Abstract
Purpose
The purpose of this study was to perform biomechanical testing of annular ligament (AL) reconstruction using the superficial head of the brachialis tendon (SHBT) as a distally based tendon graft. We hypothesized that posterior translation of the radial head following AL reconstruction with an SHBT graft does not significantly differ from intact specimens.
Methods
Six fresh-frozen elbow specimens were used. The stability of the radial head against posterior translation forces (30 N) was evaluated in 0°, 45°, 90° and 120° of elbow flexion. Posterior translation was obtained for the intact AL, the sectioned AL and the reconstructed AL. Cyclic loading (100 cycles) in 90° of elbow flexion was performed for the intact and the reconstructed AL.
Results
Posterior translation of the radial head decreased during elbow flexion in native specimens. Sectioning of the AL significantly increased instability over the full range of motion. AL reconstruction with the SHBT restored the stability of the proximal radius but—other than the native AL—was not influenced by elbow flexion. In 120° of flexion the native AL provided significantly more stability when compared to the reconstructed AL. Cyclic loading did not provide significant differences between native and reconstructed specimens.
Conclusions
We provide a feasible technique for AL reconstruction using the SHBT. The biomechanical results obtained in this study confirm the efficacy of the procedure. AL reconstruction restores the stability of the proximal radius, yet it cannot fully mimic the complex features of the intact AL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
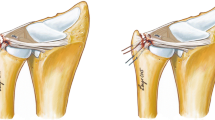
Chronic dislocation of the radial head represents a possible complication following missed or malunited Monteggia fractures [3, 27]. Depending on the angular deformity of the ulna, the radial head dislocates either anteriorly, posteriorly or laterally [1, 23]. Corrective osteotomy of the ulna is considered the mainstay of treatment to reduce the proximal radioulnar joint (PRUJ) and the radiocapitellar joint [3, 27]. Aside from osseous alignment, the interosseous membrane and the annular ligament (AL) represent important soft tissue structures regarding the integrity of the PRUJ [4, 9, 31]. The AL and the sigmoid notch of the ulna form a circle around the radial head. They act in unison to stabilize the radial head within the PRUJ during forearm rotation [21, 22].
Chronic instability of the proximal radius can cause AL insufficiency [1–3]. In such cases, AL reconstruction can be performed. Different techniques for AL reconstruction have been described using the triceps tendon or the extensor fascia [3, 8, 17, 28]. Recently, Nwoko et al. [24] described the tendon of the superficial head of the brachialis (SHBT) as a distally based graft source. The length of the SHBT was found to be sufficient for AL reconstruction [24]. The SHBT insertion distal to the coronoid could facilitate AL reconstruction as only posterior graft fixation is required. Biomechanical evaluation and description of a surgical technique are missing thus far.
The purpose of this study was to describe a surgical technique and perform biomechanical evaluation of AL reconstruction using the SHBT. We hypothesized that posterior translation of the radial head following AL reconstruction with an SHBT graft does not significantly differ from intact specimens.
Materials and methods
Specimen data
Six fresh-frozen human cadaveric specimens from six body donors were available from the university’s department of anatomy. Mean age of donors at the time of death was 77.7 years (±10.3, range: 66–89 years). Four of the donors were males and two were females. Three left-sided and three right-sided specimens were available. All specimens were stored at −20° C and were thawed at room temperature for ~24 h prior to dissection. Each of the specimens was examined clinically and by fluoroscopy. Thereby, full range of motion could be assured and specimens with deformation related to osteoarthritis or trauma could be excluded. The study was approved by the university’s ethics committee and conformed to the Declaration of Helsinki.
Testing set-up and sequence
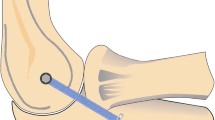
The soft tissue around the proximal humerus was removed. The specimens were then fixed to a customized hinged testing rig. The ulna was secured to the horizontal part of the testing rig with two mounting bolts. The humeral shaft was secured to the vertical part using two mounting clamps. The motion axis of the elbow was identified via X-ray. A bolt was inserted into the proximal radius 5 cm distal to the motion axis. A wire connected the bolt to the mobile traverse of the servohydraulic testing machine (model 5565, Instron, Norwood, MA, USA, maximum capacity: 5 kN). The wire was deflected through two reels and was aligned perpendicular to the horizontally placed forearm at each flexion angle. As a result, upward movement of the traverse resulted in posterior translation forces to the radius (Fig. 1). By temporary arthrodesis, specimens were secured in pronation and supination, respectively. Arthrodesis was performed at the distal radioulnar joint with two 2.0 mm K-wires. This had to be done to avoid forearm rotation when applying load to the specimens.
