Abstract
A 58-year-old female patient with a medical history of arterial hypertension and a negative family history for cerebrovascular diseases presented with a headache. MRI/MRA revealed an incidental aneurysm of the right internal carotid artery (ICA) at the origin of the anterior choroidal artery (AchoA) with a fundus diameter of 4.9 mm and a neck diameter of 2.9 mm. This aneurysm was given endovascular treatment in which a p64 flow diverter (phenox) was implanted. A follow-up DSA after 3 months showed the complete angiographic occlusion of the aneurysm with a minor in-stent stenosis. DSA runs 9 and 12 months after the endovascular treatment confirmed the persistent aneurysm occlusion, the preservation of the AchoA, and the spontaneous regression of the in-stent stenosis. Flow diverter treatment of AchoA aneurysms is the main topic of this chapter.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Anterior choroidal artery
- Flow diversion
- Microsurgical clipping
- Complications
- In-stent stenosis
- Side branch occlusion
- p64
Patient
A 58-year-old female patient with a medical history of arterial hypertension presented with a headache on the right-hand side, associated with numbness in the right-hand side of her face and right hand. The diagnostic work-up included a cranial MRI/MRA, which revealed an incidental aneurysm of the right ICA at the origin of AchoA.
Diagnostic Imaging
Performing a cranial MRI revealed a small aneurysm of the right internal carotid artery in its intradural segment. The patient then underwent a conventional catheter angiography for further evaluation. The DSA confirmed an irregular aneurysm originating from the posterior wall of the ICA at the origin of the AchoA with a posterolateral orientation and a fundus diameter of 4.9 mm and a neck diameter of 2.9 mm (Fig. 1).
The work-up for the patient’s headache showed an incidental aneurysm at the origin of the right AchoA (coronal T2W (a), TOF source image (b), MIP of the TOF angiography (c), and CTA (d)). A cerebral angiography was then performed to further characterize the aneurysm (posterior-anterior projection (e), lateral projection (f), volume rendering of the rotational angiography (g, h)). Note the irregular morphology of the fundus and the incorporation of the orifice of the AchoA at the level of the aneurysmal neck
Treatment Strategy
The prevention of further growth and rupture of this AchoA aneurysm was the goal of the treatment. Based on published data and personal experience, endovascular flow diverter treatment was deemed to be the most suitable treatment option. After discussing the case with the patient and explaining the intended treatment, alternatives, potential risks, and complications, she consented to the endovascular option with flow diversion.
Treatment
Procedure, 13.05.2016: endovascular flow diversion for the treatment of an incidental aneurysm of the right ICA located at the origin of the AchoA
Anesthesia: general anesthesia; 3000 IU unfractionated heparin (Heparin-Natrium, B. Braun) IV
Premedication: the patient received a loading dose of 1× 300 mg ASA (Aspirin, Bayer Vital) PO and 1× 600 mg clopidogrel (Plavix, Sanofi-Aventis) PO on the day prior to the treatment; a Multiplate test (Roche Diagnostics) confirmed significant dual platelet function inhibition
Access: right common femoral artery, 1× 6F sheath (Terumo); guide catheter: 1× 6F Heartrail II (Terumo); microcatheter: 1× Excelsior XT-27 (Stryker); microguidewire: 1× Synchro2 0.014″ 200 cm (Stryker)
Implant: Flow diverter: 1× p64 4/12 mm (phenox)
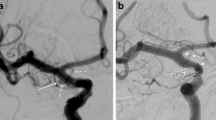
Course of treatment: the right ICA was first catheterized with a diagnostic 4F catheter, and multiple magnified projections were acquired to accurately estimate the vessel diameter and to choose an appropriately sized flow diverter stent (FDS). The ICA had a diameter of about 3.7 mm (distal) and 3.6 mm (proximal). An FDS with a diameter of 4 mm was deemed to be the most suitable size. The aforementioned guiding catheter was then placed into the midcervical segment of the right ICA, and the right middle cerebral artery (MCA) was then catheterized with an Excelsior XT-27 over a Synchro2 0.014″ microguidewire. A 12 mm-long p64 flow diverter with a diameter of 4 mm was deployed into the supraclinoid segment of the right ICA, covering the origin of the AchoA and the adjacent saccular aneurysm. The final DSA run confirmed the patency of the AchoA with contrast medium stagnation inside the covered aneurysm (Fig. 2).
