the patients (n = 48), the most common pattern was one 1 ARA on both sides- in 6.24% (n = 35) (Fig. 2). Two ARA on both sides were present in 1 patient (0.18%) who had a horseshoe kidney as a concomitant congenital kidney anomaly (Fig. 3). Seven renal arteries were discovered in one patient (0.2% of the cohort)—the patient had three ARA on the right side and two on the left side (Fig. 4). The difference in the number of ARA according to gender did not reach statistical significance on either side (p = 0.138). In males ARA was found in 25.5% on the right side and in 28.1% on the left side while the occurrence in females was, respectively, 22.4% on the right side and 22.0% on the left side.
a MDCTA VR reconstruction shows the right main renal artery originating from the aorta at the level of L1-L2 intervertebral disk. The ostium of the left main renal artery is located at the level of the superior third of L2 vertebral body. There is one inferior polar accessory renal artery on each side, originating at the level of L3. b Axial MIP reconstruction shows precaval course of the right accessory renal artery
a MDCTA VR reconstruction shows a patient with an abdominal aortic aneurysm. Horseshoe kidney was discovered on the CTA as an incidental finding. The right and left main renal arteries originate below and at the level of the aneurysm, respectively. The patient has two accessory renal artery on each side. b Axial CTA image presents the position of the horseshoe kidney in front of the infrarenal aortic aneurysm
In regard to the entry point in the kidney, hilar ARA were significantly more common than both superior and inferior polar ARA on either side (p < 0.001) (Figs. 2, 5, 6). Table 2 depicts the distribution of ARA according to their entry point.
The level of origin of the main renal arteries and ARA from the aorta ranged between Th12-L1 intervertebral disk and L5 vertebral body. The right main renal artery originated between the upper margin of the L1 vertebral body and the lower margin of L2 vertebral body in 97% of the patients and the left one- in 96.8% of the patients, with the most common levels being the L1 and L2 vertebral bodies, respectively (35.1% and 38.3%). The right main renal artery showed a higher ostium position in comparison to the left. ARA exhibited greater variability of origin location with 71.8% of right-sided ARA and 74.6% of left-sided ARA arising between the upper margin of L1 and the lower margin of L2. Three main renal arteries and eleven ARA arose from an aortic branch vessel- the common iliac arteries, internal iliac artery and from a common trunk with the median sacral artery. The distribution of the origin level of the main renal arteries and ARA is presented in Table 3.
Early branching was described in 21.7% (n = 122) of the patients. ED was noted in 72 (12.8%) of the right-sided kidneys and 50 (8.9%) of the left-sided kidneys and a significant difference was determined (p = 0.035) (Fig. 6). Bilateral ED occurred in 11 patients (1.9%). The rates of ED were similar between males (21.3%) and females (21.5%) and statistical significance was not established (p = 0.292).
One right main renal artery and seven right ARA originated from the common iliac arteries and did not cross the inferior vena cava. The remaining 560 right main renal arteries and 149 right ARA had a retro- or precaval course. In total 49 (6.9%) of all right renal arteries had a precaval disposition. Three main renal arteries (0.5% of all right main renal vessels) and 46 ARA (30.8% of right ARA) had a precaval course. This anatomic variant was significantly more common in ARA in comparison to the main vessels (p < 0.001) (Fig. 2).
Discussion
The clinical importance of arterial variations is both medical and surgical. Renal arteries are end arteries without anastomotic connections, therefore their involvement by different pathological conditions such as atherosclerosis, fibromuscular dysplasia, dissection may lead to ischemic nephropathy and functional deterioration. There is no consensus among practitioners on the role of ARA in the pathogenesis of arterial hypertension... Due to their small caliber, lower perfusion pressure and atherosclerotic changes, there is an association between the activation of the renin-angiotensin system and hypertension [15, 17]. Some researchers consider them a factor for diminished response in radiofrequency renal denervation [30].