Testing set-up at 90° of flexion—the ulna was fixed to the horizontal part of the testing rig, and the humerus was fixed to the vertical part. Temporary arthrodesis was performed at the distal radioulnar joint using two K-wires. A synthetic wire connected the mobile traverse of the testing machine to the mounting bolt at the proximal radius. Upward movement of the traverse resulted in posterior translation forces to the proximal radius
Posterior translation of the radial head was measured (upward movement of the mobile traverse; measurement accuracy: ±0.05% of displacement; ±0.015 mm test–retest reliability) for (1) the intact AL, (2) the sectioned AL and (3) the reconstructed AL with the SHBT. A maximum load of 30 N was applied in 0°, 45°, 90° and 120° of flexion for both pronation and supination [10, 14]. The crosshead speed was 1 mm/s. Additionally to single loading, cyclic loading with 100 cycles from 0 to 30 N was performed (crosshead speed 1 mm/s) in supination for the intact and the reconstructed AL. The posterolateral translation at 30 N after 100 cycles was evaluated and compared to the results at 90° prior to cyclic loading. An increase of posterolateral translation of more than 20% was considered as failure. During dissection and biomechanical testing, the specimens were kept moist by irrigation with normal saline solution.
Surgical technique
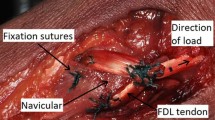
A longitudinal skin incision was performed ~3 cm lateral to the midline. The distal biceps tendon was identified by blunt preparation. Lateral to it, the radial nerve was found and protected. Underneath the biceps tendon, the lateral aspect of the SHBT was identified. We carefully harvested the graft from distal to proximal by blunt separation of the muscle fibers from the tendon (Fig. 2a, b). The median nerve and the brachial artery lie at the medial aspect of the brachialis muscle. They should be identified prior to harvesting the graft to avoid neurovascular complications (Fig. 2c).
Surgical technique and anatomical features. a Longitudinal incision 3 cm lateral of the anterior midline of the elbow. The tendon of the superficial head of the brachialis muscle (SHBT) is found underneath the distal biceps tendon (BT). b The SHBT graft is harvested. The radial nerve (RN) is identified to avoid nerve transection. BR Brachialis muscle. c At the medial aspect of the BR the median nerve (MN) and the brachial artery (BA) can be identified. d The tendon graft is passed underneath the radial nerve and around the radial head (RH). CAP capitulum. e Through Kocher’s interval, graft fixation is performed at the supinator crest of the ulna using a BicepsButton
The skin incision was now shifted laterally by creating full thickness flaps. The radial head was approached through Kocher’s interval. The SHBT was passed around the radial neck underneath the radial nerve (Fig. 2d). A 4.0-mm drill hole was applied at the supinator crest. A second drill hole was established 1.0 cm distal to the first one. A modified Krakow suture was performed at the end of the tendon graft using a #2 FiberWire (Arthrex Inc., Naples, FL, USA). The FiberWire was passed through the proximal drill hole with the graft. The suture then exited the distal drill hole. Graft fixation was then performed over the distal drill hole using a BicepsButton (Arthrex Inc., Naples, FL, USA). The graft was tightened to 10 N (Fig. 2e). The soft tissue—especially the extensor fascia—was carefully re-adapted after sectioning and reconstruction of the AL.
Statistical analysis
Mean and standard deviation of measured values for the native AL, the sectioned AL and the reconstructed AL were calculated. Standard distribution of the data was assured using a Levene test. A one-sided analysis of variance (ANOVA) with a post hoc Scheffé test was performed to evaluate significant differences of the study groups. The level of significance was set to p < 0.05. Correlation between elbow flexion and posterior translation was evaluated using Pearson’s correlation coefficient (r). 95% confidence intervals (CI) were obtained to assess significance of correlation. The software SPSS 23 (IBM Inc., Armonk, NY, USA) was used for statistical analysis.