Flow diversion as treatment of an incidental aneurysm of the right ICA at the origin of the AchoA. Using an appropriate working projection, the microcatheter was advanced into the right MCA, bypassing the aneurysm (road map in the working projection (a)). An appropriately sized p64 flow diverter stent was then advanced through the microcatheter and deployed into the supraclinoid segment of the right ICA (partial FD deployment (b), full FD deployment prior to detachment (c)). After confirming that the FDS was in the correct position, it was then mechanically detached by releasing the proximal markers (d). The final angiographic runs (early arterial phase (e), late arterial phase (f), posterior-anterior whole-head run (g)) showed flow stagnation within the covered aneurysm and confirmed the patency of the AchoA
Duration: 1st–14th DSA run: 42 min; fluoroscopy time: 20 min
Complications: none
Post medication: oral dual antiplatelet medication of 1× 100 mg ASA PO daily and 1× 75 mg clopidogrel PO daily for 1 year, followed by 1× 100 mg ASA PO daily lifelong
Clinical Outcome
The postprocedural phase was uneventful and the patient was discharged home on the 4th day after the endovascular procedure. The MRI/MRA prior to discharge was within normal limits with no evidence of ischemic or hemorrhagic complications (Fig. 3).
MRI/MRA after the uneventful flow diverter treatment of the AchoA aneurysm showing no evidence of ischemic changes in the territory of the AchoA. The apparent separation of the aneurysm from the right ICA is due to signal artifacts of the FDS mesh along the vessel wall (axial DWI (a), axial T2w (b), MIP of TOF angiography (c, d))
Follow-Up Examinations
The first angiography follow-up was performed 3 months after the endovascular treatment and showed complete exclusion of the aneurysm and confirmed the patency and unchanged diameter of the AchoA. A minor luminal stenosis along the FDS was evident; this is a common finding and usually self-limiting (Fig. 4). A follow-up MRI was also performed and showed no evidence of ischemic changes in the right AchoA supply territory. The second angiographic follow-up was performed after a period of 10 months and confirmed the persistent complete exclusion of the aneurysm and patency of the AchoA. The in-stent stenosis evident in the previous MRI had almost completely resolved (Fig. 5). The most recent angiographic follow-up performed 2 years after the treatment showed complete resolution of the in-stent stenosis (Fig. 6).
The first angiographic follow-up 3 months after the treatment of the AchoA aneurysm by flow diversion showing complete exclusion of the aneurysm with minor stenosis of the ICA along the FDS (radiographic image of the p64 FDS (a), DSA in posterior-anterior projection (b): note the smaller diameter of the contrasted lumen in comparison to the diameter of the stent). The AchoA remained patent and unchanged in diameter (lateral subtracted projection (c)). The MRI showed evident thrombosis of the aneurysm by showing up the high T1WI signal methemoglobin, which can be mistaken for persistent flow in the TOF angiography at first glance (coronal T1WI (d), MIP of the TOF angiography (e))
The second angiographic follow-up 10 months after the treatment with complete exclusion of the aneurysm from blood circulation and almost complete resolution of the previous luminal narrowing along the stent (radiographic image of the stent (a), DSA in posterior-anterior projection (b)). The AchoA is still patent with no change in caliber (lateral subtracted projection (c)). Note that the aneurysm is now completely shrunken with no evidence of it on the follow-up MRI (coronal T1WI (d), MIP of the TOF angiography (e))
The latest angiographic follow-up performed 2 years after the endovascular treatment confirming the persistent exclusion of the AchoA aneurysm and showing complete resolution of the previous luminal stenosis along the stent (posterior-anterior view (a), lateral view (b), volume rendered rotational DSA (c)), note the patency of the AchoA
Discussion
The AchoA arises from the posterior aspect of the intradural internal carotid artery distal to the origin of the posterior communicating artery (PcomA) with rare variations in its anatomic origin. Its course is usually divided into a cisternal and a plexal segment, and its vascular supply territory includes the optic tract, internal capsule, globus pallidus, tail of the caudate nucleus, parts of the cerebral peduncle, lateral geniculate body, and parts of the mesial temporal lobe, making it an extremely eloquent vessel despite its small size (Morandi et al. 1996; Tanriover et al. 2014). The clinical manifestations of infarcts in the AchoA territory are variable and include contralateral hemiparesis, hemianesthesia, and homonymous hemianopia (Pezzella and Vadalà 2012; Nadaf et al. 2018).
Aneurysms of the AchoA are relatively uncommon, representing 2–5% of all intracranial aneurysms, and their management can be challenging (Lehecka et al. 2010). Beside the microneurosurgical option, several endovascular techniques have been described for the treatment of aneurysms arising from or at the origin of the AchoA including straightforward coil occlusion, stent-assisted coil embolization with jailing of the coiling microcatheter, coil embolization with the use of low-angled microcatheter to avoid packing the origin of the AchoA, and the placement of a small microcatheter into the AchoA during coil insertion (Friedman et al. 2001; André et al. 2017; Hou et al. 2018; Sheen and Suh 2017; Gimonet et al. 2016).
