Abstract
Background and purpose
The subcallosal artery [SCA, a branch of the anterior communicating artery (ACoA)] is not well described in the literature. However, the memory disorders that can occur after surgical repair of ruptured ACoA aneurysms might be related to infarction of the SCA. The objective of the present study was to perform a thorough anatomical assessment of the SCA.
Methods
The study was carried out over a 6-month period in a University Hospital’s anatomy laboratory, using brains extracted from human cadavers. The brains were injected with colored neoprene latex and dissected to study the SCA’s origin, path, termination, diameter, length, and vascularized territories.
Results
21 cadaveric specimens were studied. The mean ± standard deviation diameter and length of the SCA were 0.83 ± 0.57 mm and 38.14 ± 25.11 mm, respectively. The predominantly vascularized territories were the paraterminal gyrus (100%), the parolfactory gyrus (78.95%), the rostrum (84.21%) and genu (78.95%) of the corpus callosum, the lamina terminalis (78.95%), the anterior commissure (63.16%), the anterior cingulate gyrus (47.37%), and the fornix (26.32%). When the SCA supplied the fornix and the anterior cingulate gyrus, it was significantly longer and broader (p < 0.05).
Conclusion
Anatomic knowledge of the SCA is crucial—especially for the treatment of ACoA aneurysms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior communicating artery (ACoA) is part of the network of anterior cerebral arteries, and connects to their initial portions. According to Serizawa et al. [17], the ACoA has several types of perforating arteries: chiasmatic arteries, hypothalamic arteries, and the subcallosal artery (SCA). The latter has not been well described in the literature [4, 8,9,10, 17, 18].
The SCA is considered to be the ACoA’s largest perforating branch, and originates from the posterior or posterosuperior part of the ACoA (mainly in the medial and left lateral thirds) [17]. It runs along the cistern of the lamina terminalis, and ends in the right and left subcallous areas—sometimes with bilateral hypothalamic ramifications.
Furthermore, the SCA has proximal branches that supply the lamina terminalis, the anterior commissure, the supraoptic nuclei of the hypothalamus, the septal area [9], and (sometimes) the subcallosal area. The distal branches of the SCA supply the genu and rostrum of the corpus callosum and the ventral portion of the cingulate gyrus [9].
The SCA is sometimes replaced by a larger artery, referred to as the median corpus callosum artery (MCCA) or the callosomarginal artery by some researchers [6, 8, 14]. This artery is longer, bypasses the corpus callosum (while supplying it) and ends in the precuneus region. In rare cases, the SCA is replaced by an azygos anterior cerebral artery (ACA), the latter is a single, post-communicating ACA that gives rises to both the pericallosal and callosomarginal arteries [5, 16].
Anatomical knowledge of variations of the SCA is crucial for the surgical treatment of ACoA aneurysms—the most frequent aneurysms of the encephalon [9]. Recently, Matsushige et al. performed a 7 Tesla MRI angiography study of the SCA [10] in a series of 73 patients. The SCA’s origin, path, and termination were noted. However, the MRI technique could not reliably determine which the territories were vascularized.
The primary objective of the present anatomical study of latex-injected brains from human cadavers was to describe the path of the SCA (from its origin to its termination) and the vascularized territories. The secondary objective was to gather data that might explain the neurological disorders (e.g. memory disorders) resulting from ischemia of the SCA [11, 13, 15, 19].
Methods
The study was conducted in the Anatomy and Organogenesis Laboratory at our Faculty of Medicine from January 2nd to June 29th, 2018. 21 freshly frozen cadavers were studied. The head was removed at the sixth cervical vertebra. The left and right common carotid and vertebral arteries were catheterized, and washed with saline solution to remove post-mortem blood clots. The blood vessels were then perfused with a red neoprene latex suspension. The head was then embalmed in a 10% formalin solution for 3 weeks. After resection of the cranial vault, the encephalon was carefully removed. Dissections were performed using microsurgical instruments and a surgical microscope (ZEISS® OPMI 1 from Carl Zeiss® Co., Oberkochen, Germany), with the magnification ranging from 4 to 40 × . Photographs were taken with a Sony® EOS 1100D camera (Sony Corporation, Tokyo, Japan).
