Abstract
Background and importance
Aneurysms of the anterior choroidal artery (AChoA) are rare and often difficult to treat. Variations may be present and must be identified prior to treatment. We report a unique case of a ruptured aneurysm located at the origin of a duplicate branch of the AChoA.
Clinical presentation
A 56-year-old male was admitted to our university hospital for coma. A brain CT scan showed a subarachnoid hemorrhage, and CT angiography revealed a duplication of the right AChoA, with an aneurysm located at the branch’s origin. We decided to embolize this aneurysm. Four weeks later, our patient was able to transfer to the rehabilitation unit.
Conclusion
To the best of our knowledge, this is one of the first descriptions of an aneurysm located at the origin of a duplicate branch of the AChoA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior choroidal artery (AChoA) feeds a number of important structures in the brain. Infarction of the AChoA is often associated with proportional contralateral hemiplegia and hemianesthesia, and homonymous hemianopia (due to damage to the posterior limb of the internal capsule).
Aneurysms of the AChoA are quite rare and account for just 4 % of all intracranial aneurysms. According to Rhoton’s classification, aneurysms of the AChoA can appear either in the cisternal or plexal (intraventricular) segments [10].
Anatomic variations of the AChoA have already been described [1, 3, 5, 6, 8], the most common of which is an ectopic origin. Duplication of the origin is very rare [1, 3, 6].
We report a unique case of an aneurysm located at the origin of a duplicate branch of the right AChoA in a patient with subarachnoid hemorrhage. Embolization of the aneurysm led to a satisfactory outcome.
Case report
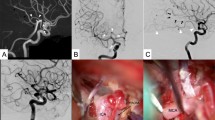
A 56-year-old male with a history of untreated hypertension and active smoking had been found unconscious in the street. The initial Glasgow Coma Scale was 4. Computed tomography (CT) showed subarachnoid hemorrhage in both left and right Sylvian fissures, in the ambient cistern and within the fourth ventricle (Fischer IV). CT angiography of the brain revealed a 6 × 5 mm aneurysm within the cisternal segment of the right AChoA. Conventional angiography was performed within 48 h of the hemorrhage. After a thorough inspection of the final portion of the right internal carotid artery, we noticed a duplication of the right AChoA. In fact, the aneurysm was located at the origin of the superior branch of the AChoA (Figs. 1, 2).
A three-dimensional reconstruction of the brain angiogram, showing a posterior view of the termination of the right ICA (3) and the carotid bifurcation with the right anterior cerebral artery (6), the right middle cerebral artery (5), duplication of the right AChoA (1 inferior branch, 2 superior branch) and the aneurysm (4)
After microcatheterization of the aneurysm, coil embolization was used to obtain complete occlusion (Fig. 3).
The patient came out of the coma a few days later. He displayed temporospatial disorientation and agitation. A neurological examination revealed dyspraxia of the left arm, left homonymous hemianopia and memory disturbances but no sensory or motor impairments. He also presented with Terson syndrome. Neither ischemic lesions nor hydrocephalus were seen on a post-embolization CT scan. Four weeks later, the patient was admitted to a rehabilitation unit.
Discussion
In embryonic development, the AChoA emerges from the anterior circulation and the internal carotid artery (ICA). According to Lasjaunias [9], the origin of the AChoA is located posterolateral to the ICA’s seventh segment (C7) (the supracavernous portion), between the posterior communicating artery (PCoA) and the carotid bifurcation. In a large cadaveric study [6], the mean distances observed between the bifurcation and the AChoA and between the PCoA and the AChoA were, respectively, 5.2 mm and 3.2 mm.
Duplication of the AChoA is very rare. During microsurgical exploration of 154 anterior choroidal arteries, Hussein [6] found seven cases with a double origin and even two cases with a triple origin. More recently, Akar’s [1] intraoperative study of 130 patients (using a pterional approach) described 17 double branches of the AChoA (13 %) and 3 triple branches (2.4 %). According to Morandi’s cadaveric study [8], (1) AChoA duplication is very rare and (2) the anterior uncal artery (the first cortical branch of the M1 segment of the middle cerebral artery) can be misinterpreted as a branch of the AChoA, creating a “pseudo-duplication” image of the latter’s origin.
Moreover, Fernández-Miranda [3] found two types of pseudo-duplication of the AChoA in 47 hemispheres, as described previously by Rhoton [10]: a single trunk formed by two different arteries from the ICA, and the single trunk that divided into two early branches.
The existence of a duplicate origin can be explained by the persistence of an anterior choroidal embryonic system avian pattern [9] or by an ICA fenestration which may be confused with duplicated origin of the AChoA [11].