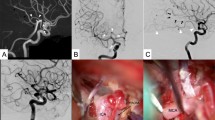
As for microneurosurgery, the preservation of the patency of the AchoA while attempting complete exclusion of the aneurysm is a major concern (Lehecka et al. 2010). An example of this worrisome complication is demonstrated in Fig. 7. The reported rate of complications after surgical management of AchoA aneurysms is variable. In a surgical series of 51 AchoA aneurysms, 8 patients (16%) suffered postoperative clinical and radiological infarcts in the AchoA territory, and when only considering aneurysms arising directly from the origin of the AchoA, the postoperative ischemic complication rate was 44% (Friedman et al. 2001). Li et al. (2012) reported a 15% rate of confirmed AchoA infarctions in their series of 102 patients from a single institution over 16 years. In their retrospective series of 62 patients with AchoA aneurysms, Lee and Park (2013) reported a 5.6% rate of transient AchoA syndrome after surgery with no permanent deficits. Bohnstedt et al. (2013) reported a 53% complication rate in their study of 127 AchoA aneurysms (112 treated with clipping) with postoperative ischemia in 12%. These authors reported a high correlation of ischemic complications with the use of temporary clips.
This series of images demonstrates the course of a 49-year-old male patient who presented with subarachnoid hemorrhage (Hunt and Hess I, Fisher 3). That diagnostic work-up showed a 3 mm-sized aneurysm at the origin of the left AchoA (arrow, a) and a 3 mm-sized aneurysm of the right ICA adjacent to the origin of the ophthalmic artery. The AchoA aneurysm was deemed to be the source of the bleeding and was subsequently treated by microsurgical clipping. Postoperatively, the patient developed a right-hand hemiparesis and expressive aphasia. The performed MRI showed acute ischemia of the left AchoA supply territory (T2WI (b), DWI (c), T2WI 3 months later (f)). The performed conventional angiography confirmed the iatrogenic occlusion of the left AchoA with incomplete exclusion of the aneurysm (DSA lateral view (d, e), DSA 3 months later (g)). The subsequent angiographic follow-ups (performed after treatment of the right-sided ICA aneurysm with a Pipeline device) showed regrowth of the remnant after clipping of the left AchoA aneurysm (h, i). Retreatment was thus carried out by placing a p64 FDS into the left supraclinoid ICA covering the remnant of the AchoA aneurysm and simultaneously the infundibular origin of the left PcomA (j–l). The patient developed postinterventional transient global aphasia due to multiple left parietal subcortical ischemic lesions (m). The follow-up studies performed 3 months after the treatment showed transient in-stent stenosis of the left ICA (n, o) that had completely resolved by the 1 year follow-up (p, q)
In a mixed surgical and endovascular series of 47 AchoA aneurysms, André et al. (2017) reported 5 major and 6 minor complications with no significant difference in complication rate between the surgical and endovascular treatments. The majority of endovascular treatments in this series were regular coil occlusion with or without a remodeling balloon. The authors reported four cases of occlusion of the AchoA with one resulting in a major ischemia and subsequent fatal hemorrhage after an IV infusion of abciximab.
The recent advent of FDS for the treatment of wide-necked side-wall aneurysms has offered an effective alternative approach for the treatment of aneurysms arising from the intradural segment of the ICA. In the case of aneurysms arising from the AchoA or its vicinity, the patency of this eloquent vessel is a concern once its orifice has been covered by an FDS. Several reports showed, however, that the concerning AchoAs remained patent with no change of caliber or flow once covered by an FDS (Brinjikji et al. 2015; Kühn et al. 2015; Neki et al. 2015; Raz et al. 2015; Srinivasan et al. 2017; Bhogal et al. 2018). Although late angiographic occlusion of the AchoA after flow diversion has been reported, the authors observed no clinical or radiological evidence of AchoA infarcts (Brinjikji et al. 2015; Raz et al. 2015). Interestingly, despite evident hemodynamic changes or occlusion of other simultaneously covered branches of the ICA such as the PcomA, the ophthalmic artery, and the ACA, there were no such changes reported for the AchoA (Kühn et al. 2015; Srinivasan et al. 2017; Bhogal et al. 2017).
In our experience, the use of FDS for the treatment of AchoA aneurysms is safe and efficacious (Bhogal et al. 2018). The patient in the presented case was treated with a single FDS, which resulted in completely excluding the covered aneurysm while preserving the patency of the AchoA and with no evidence of clinical or radiological infarct in the AchoA territory over the follow-up period of 2 years. Although the first angiographic follow-up showed minor stenosis of the covered ICA, in our experience this is a common and self-limiting phenomenon with no clinical symptoms (Aguilar Pérez et al. 2017).