From a surgical perspective, it is well known that crossing inferior polar ARA is an etiological factor for extrinsic ureteropelvic junction compression and hydronephrosis in children with a reported incidence of 11–25% [5, 13]. With the advances in laparoscopic and robot-assisted surgery, vascular preoperative evaluation is essential for the planning of a number of abdominal and pelvic surgical interventions, thus, lowering the risk of iatrogenic injury [31]. Although ARA are an undesirable factor in transplantation and are associated with a higher rate of complications, recent studies report similar short and long-term outcomes in multiple and single renal artery grafts [9, 33]. Furthermore, it is important to report ED because sufficient arterial length is needed to ensure safe vascular anastomosis.
Presence of ARA and their origin location is also important during surgical or endovascular treatment of abdominal aortic aneurysms. Although data is inconsistent, some researchers report a higher incidence of acute kidney injury and long-term worsening of renal function after the sealing of ARA [28].
There are big differences in the occurrence of anatomic variations among different populations. Our study shows a higher number of anatomic renal arterial variations compared to most previously published results. Only 46.3% of the patients exhibited classic renal arterial anatomy. Similar to other studies, the most common variation was the presence of ARA, discovered in 41.2% of our subjects (n = 231) [11, 14, 22]. These results are higher than the average reported frequency of 10–30% in other studies [3, 10, 20]. Possible reasons for this variability of the results could be the size of the study group and adopted classification, which also varies greatly among researchers. Another reason could be attributed to the type of study- cadaveric, radiologic or surgical. According to laterality, published results are inconsistent. Some researchers report higher prevalence on the right side [26] and others on the left [14] but without significant differences. Our results demonstrated that ARA are more common on the left side in comparison to the right side (25.5 vs. 24.3%, respectively) without reaching statistical significance. In our study, similar to most researchers greater proportion of males exhibited the presence of ARA [18]. However, a statistically significant difference between genders was not observed. Contrary to our results Sośnik stated a significantly greater occurrence of ARA in males [24].
The occurrence frequency of hilar ARA (72.6%) in our study was remarkably higher compared to polar ARA (15.2% superior polar ARA and 12.2% inferior polar ARA). Similar to our results Çınar et al. described a greater frequency of hilar ARA (84.5%) compared to polar ARA (15.5%) and a greater proportion of superior than inferior polar ARA [7]. One study by Johnson et al. also report a higher prevalence of hilar ARA compared to polar ARA. However, ARA entering the kidney through the lower pole were more common in comparison to superior polar ARA [14].
In our study, the most common site of both main and accessory renal arteries origin was the abdominal aorta between the level of L1 and L2 vertebrae. These findings are consistent with the classical teaching and previously published data reporting that 94–95% of the main renal arteries and 78–80% of ARA arise from the aorta at the L1-L2 level [11, 20]. Similar to our results Natsis states that the majority of right main renal arteries originate at the level of L1 and the majority of left main renal arteries- at the level of L2, while the most common level described by Palmieri is the L1-L2 intervertebral disk on both sides [20, 21]. ARA exhibits a bigger range and more inferior ostium location compared to the main renal arteries. In the current study, both the main and accessory renal arteries on the left showed a lower ostium position compared to the contralateral side, coinciding with previous literature reports [11, 20]. Three main renal arteries (0.3%) and eleven ARA (3.4%) did not arise from the aorta. The most common origin arteries were the common iliac arteries. One left main renal artery showed a very rare variant and originated from a common trunk with the median sacral artery. One left ARA arose from the left internal iliac artery. The renal arteries are known to originate from the iliac arteries, superior and inferior mesenteric arteries, contralateral renal artery, lumbar artery or the thoracic aorta. Kim et al. described a case in which the main renal artery supplying the right kidney originated from the left proximal common iliac artery. In our cohort, such variation was not observed. However, in one patient an accessory renal artery to the right kidney arose from the contralateral left common iliac artery [16]. The variations in the ostium location and number of renal arteries are commonly encountered together with other concomitant genitourinary anomalies such as horseshoe or ectopic kidney [10]. This data is confirmed in a study by Majos et al. published in 2018. The authors describe a significantly greater number of arteries in horseshoe kidneys compared to separate kidneys (p < 0.0001) as well as greater variability in arterial origin location in the horseshoe kidney group (p = 0.0001). The arteries supplying the horseshoe kidneys originated from the common iliac arteries in 14.78% of the cases and below them in less than 1% whereas such variations were not described in the separate kidney group [19]. Moreover, apart from originating from a different vessel than the aorta, the renal arteries give rise to different vessels which also exhibit multiple variations. A recently published case report by Vas et al. describes an extremely rare variant in which a replaced right hepatic artery originated from the right distal renal artery and exhibited a cranial course to the porta hepatis [29]. Such variation was not observed in our large cohort.