Results
Pronation and supination did not significantly influence either of the test results (0.548 ≤ p ≤ 0.902). In the following, results are only reported for the supinated forearm. None of the native or reconstructed AL failed during single or cyclic loading.
In full extension, posterior translation of the native specimens was 13.5 mm (±1.4 mm, range: 12.1–15.7 mm). Translation increased to 23.2 mm (±3.5 mm, range: 18.5–27.0 mm) after sectioning of the AL. Reconstruction of the AL restored stability (13.1 ± 2.4 mm, range: 9.5–16.0 mm). Values for native and reconstructed specimens did not show significant differences (p = 0.729). Sectioning of the AL lead to a significant increase of radial head translation (0.0003 ≤ p ≤ 0.0006).
Native specimens showed posterior translation of 12.8 mm (±1.6 mm, range: 11.3–15.8 mm) in 45° of flexion. After sectioning of the AL, translation increased to 24.3 mm (±2.5 mm, range: 22.1–27.6 mm). Once again, reconstruction restored radial head stability with a mean posterior translation of 12.6 mm (±2.0 mm, range: 10.1–14.9 mm). Native and reconstructed AL showed equal results (p = 0.803). Radial head translation increased significantly after AL sectioning (0.00001 ≤ p ≤ 0.0002).
Similarly, native and reconstructed specimens provided comparable results in 90° of flexion (native AL: 11.8 ± 1.4 mm, 10.6–14.0 mm; reconstructed AL: 12.8 ± 1.8 mm, 10.4–15.2 mm; p = 0.308). After sectioning of the AL, posterior translation increased significantly (24.6 ± 3.4 mm, range: 19.0–27.6 mm; 0.00005 ≤ p ≤ 0.0003).
In 120° of flexion, posterior translation was significantly less pronounced in native specimens when compared to reconstructed specimens (native AL: 9.5 ± 2.0 mm, 7.0–13.1 mm; reconstructed AL: 12.9 ± 1.5 mm, 11.1–14.9 mm; p = 0.01). Sectioning of the AL resulted in significantly decreased radial head stability (24.3 ± 2.5 mm, range: 21.3–27.2 mm; 0.00001 ≤ p ≤ 0.0004).
Cyclic loading in 90° of flexion and supination did not provide significant differences (p = 0.632) between the native AL (12.1 ± 1.5 mm, range: 10.7–14.6 mm) and the reconstructed AL (13.6 ± 2.1 mm, range: 10.5–16.0 mm).
The flexion angle of the elbow inversely correlates with posterior translation in native specimens (r = −0.939; 95% CI: −0.993; −0.536). After reconstruction of the AL though, these values did no longer correlate (r = −0.245; 95% CI: −0.880; +0.706).
Results of biomechanical testing are depicted in Table 1 and Fig. 3.
Posterior translation of the radial head following translation forces of 30 N were applied. Asterisk: statistical significance (p < 0.05). The elbow flexion angle correlated inversely with posterior translation in intact specimens specimens (r = −0.939; 95% CI: −0.993; −0.536) but did no longer correlate following AL reconstruction (r = −0.245; 95% CI: −0.880; +0.706)
Discussion
This study aimed to analyze the biomechanical properties of AL reconstruction using an SHBT graft. We hypothesized that posterior translation of the radial head following AL reconstruction with an SHBT graft does not significantly differ from intact specimens. Our study yielded results that support the hypothesis for 0°–90° of elbow flexion. Sectioning of the AL led to a significant increase of posterior translation of the radial head. Stability was restored ensuing AL reconstruction. The tendon graft effectively withstood both single and cyclic loading. However, the flexion angle of the elbow did not influence the posterior translation of the radial head after AL reconstruction. In native specimens, an increase of the flexion angle resulted in a less pronounced posterior translation though. As a result, the intact specimens were significantly more stable in 120° of flexion when compared to AL reconstruction with a mean difference of posterior translation of 3.4 mm (Table 2).