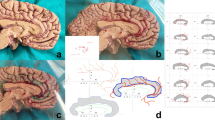
Firstly, the dissection of the encephalon was initiated below the circle of Willis, with exposure of the ACoA. Perforating branches of the ACoA were removed (Fig. 1) and the interhemispheric fissure was dissected to expose the genu and rostrum of the corpus callosum (Fig. 2). The SCA’s origin could be noted.
Inferior view of the interhemispheric fissure: ACoA (1); left post-communicating ACA (2); right precommunicating ACA (3); right post-communicating ACA (4); SCA (5); a perforating branch, vascularizing the lamina terminalis, and rostrum of the corpus callosum (6); the path of the SCA, in front of the genu of the corpus callosum (7)
Next, the encephalon was sectioned in the parasagittal plane, exposing the left hemisphere (Fig. 3) and the SCA’s origin on the ACoA (Fig. 4).
The SCA’s origin, initial diameter, path, termination, total length, and vascularized areas were noted. The ACoA’s length, and the distance between the SCA and the A1A2 angle were also noted. A digital caliper was used to measure diameters and distances.
Pearson’s correlation test was used to assess the correlation between quantitative variables, and student’s test was used for intergroup comparisons of quantitative variables. All tests were two-sided. All statistical analyses were performed with R software (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/).
Results
One of the 21 latex-injected cadaver brains could not been studied, due to poor conservation (specimen 20, Table 1). A second brain could not been studied as a result of excessive diffusion of the latex suspension between the hemisphere (specimen 14). The SCA was located in all cases.
Origin of the SCA
The SCA’s origin was almost always located at the posterior or posterosuperior part of the ACoA (95%), except for specimen 12 (the right post-communicating ACA).
Length of the SCA
The mean ± standard deviation (SD) length of the ACoA was 2.54 ± 1.09 mm, and the mean ± SD distance between the origin of the SCA origin and the left A1A2 angle was 1.26 ± 0.74 mm (Table 1). The main SCA arose from the medial third (53%) or the left lateral third of the ACoA (29%).
Path of the SCA
Seventeen SCAs followed a C-shaped course to the paraterminal gyrus (Fig. 5) and the other three followed a straight course to the parolfactory gyrus (Fig. 6).
Termination of the SCA
The SCA mainly terminated in either the body (36.8%) or the genu of the corpus callosum (36.8%). The remaining SCAs terminated at the rostrum of the corpus callosum (27.4%).
Diameter of the SCA
The mean ± SD diameter of the SCA was 0.83 ± 0.57 mm, and 4 SCAs were over 1 mm in diameter. The mean ± SD length of the SCA was 38.14 ± 25.11 mm, and 4 were longer than 69 mm (Table 1). These two variables were significantly correlated (Pearson’s correlation coefficient: 0.79; p < 0.001).
Vascularized territories
The brain parts vascularized by the SCA are listed in Table 2. The most frequently vascularized parts were the paraterminal and parolfactory gyri, the rostrum, and genu of the corpus callosum, the lamina terminalis, and the anterior commissure. In five cases, we found a perforating SCA branch that vascularized the fornix (Figs. 7, 8).
Subgroups analyses
The results of a subgroup analysis are summarized in Table 3. The diameter of the SCA was significantly greater in the “fornix vascularized” (fornix +), “corpus callosum vascularized,” (CC +) and “anterior cingulate gyrus vascularized” (ACG +) groups than in the “fornix not vascularized” (fornix-), “corpus callosum not vascularized” (CC −) and “anterior cingulate gyrus not vascularized” (ACG-) groups. The SCA was significantly longer in the fornix + , AC + , CC + , and ACG + groups than in the fornix −, AC −, CC −, and ACG-groups.
In specimens 5 and 11 in particular, the SCA had a large diameter and replaced the pericallosal artery.
Discussion
The present study provided a precise anatomic description of the SCA in human brains. In a few cases, we encountered a number of practical difficulties: either the latex diffused throughout the brain or the latex did not diffuse into small perforating branches (see Table 1). According to the literature data [9, 17], the SCA usually originates from the left side of the ACoA. This is why we have chosen to perform a right parasagittal section of the encephalon and thus expose the left hemisphere. This choice prevented us from studying bilaterally vascularized brain structures.