In the present case, we observed two different branches at the origin of the AChoA, which came from the ICA. The aneurysm was 2.4 mm from the ACoP and 4.7 mm from carotid bifurcation. It is quite similar as the others studies [5, 6]. We consider that this duplicate origin is not a “pseudo-duplication” because the two branches join together a few mm after the origin (within the cisternal segment) and form a single trunk of the AChoA. An aneurysm was located at the origin of the superior branch; this has never previously been described. Moreover, the short distance between the two branches means that this variant cannot be considered as an ICA fenestration.
In view of the neck/fundus ratio and the patient’s poor initial neurological status, we decided to embolize the aneurysm, with interruption of the blood flow in the superior branch of the AchoA. Treatment of AChoA aneurysms is often associated with high rates of morbidity and mortality; these are due (at least in part) to the aneurysm’s site and the major blood supply to the structures involved [2, 4, 7].
Bohnstedt et al. recently described a large cohort of 127 AChoA aneurysms (treated by clipping in 88 % of cases) [2]. One-third of the aneurysms had caused a subarachnoid hemorrhage. Ischemic complications occurred in 15 patients (12.6 %) and in 15.5 % of patients in whom the AChoA aneurysm had ruptured prior to clipping. Half of these complications were observed in aneurysms originating simultaneously from the ICA and the AChoA (6 patients).
Another study of 51 clipped AChoA aneurysms found that 16 % of the latter were associated with infarctions in the AChoA territory [4]. Half of the aneurysms had ruptured prior to treatment. Friedman et al. emphasized the importance of the aneurysm’s site, especially when this involved the AChoA directly. Hence, the risk of stroke is greater here than at other sites.
Endovascular embolization of AChoA aneurysms appears to be a good alternative to surgery, since it is associated with a lower incidence of ischemic complications [2, 4]. Kang et al. reported on AChoA endovascular embolization in 88 patients [7]. One-third had a subarachnoid hemorrhage. Embolization was near-complete in 77 % of the cases. The lower incidence of ischemic complications may be due to a better view of blood flow and the ability to perform peroperative thrombolysis. The mortality rate (5 % of the treated patients) was similar in all these studies.
In the present case, we considered that the risk of per- and post-embolization complications was low because of the presence of duplicate branches of the AChoA. Once the aneurysm was totally embolized, blood flow was interrupted in the superior branch of the AChoA but not in the inferior branch. Hence, blood flow in the AChoA as a whole was maintained.
Conclusion
Aneurysms of AChoA are quite rare and are particularly difficult to treat (mainly because they are located deep within the brain). The anatomy of the AChoA and the termination of the ICA must be well characterized before treatment is initiated.
References
Akar A, Sengul G, Aydin IH (2009) The variations of the anterior choroidal artery: an intraoperative study. Turk Neurosurg 19(4):349–352
Bohnstedt BN, Kemp WJ 3rd, Li Y, Payner TD, Horner TG, Leipzig TJ, Cohen-Gadol AA (2013) Surgical treatment of 127 anterior choroidal artery aneurysms: a cohort study of resultant ischemic complications. Neurosurgery 73(6):933–939 (discussion 939–940)
Fernández-Miranda JC, de Oliveira E, Rubino PA, Wen HT, Rhoton AL Jr (2010) Microvascular anatomy of the medial temporal region: part 1: its application to arteriovenous malformation surgery. Neurosurgery 67(3 Suppl Operative):ons237–ons276 (discussion ons276)
Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, Hansen KK (2001) Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg 94(4):565–572
Fujii K, Lenkey C, Rhoton AL Jr (1980) Microsurgical anatomy of the choroidal arteries. Fourth ventricle and cerebellopontine angles. J Neurosurg 52(4):504–524
Hussein S, Renella RR, Dietz H (1988) Microsurgical anatomy of the anterior choroidal artery. Acta Neurochir (Wien) 92(1–4):19–28
Kang H-S, Kwon BJ, Kwon O-K, Jung C, Kim JE, Oh CW, Han MH (2009) Endovascular coil embolization of anterior choroidal artery aneurysms. Clinical article. J Neurosurg 111(5):963–969
Morandi X, Brassier G, Darnault P, Mercier P, Scarabin JM, Duval JM (1996) Microsurgical anatomy of the anterior choroidal artery. Surg Radiol Anat 18(4):275–280
Lasjaunias P, Berenstein A, ter Brugge KG (2006) Surgical Neuroangiography, 2nd edn. Springer, Berlin
Rhoton AL Jr, Fujii K, Fradd B (1979) Microsurgical anatomy of the anterior choroidal artery. Surg Neurol 12(2):171–187
Uchino A, Kamiya K, Suzuki C (2013) Duplicate origin of the posterior communicating artery diagnosed by magnetic resonance angiography. Surg Radiol Anat 35(8):741–743
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chenin, L., Chivot, C., Toussaint, P. et al. An unusual, duplicate origin of the anterior choroidal artery with aneurysm: a case report. Surg Radiol Anat 37, 1273–1275 (2015). https://doi.org/10.1007/s00276-015-1499-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-015-1499-3