This can be attributed to the anatomical features of the AL [18]. At its anterior aspect, it coalesces with the radial collateral ligament (RCL). Posteriorly, the lateral ulnar collateral ligament (LUCL) inserts into the AL. Together, the AL, the RCL and the LUCL form the Y-shaped lateral collateral ligament (LCL) [29]. The LCL complex acts as an important stabilizer of the radial column [9]. It neutralizes varus as well as rotational forces and represents a primary constraint against posterolateral rotatory instability (PLRI) [6]. Dunning et al. [6] and McAdams et al. [19] found that PLRI only occurs in case of tears of both the RCL and the LUCL if the AL is intact. This emphasizes the importance of an intact AL regarding the kinematics of the elbow joint. The anatomic studies of Wavreille et al. [30] as well as Moritomo et al. [20] showed that the LUCL lengthens during flexion of the elbow. As the LUCL coalesces with the AL at its insertion [5], elbow flexion leads to tightening of both the LUCL and the AL. This could explain why we found a significant decrease of the posterior translation in native specimens during flexion of the elbow. The reconstructed AL does not have a connection to the LCL complex and, therefore, it is not influenced by the flexion angle. Hence, AL reconstruction with a tendon graft can restore the stability of the PRUJ, yet it fails to fully mimic the complex features of the intact AL.
The anatomy of the brachialis muscle has been described to great detail by Leonello et al. [16]. The authors described that the muscle possesses a deep and a superficial head. The latter was described to terminate in a thick tendon, which could make it a useful donor for ligament reconstruction around the elbow [16]. Nwoko et al. [24] picked up on that idea and performed an anatomic study to evaluate the feasibility of AL reconstruction with the SHBT. In all their 24 cadaveric specimens, they found the SHBT to be sufficient in length for reconstruction of the AL. The average excess length of the tendon was 12 mm and ranged from 4 to 22 mm [24]. The SHBT averaged 81 and 87 mm of length, respectively [16, 24].
Our results support the findings of Leonello et al. [16] and Nwoko et al. [24]. The SHBT was reliably found to be a thick, circular tendon. The length of the tendon graft sufficed to perform fixation at the supinator crest of the ulna in all of our six specimens. Moreover, we were able to describe a surgical technique. We recommend the incision to be placed about 3 cm lateral to the midline of the anterior aspect of the elbow. Thereby, harvesting and fixation of the graft can be performed through a single incision. When harvesting the graft, great care has to be taken to spare the surrounding neurovascular structures. The brachialis muscle can receive motor fibers from the musculocutaneous as well as the radial and median nerve [26, 32]. The radial nerve always has to be identified since the tendon graft needs to be passed around the radial neck underneath the nerve and the forearm extensors. Placing the graft above the nerve by mistake would cause compression with possible subsequent radial nerve palsy. While the native AL courses around the radial head, the SHBT surrounds the radial neck as its insertion at the coronoid process is located distal to the AL. Graft fixation was, therefore, performed at the level or slightly distal to the supinator crest of the ulna.
Using the SHBT for AL reconstruction is convenient since the distally based graft only has to be fixed posterior to the radial head. Other grafts require fixation on both sides [3, 8, 17, 28]. On the other hand, sacrificing the SHBT could potentially infringe with the kinematics of the elbow joint. The brachialis muscle acts as an active stabilizer of the elbow and is important for elbow flexion. Even though the deep head of the brachialis remains intact in the described technique, flexion strength could decrease as a result of the surgery [16].
Malalignment of the ulna following a Monteggia fracture results in chronic (sub)luxation of the radial head [27]. To reduce the radial column, osteotomy of the ulna has to be performed to realign the forearm. Both the angulation and the length of the ulna have to be addressed to adequately restore the anatomy of the PRUJ and the radiocapitellar joint [7]. Most techniques for osteotomy of the ulna are performed by plate or intramedullary fixation [13, 25, 27]. Recently, Rajasekaran et al. [25] described a new technique for ulnar fixation with a single screw. The authors used a Z-shaped osteotomy, which allowed them to restore both the angulation and the length of the ulna [25].
Osteotomy of the proximal ulna is a widely agreed principle to treat chronic Monteggia lesions. Controversy remains though regarding the necessity of reconstruction of the AL [2]. Sandman et al. [27] evaluated the influence of ulnar angulation and AL integrity on subluxation of the radial head. The results of their biomechanical study show that an intact AL enhances the alignment of the radial head to a significant extent. The authors conclude that correcting the angulation of the ulna does not suffice to anatomically restore the biomechanics of the elbow. Hence, they recommend reconstructing the AL if insufficiency is present [27].