The prevalence of ED in the literature ranged between 4 and 35.9% [8, 27]. The frequency of 21.7% in our study could be considered moderate and aligns with previously published data. The majority of the studies do not establish a significant association between ED and laterality, although it was more prevalent on the right side [6, 18, 27]. However, according to our findings, ED is significantly more common on the right- 12.8 vs. 8.9%, p = 0.035. With respect to gender, two studies report significantly higher ED occurrence in males [6, 11]. On the other hand, Kumaresan et al. report a higher prevalence in females but without statistical significance [18]. However, in our study the rates of early bifurcation were similar between genders- 21.3% in males and 21.5% in females. Discrepancies in branching pattern could be attributed to the methodology of data sampling and the adopted definition of ED in each study, which often varies between studies.
Traditionally, right-sided renal arteries pass behind the IVC. The reported prevalence of the precaval course of the renal arteries is 4.5–9.17% [2, 8]. It is important to acknowledge precaval renal arteries because their presence has been associated with asymmetrical obstruction of the renal collecting system although the incidence of symptomatic hydroneprhosis is still unknown [32]. In the present study, 6.9% of the right renal arteries had a precaval course which is in agreement with other studies. Similar to the studies of Yeh et al. and Bouali et al. the overwhelming majority of the precaval renal arteries were accessory [2, 32]. Precaval course was noted in only 0.5% of the main renal arteries and in 30% of ARA and a significant difference was determined (p < 0.001). Precaval course of a single and main artery was found in one patient which is a very rare variant, and only a few cases are described in the literature [32].
Conclusion
The renal arterial vasculature exhibits complex anatomy and a large number of variations. The interdisciplinary significance of these variations is undisputed. The present study provides novel data on the prevalence of renal arterial variations in the Bulgarian population. Anatomic renal arterial vasculature variants are common and the most frequently encountered variation was an accessory renal artery. Therefore awareness and thorough preoperative radiological assessment are essential for the success and safety of surgical and endovascular interventions.
Data availability
Data are available on demand.
References
Arevalo Pérez J, Gragera Torres F, Marín Toribio A, Koren Fernández L, Hayoun C, Daimiel Naranjo I (2013) Angio CT assessment of anatomical variants in renal vasculature: its importance in the living donor. Insights Imaging 4:199–211. https://doi.org/10.1007/s13244-012-0217-5
Bouali O, Labarre D, Molinier F, Lopez R, Benouaich V, Lauwers F, Moscovici J (2012) Anatomic variations of the renal vessels: focus on the precaval right renal artery. Surg Radiol Anat 34:441–446. https://doi.org/10.1007/s00276-011-0923-6
Bouzouita A, Saadi A, Hermi A, Chakroun M, Bouchiba N, Allouche M, Hamdoun M, Mighri MM, Chebil M (2021) Cadaveric study of arterial renal anatomy and its surgical implications in partial nephrectomy. Surg Radiol Anat 43:1449–1459. https://doi.org/10.1007/s00276-021-02769-8
Cenal U, Erturk T, Karayagiz AH, Ozdemir E, Polatkan SV, Cakir U, Berber I (2019) Geographic distribution of multiple arteries and veins of 878 kidney donors from a transplant center in Turkey. Transplant Proc 51:1086–1088. https://doi.org/10.1016/j.transproceed.2019.01.100