The SCA’s diameter and total length were significantly correlated (Pearson correlation coefficient: 0.79; p < 0.001); we therefore hypothesize that the longer the artery, the greater the number of vascularized brain structures and therefore the greater the diameter.
In some specimens, we observed perforating branches of the SCA artery supplying the fornix (Fig. 7). In 5 of the 19 specimens, the SCA supplied the anterior fornix; these arteries were significantly broader and longer than those not supplying the fornix. Moreover, 5 of the 6 SCA with a diameter greater than 0.7 mm supplied the fornix.
Similarly, the SCAs supplying the anterior commissure, the body of the corpus callosum, and the anterior cingulate gyrus were larger and longer than those that did not (Table 3). Since the fornix and cingulate gyrus belong to the Papez circuit [15], we hypothesize that memory disorders might be due to ischemia of the SCA.
The ACoA phylogenetically appears with the reptiles that have 2 hemispheres supplied by the “2 primitive olfactory arteries”. In humans, classically, the subcallosal artery always arise from the posterior aspect of the ACoA and appears in embryo [20–24 mm, Stage 6 of Paget or stage 20 of Streeter (51 days post-ovulation)]; the anterior commissure appears during the 7th week but the corpus callosum starts its development only from the 10th week (Larsen).
The subcallosal artery (also named precallosal artery for Dunker, anterior median cerebral artery for Lazorthes [7], superior callosal artery for Perlmutter) should be differentiated from the perforators arising from the ACoA area and should probably be considered as a short median artery of the CC (Lazorthes) supplying only the rostrum.
The MCCA is the only artery arising from the posterior aspect of the ACoA [the others perforators are arising from the junction A1-A2-ACoA (opposite of Heubner artery)] or from the origin of A2. The MCCA supplies the genu and body of the CC and can arise not only from ACoA but also from A2, A3, and A4.
We did not measured the width of the ACoA in our study, but we found that specimen with shorter ACoA (less than 2 mm) was associated with an origin of the SCA at the left third of the ACoA (mean ratio 0.28) while long ACoA (more than 2 mm) was associated with an origin of the SCA at the middle third of ACoA (mean ratio 0.54).
When a SCA supplied the genu and body of CC (also called the MCCA), we found that it arose from the medial third (mean ratio of 0.55). But a SCA supplying only the rostrum (specimen 8 and 10) arose from the left third of ACoA (mean ratio 0.25).
A few literature studies of the ACoA have precisely described its perforating branches [9, 17, 18]. However, only one study (an MRI study) has focused on the SCA [10].
Marinkovic et al. [9] provided an anatomical description of the ACoA from 22 human cadavers. The researchers described two types of ACoA branches: short branches (diameter < 0.15 mm) supplying the lamina terminalis and the hypothalamus, and long branches (the SCA in 91% of cases; mean diameter: 0.486 mm and the MCCA 9% of cases, long and broader). The SCA arose from the superior lateral part of the ACoA and ended at the rostral part of the CC.
Türe et al. studied the vascularisation of the corpus callosum [18]. The researchers described three branches of the ACoA: hypothalamic branches (in 20% of cases), the SCA (50%), and the MCCA (30%). The SCA supplied the median parts of the rostrum and genu of the corpus callosum, the hypothalamus, the anterior cingulate gyrus, and the septum pellucidum. The SCA’s mean diameter of 0.5 mm was lower than that of the MCCA (0.9 mm). The MCCA supplied the body and splenium of the corpus callosum more frequently. We consider that these arteries are quite similar to the largest SCAs observed in the present study. However, the mean diameter in our study was much greater (1.59 mm). Furthermore, Türe et al. did not observe a branch that directly supplied the fornix.
In 1997, Serizawa et al. [17] described the anatomy of the ACoA in 30 human cadaver brains. The researchers also observed chiasmatic (small-diameter), hypothalamic and subcallosal (large-diameter) branches. The latter supplied the rostrum and genu of the corpus callosum, the anterior commissure, the anterior cingulate gyrus, the parolfactory, and paraterminal gyri, the septum pellucidum, the anterior column of fornix, and a limbic area. The SCA was described as a single, large perforating branch of the ACoA in 27 of the 30 cases, and the mean diameter was 0.50 mm. In most cases, the SCA’s origin was located in the posterosuperior or superior part of the ACoA. In three cases, the SCA was replaced by an MCCA or an azygos ACA. According to Serizawa et al., the MCCA is an SCA that ends beyond the genu of the corpus callosum and supplies the dorsal part of cingulate gyrus up to the paracentral lobule of both hemispheres. However, Serizawa et al. did not give a precise description of the branch that supplied the fornix. Lastly, Serizawa et al. suggested that memory disorders after ACoA aneurysm surgery were potentially linked to the presence of an SCA.