This study is limited by its in vitro design and by its limited sample size of specimens with advanced age. In a post hoc power analysis, the power (ß-1) of our results comparing the intact specimens and AL reconstructions at 0°–90° of elbow flexion was found to be fairly low (0.071–0.260). Hence, a higher sample size might have detected slight differences between the study groups. Proximal instability of the radial head does not only affect posterior translation but can also occur in every direction. We chose testing the posterior translation as Bado type 2 fractures represent the most common Monteggia (like) lesions in adults with a posterior dislocation of the radial head. Hence, this might represent the most relevant direction of dislocation in clinical practice [15]. In addition to that, we did not apply failure loads to be able to perform testing of each specimen over the full range of motion. Thereby, direct comparison of the results for the intact, the sectioned and the reconstructed AL of each specimen was possible. While failure loads would generally be of interest, they would have caused additional soft tissue injuries causing a bias regarding the comparability of the study groups (intact specimens, sectioned AL, reconstructed AL). The in vitro design of the study neglects the effect of active stabilizers. Therefore, the stabilizing influence of the surrounding forearm muscles could not be evaluated. As mentioned by Nwoko et al. [24], harvesting of the tendon graft could lead to heterotopic ossification and subsequent joint stiffness. Moreover, posttraumatic scarring could complicate the graft harvest. This could increase the risk of neurologic as well as vascular complications [11, 12].
While certain limitations apply to our study, we provide a feasible technique for AL reconstruction with the SHBT. The biomechanical results confirm that AL reconstruction with a SHBT graft can counteract posterior translation forces of the proximal radius from 0° to 90° of elbow flexion. Above 90° of elbow flexion, however, it provides less stability when compared to intact specimens. Future studies should consider the clinical results of this technique to evaluate its applicability in practice.
References
Bado JL (1967) The Monteggia lesion. Clin Orthop Relat Res 50:71–86
Bhaskar A (2009) Missed Monteggia fracture in children: is annular ligament reconstruction always required? Indian J Orthop 43:389–395. doi:10.4103/0019-5413.55978
Boyd HB, Boals JC (1969) The Monteggia lesion. A review of 159 cases. Clin Orthop Relat Res 66:94–100
Bozkurt M, Acar HI, Apaydin N et al (2005) The annular ligament: an anatomical study. Am J Sports Med 33:114–118
Cohen MS, Hastings H 2nd (1997) Rotatory instability of the elbow. The anatomy and role of the lateral stabilizers. J Bone Jt Surg Am 79:225–233
Dunning CE, Zarzour ZD, Patterson SD et al (2001) Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Jt Surg Am 83-A:1823–1828
Francisco FF, Langendörfer M, Wirth T et al (2014) Korrektur von veralteten Monteggia-Verletzungen im Kindes-und Jugendalter. Obere Extremität 9:178–185. doi:10.1007/s11678-014-0266-0
Gyr BM, Stevens PM, Smith JT (2004) Chronic Monteggia fractures in children: outcome after treatment with the Bell-Tawse procedure. J Pediatr Orthop B 13:402–406
Hackl M, Bercher M, Wegmann K et al (2016) Functional anatomy of the lateral collateral ligament of the elbow. Arch Orthop Trauma Surg 136:1031–1037. doi:10.1007/s00402-016-2479-8
Hackl M, Heinze N, Wegmann K et al (2016) The circumferential graft technique for treatment of multidirectional elbow instability: a comparative biomechanical evaluation. J Shoulder Elb Surg 25:127–135. doi:10.1016/j.jse.2015.07.016
Hackl M, Lappen S, Burkhart KJ et al (2015) Elbow positioning and joint insufflation substantially influence median and radial nerve locations. Clin Orthop Relat Res 473:3627–3634. doi:10.1007/s11999-015-4442-3
Hackl M, Wegmann K, Lappen S et al (2015) The course of the posterior interosseous nerve in relation to the proximal radius: is there a reliable landmark? Injury 46:687–692. doi:10.1016/j.injury.2015.01.028