Chiarenza SF, Bleve C, Fasoli L, Battaglino F, Bucci V, Novek S, Zolpi E (2016) Ureteropelvic junction obstruction in children by polar vessels. Is laparoscopic vascular hitching procedure a good solution? Single center experience on 35 consecutive patients. J Pediatr Surg 51:310–314. https://doi.org/10.1016/j.jpedsurg.2015.10.005
Cicek SK, Ergun S, Akıncı O, Sarıyar M (2021) Renal vascular and ureteral anatomic variations in 1859 potential living renal donors. Transplant Proc 53:2153–2156. https://doi.org/10.1016/j.transproceed.2021.07.030
Çınar C, Türkvatan A (2016) Prevalence of renal vascular variations: evaluation with MDCT angiography. Diagn Interv Imaging 97:891–897. https://doi.org/10.1016/j.diii.2016.04.001
Famurewa OC, Asaleye CM, Ibitoye BO, Ayoola OO, Aderibigbe AS, Badmus TA (2018) Variations of renal vascular anatomy in a nigerian population: A computerized tomography studys. Niger J Clin Pract 21:840–846
Garcia LE, Parra N, Gaynor JJ, Baker L, Guerra G, Ciancio G (2021) Clinical outcomes following single vs. multiple vessel living-donor kidney transplantation: a retrospective comparison of 210 patients. Front Surg. https://doi.org/10.3389/fsurg.2021.693021
Gulas E, Wysiadecki G, Szymański J, Majos A, Stefańczyk L, Topol M, Polguj M (2015) Morphological and clinical aspects of the occurrence of accessory (multiple) renal arteries. Arch Med Sci 14:442–453. https://doi.org/10.5114/aoms.2015.55203
Gümüş H, Bükte Y, Ozdemir E, Cetinçakmak MG, Tekbaş G, Ekici F, Onder H, Uyar A (2012) Variations of renal artery in 820 patients using 64-detector CT-angiography. Ren Fail 34:286–290. https://doi.org/10.3109/0886022X.2011.647295
Hlaing KP, Das S, Sulaiman IM, Abd-Latiff A, Abd-Ghafar N, Suhaimi FH, Othman F (2010) Accessory renal vessels at the upper and lower pole of the kidney: a cadaveric study with clinical implications. Bratisl Lek Listy 111:308–310
Hoffer FA, Lebowitz RL (1985) Intermittent hydronephrosis: a unique feature of ureteropelvic junction obstruction caused by a crossing renal vessel. Radiology 156:655–658. https://doi.org/10.1148/radiology.156.3.4023225
Johnson PB, Cawich SO, Shah SD, Aiken W, McGregor RG, Brown H, Gardner MT (2013) Accessory renal arteries in a Caribbean population: a computer tomography based study. Springer Plus. https://doi.org/10.1186/2193-1801-2-443
Kang K, Ma Y, Jia C, Cheng Y, Yang Y, Wang L, Jiang Y, Lu Y (2020) Relationship between accessory renal artery and clinical characteristics of middle-aged patients with primary hypertension. Int J Hypertens. https://doi.org/10.1155/2020/7109502
Kim J, Lee JM, Cho SG, Hong JU (2022) Ectopic vascularization of the right kidney arising from contralateral common iliac artery. Surg Radiol Anat 44:979–981. https://doi.org/10.1007/s00276-022-02970-3
Kuczera P, Włoszczyńska E, Adamczak M, Pencak P, Chudek J, Wiecek A (2009) Frequency of renal artery stenosis and variants of renal vascularization in hypertensive patients: analysis of 1550 angiographies in one centre. J Hum Hypertens 23:396–401. https://doi.org/10.1038/jhh.2008.149
Kumaresan M, Saikarthik J, Sangeetha A, Saraswathi I, Senthil Kumar K, Roselin P (2021) Peri-hilar branching pattern and variations of the renal artery among Indian kidney donors using pre-operative computed tomography angiography: an anatomical study and review. Folia Morphol (Warsz). https://doi.org/10.5603/FM.a2021.0103
Majos M, Polguj M, Szemraj-Rogucka Z, Arazińska A, Stefańczyk L (2018) The level of origin of renal arteries in horseshoe kidney vs. in separated kidneys: CT-based study. Surg Radiol Anat 40:1185–1191. https://doi.org/10.1007/s00276-018-2071-8
Natsis K, Paraskevas G, Panagouli E, Tsaraklis A, Lolis E, Piagkou M, Venieratos D (2014) A morphometric study of multiple renal arteries in Greek population and a systemic review. Rom J Morphol Embryol 55:1111–1122