More recently, Matsushige et al. [10]. performed a 7 Tesla MRI study of the SCA in 75 patients. The SCA was defined as the only vessel coming from the ACoA and that never ended further than at the genu of the corpus callosum. If it went further, it was considered to be an accessory ACA or an MCCA. In all, 93.7% of the patients presented a SCA. The researchers also described three types of path: a “C-shape” (55.9%), a “S-shape” (27.2%) and a straight path (16.9%). The mean diameter and length were, respectively, 0.49 mm and 15.8 mm, and the two were linearly correlated. Furthermore, the “C-shaped” SCA gave rise to branches that supplied the lamina terminalis, paraterminal gyrus, anterior commissure, fornix, anterior cingulate gyrus, and rostrum and genu of the corpus callosum. We found similar results here; of the three patients with a “straight” SCA, none presented a branch supplying the fornix. In contrast, all SCAs with a branch supplying the fornix were “C-shaped”.
These anatomical findings may be of direct value for the treatment of ACoA aneurysms in general and for surgery in particular. Indeed, a complete preoperative assessment (an angio-CT scan, angio-MRI, and an angiogram) of the branches of the ACoA might reveal the SCA’s shape (straight, “S-shaped” or “C-shaped”), diameter, and even length. These variables might be predictive of the risk of post-treatment memory disorders. The therapeutic strategy could therefore be chosen according to the presence or absence of a large SCA (exclusion surgery versus endovascular treatment).
Some researchers [1,2,3, 12] have linked the occurrence of neuropsychological symptoms to the surgical treatment of ACoA aneurysms. In 1982, Gade [3] reported that 15 out of 48 patients having undergone surgical aneurysm repair presented with amnesic syndrome and a severe, isolated impairment of episodic memory. These conditions had not significantly improved 2 years later. Gade also noted that most of the patients presented an impairment of abstract reasoning and a lack of initiative [3].
More recently, Böttger et al. study [1] of 30 patients found that neurocognitive disorders (attention, memory, learning, and planning disorders) were frequent 5 months after surgery. Orientation disorders, confabulations, anosognosia, apathy, and mood disorders were much less prevalent. Böttger et al. were able to constitute several subgroups as a function of the symptoms. However, in view of the subgroups’ heterogeneity, the researchers decided that “ACoA syndrome” per se could not be defined.
Lastly, Mortimer et al. [12] compared patients having under surgical repair (clipping) of an ACoA aneurysm with those treated via an endovascular method (coiling). The researchers observed significantly more cerebral ischemic lesions in the “clipping” group than in the “coiling” group; these lesions were attributed to the ACoA’s perforating arteries (the recurrent artery of Heubner and the SCA).
Conclusion
We performed a detailed morphological and anatomical study of the SCA (the main perforating branch of the ACoA) in human cadavers. We recorded the SCA’s origin, path, termination, diameter, length, and vascularized territories. Our present results suggest that SCA branches vascularizing the fornix and anterior cingulate gyrus are longer and have a larger diameter. Thus, ischemia of the SCA (during the surgical or endovascular treatment of ACoA aneurysms, for example) might be more likely to result in memory disorders—especially if for large-diameter arteries. A prospective radiological study (based on pretreatment angio-CT scans and/or cerebral angiograms of patients with ACoA aneurysms) are warranted with a view to predict the post-treatment memory disorders.