Hirayama T, Takemitsu Y, Yagihara K et al (1987) Operation for chronic dislocation of the radial head in children. Reduction by osteotomy of the ulna. J Bone Jt Surg Br 69:639–642
King GJ, Dunning CE, Zarzour ZD et al (2002) Single-strand reconstruction of the lateral ulnar collateral ligament restores varus and posterolateral rotatory stability of the elbow. J Shoulder Elb Surg 11:60–64. doi:10.1067/mse.2002.118483
Konrad GG, Kundel K, Kreuz PC et al (2007) Monteggia fractures in adults: long-term results and prognostic factors. J Bone Jt Surg Br 89:354–360. doi:10.1302/0301-620X.89B3.18199
Leonello DT, Galley IJ, Bain GI et al (2007) Brachialis muscle anatomy. A study in cadavers. J Bone Jt Surg Am 89:1293–1297. doi:10.2106/JBJS.F.00343
Lloyd-Roberts GC, Bucknill TM (1977) Anterior dislocation of the radial head in children: aetiology, natural history and management. J Bone Jt Surg Br 59-B:402–407
Mak S, Beltran LS, Bencardino J et al (2014) MRI of the annular ligament of the elbow: review of anatomic considerations and pathologic findings in patients with posterolateral elbow instability. AJR Am J Roentgenol 203:1272–1279. doi:10.2214/AJR.13.12263
McAdams TR, Masters GW, Srivastava S (2005) The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elb Surg 14:298–301. doi:10.1016/j.jse.2004.08.003
Moritomo H, Murase T, Arimitsu S et al (2007) The in vivo isometric point of the lateral ligament of the elbow. J Bone Jt Surg Am 89:2011–2017. doi:10.2106/JBJS.F.00868
Morrey BF, An KN (1983) Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med 11:315–319
Morrey BF, An KN (1985) Functional anatomy of the ligaments of the elbow. Clin Orthop Relat Res 201:84–90
Nijs S, Hackl M, Devriendt S (2014) Proximal ulna fractures. Obere Extremität 9:192–196. doi:10.1007/s11678-014-0286-9
Nwoko OE, Patel PP, Richard MJ et al (2013) Annular ligament reconstruction using the distal tendon of the superficial head of the brachialis muscle: an anatomical feasibility study. J Hand Surg Am 38:1315–1319. doi:10.1016/j.jhsa.2013.04.008
Rajasekaran S, Venkatadass K (2014) “Sliding angulation osteotomy”: preliminary report of a novel technique of treatment for chronic radial head dislocation following missed Monteggia injuries. Int Orthop 38:2519–2524. doi:10.1007/s00264-014-2514-8
Sanal HT, Chen L, Negrao P et al (2009) Distal attachment of the brachialis muscle: anatomic and MRI study in cadavers. AJR Am J Roentgenol 192:468–472. doi:10.2214/AJR.08.1150
Sandman E, Canet F, Petit Y et al (2014) Radial head subluxation following malalignment of the proximal ulna: a biomechanical study. J Orthop Trauma 28:464–469. doi:10.1097/BOT.0000000000000058
Seel MJ, Peterson HA (1999) Management of chronic posttraumatic radial head dislocation in children. J Pediatr Orthop 19:306–312
Seki A, Olsen BS, Jensen SL et al (2002) Functional anatomy of the lateral collateral ligament complex of the elbow: configuration of Y and its role. J Shoulder Elb Surg 11:53–59. doi:10.1067/mse.2002.119389
Wavreille G, Seraphin J, Chantelot C et al (2008) Ligament fibre recruitment of the elbow joint during gravity-loaded passive motion: an experimental study. Clin Biomech (Bristol, Avon) 23:193–202. doi:10.1016/j.clinbiomech.2007.09.014
Weiss AP, Hastings H 2nd (1992) The anatomy of the proximal radioulnar joint. J Shoulder Elb Surg 1:193–199. doi:10.1016/1058-2746(92)90013-S
Won SY, Cho YH, Choi YJ et al (2015) Intramuscular innervation patterns of the brachialis muscle. Clin Anat 28:123–127. doi:10.1002/ca.22387
Acknowledgements
We would like to thank A. Rowlin (AVMZ, University of Magdeburg) for the schematic drawings presented in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
All named authors hereby declare that they have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Hackl, M., Wegmann, K., Ries, C. et al. Annular ligament reconstruction with the superficial head of the brachialis: surgical technique and biomechanical evaluation. Surg Radiol Anat 39, 585–591 (2017). https://doi.org/10.1007/s00276-016-1774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-016-1774-y