Palmieri J, Petroianu A, Silva LC, Andrade LM, Alberti LR (2011) Study of arterial pattern of 200 renal pedicle through angiotomography. Rev Col Bras Cir 38:116–121. https://doi.org/10.1590/s0100-69912011000200009
Regmi PR, Amatya I, Kayastha P, Paudel S, Suwal S, Ghimire RK (2020) Normal anatomy and variants of renal vasculature with multidetector computed tomography in a tertiary care hospital: a descriptive cross-sectional study. J Nepal Med Assoc 58:911–914
Sarier M, Callioglu M, Yuksel Y, Duman E, Emek M, Usta SS (2020) Evaluation of the renal arteries of 2,144 living kidney donors using computed tomography angiography and comparison with intraoperative findings. Urol Int 104:637–640. https://doi.org/10.1159/000507796
Sośnik H, Sośnik K (2017) Investigations on renal vascularisation pathology in the Polish population. 1. Incidence of multiple kidney arteries. Folia Morphol (Warsz) 76:226–231. https://doi.org/10.5603/FM.a2016.0073
Standring S (2008) Gray’s anatomy: the anatomical basis of clinical practice, 40th edn. Churchill Livingstone Elsevier, Edinburgh
Tardo DT, Briggs C, Ahern G, Pitman A, Sinha S (2017) Anatomical variations of the renal arterial vasculature: an Australian perspective. J Med Imaging Radiat Oncol 61:643–649. https://doi.org/10.1111/1754-9485.12618
Tarzamni MK, Nezami N, Rashid RJ, Argani H, Hajealioghli P, Ghorashi S (2008) Anatomical differences in the right and left renal arterial patterns. Folia Morphol (Warsz) 67:104–110
Tenorio ER, Kärkkäinen JM, Marcondes GB, Lima GBB, Mendes BC, DeMartino RR, Macedo TA, Oderich GS (2021) Impact of intentional accessory renal artery coverage on renal outcomes after fenestrated-branched endovascular aortic repair. J Vasc Surg 73:805–818. https://doi.org/10.1016/j.jvs.2020.06.123
Vas D, Moreno Rojas J, Solà Garcia M (2022) Replaced right hepatic artery arising from the distal renal artery, a new variation. Surg Radiol Anat 44:1339–1342. https://doi.org/10.1007/s00276-022-03017-3
VonAchen P, Hamann J, Houghland T, Lesser JR, Wang Y, Caye D, Rosenthal K, Garberich RF, Daniels M, Schwartz RS (2016) Accessory renal arteries: Prevalence in resistant hypertension and an important role in nonresponse to radiofrequency renal denervation. Cardiovasc Revasc Med 17:470–473. https://doi.org/10.1016/j.carrev.2016.07.009
Wang J, Li Y, Xiao N, Duan J, Yang N, Bao J, Li Y, Mi J (2014) Retroperitoneoscopic resection of primary paraganglioma: single-center clinical experience and literature review. J Endourol 28:1345–1351. https://doi.org/10.1089/end.2014.0345
Yeh BM, Coakley FV, Meng MV, Breiman RS, Stoller ML (2004) Precaval right renal arteries: Prevalence and morphologic associations at spiral CT. Radiology 230:429–433. https://doi.org/10.1148/radiol.2302021030
Zorgdrager M, Krikke C, Hofker SH, Leuvenink HG, Pol RA (2016) Multiple renal arteries in kidney transplantation: a systematic review and meta-analysis. Ann Transplant 21:469–478
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
EM: conceptualization, data collection and analysis, original manuscript preparation. VG: Data analysis, work supervision, manuscript review. MN: Material preparation, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Research was conducted on already available patient data obtained for clinical purposes. Informed consent and ethical approval were waived by local ethics due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mihaylova, E., Groudeva, V. & Nedevska, M. Multidetector computed tomography angiography study of the renal arterial vasculature anatomy and its variations in a Bulgarian adult population. Surg Radiol Anat 45, 289–296 (2023). https://doi.org/10.1007/s00276-023-03092-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-023-03092-0