References
Böttger S, Prosiegel M, Steiger HJ, Yassouridis A (1998) Neurobehavioural disturbances, rehabilitation outcome, and lesion site in patients after rupture and repair of anterior communicating artery aneurysm. J Neurol Neurosurg Psychiatry 65:93–102
DeLuca J, Diamond BJ (1995) Aneurysm of the anterior communicating artery: a review of neuroanatomical and neuropsychological sequelae. J Clin Exp Neuropsychol 17:100–121. https://doi.org/10.1080/13803399508406586
Gade A (1982) Amnesia after operations on aneurysms of the anterior communicating artery. Surg Neurol 18:46–49
Jackowski Meneses, Ramina Marrone, Stefani Aquini, Winkelmann Schneider (1999) Perforating and leptomeningeal branches of the anterior communicating artery: an anatomical review. Crit Rev Neurosurg 9:287–294
Kakou M, Velut S, Destrieux C (1998) Arterial and venous vascularization of the corpus callosum. Neurochirurgie 44:31–37
Kannath SK, Malik V, Rajan JE (2017) Isolated subcallosal artery infarction secondary to localized cerebral vasospasm of anterior communicating artery complex following subarachnoid hemorrhage. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.07.052
Lazorthes G, Gouazé A, Salamon G (1976) Vascularisation et circulation de l’encéphale. Anatomie descriptive et fonctionnelle, vol 1. Paris, Masson, pp 101–113
Marinković S, Gibo H, Milisavljević M (1996) The surgical anatomy of the relationships between the perforating and the leptomeningeal arteries. Neurosurgery 39:72–83
Marinković S, Milisavljević M, Marinković Z (1990) Branches of the anterior communicating artery. Microsurgical anatomy. Acta Neurochir (Wien) 106:78–85
Matsushige T, Chen B, Dammann P, Johst S, Quick HH, Ladd ME, Forsting M, Sure U, Wrede KH (2016) Microanatomy of the subcallosal artery: an in vivo 7 T magnetic resonance angiography study. Eur Radiol 26:2908–2914. https://doi.org/10.1007/s00330-015-4117-1
Meila D, Saliou G, Krings T (2015) Subcallosal artery stroke: infarction of the fornix and the genu of the corpus callosum. The importance of the anterior communicating artery complex. Case series and review of the literature. Neuroradiology 57:41–47. https://doi.org/10.1007/s00234-014-1438-8
Mortimer AM, Steinfort B, Faulder K, Erho T, Scherman DB, Rao PJ, Harrington T (2016) Rates of local procedural-related structural injury following clipping or coiling of anterior communicating artery aneurysms. J Neurointerv Surg 8:256–264. https://doi.org/10.1136/neurintsurg-2014-011620
Moussouttas M, Giacino J, Papamitsakis N (2005) Amnestic syndrome of the subcallosal artery: a novel infarct syndrome. Cerebrovasc Dis 19:410–414. https://doi.org/10.1159/000086104
Mugikura S, Kikuchi H, Fujii T, Murata T, Takase K, Mori E, Marinković S, Takahashi S (2014) MR imaging of subcallosal artery infarct causing amnesia after surgery for anterior communicating artery aneurysm. AJNR Am J Neuroradiol 35:2293–2301. https://doi.org/10.3174/ajnr.A4057
Onate Miranda M, Alba Suarez EM, Frutos R, Escribano Vera J, Palomo Ferrer F, Alvarez-Linera Prado J (2015) Amnestic syndrome of the subcallosal artery with additional penetrating vessel involvement. J Neurol Sci 359:438–439. https://doi.org/10.1016/j.jns.2015.10.014
Parmar H, Sitoh YY, Hui F (2005) Normal variants of the intracranial circulation demonstrated by MR angiography at 3T. Eur J Radiol 56:220–228. https://doi.org/10.1016/j.ejrad.2005.05.005
Serizawa T, Saeki N, Yamaura A (1997) Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery 40:1211–1216 (discussion 1216–1218)
Türe U, Yaşargil MG, Krisht AF (1996) The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery 39:1075–1084 (discussion 1084–1085)
Turine G, Gille M, Druart C, Rommel D, Rutgers MP (2016) Bilateral anterior fornix infarction: the “amnestic syndrome of the subcallosal artery”. Acta Neurol Belg 116:371–373. https://doi.org/10.1007/s13760-015-0553-6
Author information
Authors and Affiliations
Contributions
Protocol/project development: LC, AK, PF, EH, JP. Data collection or management: LC, JP. Data analysis: LC, JP. Manuscript writing: LC.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chenin, L., Kaoudi, A., Foulon, P. et al. Microsurgical anatomy of the subcallosal artery. Surg Radiol Anat 41, 1037–1044 (2019). https://doi.org/10.1007/s00276-019-02279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-019-02279-8