Therapeutic Alternatives
-
Coil Occlusion
-
Conservative Management
-
Microsurgical Clipping
-
Stent-Assisted Coil Occlusion
-
Telescoping Stents
References
Aguilar Pérez M, Bhogal P, Henkes E, Ganslandt O, Bäzner H, Henkes H. In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol. 2017; https://doi.org/10.1007/s00062-017-0591-y.
André A, Boch AL, Di Maria F, Nouet A, Sourour N, Clémenceau S, Gabrieli J, Degos V, Zeghal C, Chiras J, Cornu P, Clarençon F. Complication risk factors in anterior choroidal artery aneurysm treatment. Clin Neuroradiol. 2017; https://doi.org/10.1007/s00062-017-0575-y.
Bhogal P, Ganslandt O, Bäzner H, Henkes H, Pérez MA. The fate of side branches covered by flow diverters – results from 140 patients. World Neurosurg. 2017;103:789–98. https://doi.org/10.1016/j.wneu.2017.04.092.
Bhogal P, Ganslandt O, Bäzner H, Henkes H, Aguilar PM. Treatment of unruptured, saccular, anterior choroidal artery aneurysms with flow diversion: single Centre experience. Clin Neuroradiol. 2018; https://doi.org/10.1007/s00062-018-0677-1.
Bohnstedt BN, Kemp WJ 3rd, Li Y, Payner TD, Horner TG, Leipzig TJ, Cohen-Gadol AA. Surgical treatment of 127 anterior choroidal artery aneurysms: a cohort study of resultant ischemic complications. Neurosurgery. 2013;73(6):933–9.; discussion 939–940. https://doi.org/10.1227/NEU.0000000000000131.
Brinjikji W, Kallmes DF, Cloft HJ, Lanzino G. Patency of the anterior choroidal artery after flow-diversion treatment of internal carotid artery aneurysms. AJNR Am J Neuroradiol. 2015;36(3):537–41. https://doi.org/10.3174/ajnr.A4139.
Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, Hansen KK. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94(4):565–72. https://doi.org/10.3171/jns.2001.94.4.0565.
Gimonet H, Desal HA, Mosimann PJ, Stracke P, Daumas-Duport B, Lintia-Gaultier A, Bourcier R, Chapot R. A new endovascular technique for small anterior choroidal artery aneurysms. A consecutive series using the 3-catheter-protective technique. J Neuroradiol. 2016;43(3):223–6. https://doi.org/10.1016/j.neurad.2016.02.004.
Hou SY, Kühn AL, Puri AS, Wakhloo AK. Open-cell stent and use of cone-beam CT enables a safe and effective coil embolization of true ophthalmic artery and anterior choroidal artery aneurysms with preservation of parent vessel: clinical and angiographic results. Interv Neuroradiol. 2018;24(2):135–9. https://doi.org/10.1177/1591019917747246.
Kühn AL, Hou SY, Perras M, Brooks C, Gounis MJ, Wakhloo AK, Puri AS. Flow diverter stents for unruptured saccular anterior circulation perforating artery aneurysms: safety, efficacy, and short-term follow-up. J Neurointerv Surg. 2015;7(9):634–40. https://doi.org/10.1136/neurintsurg-2014-011237.
Lee YS, Park J. Anterior choroidal artery aneurysm surgery: ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013;54(2):86–92. https://doi.org/10.3340/jkns.2013.54.2.86.
Lehecka M, Dashti R, Laakso A, van Popta JS, Romani R, Navratil O, Kivipelto L, Kivisaari R, Foroughi M, Kokuzawa J, Lehto H, Niemelä M, Rinne J, Ronkainen A, Koivisto T, Jääskelainen JE, Hernesniemi J. Microneurosurgical management of anterior choroid artery aneurysms. World Neurosurg. 2010;73(5):486–99. https://doi.org/10.1016/j.wneu.2010.02.001.
Li J, Mukherjee R, Lan Z, Liu Y, He M. Microneurosurgical management of anterior choroidal artery aneurysms: a 16-year institutional experience of 102 patients. Neurol Res. 2012;34(3):272–80. https://doi.org/10.1179/1743132812Y.0000000008.
Morandi X, Brassier G, Darnault P, Mercier P, Scarabin JM, Duval JM. Microsurgical anatomy of the anterior choroidal artery. Surg Radiol Anat. 1996;18(4):275–80.
Nadaf S, Chakor RT, Kothari KV, Patel BA. Anterior choroidal artery infarction. BMJ Case Rep. 2018. pii: bcr-2017-222414; https://doi.org/10.1136/bcr-2017-222414.
Neki H, Caroff J, Jittapiromsak P, Benachour N, Mihalea C, Ikka L, Moret J, Spelle L. Patency of the anterior choroidal artery covered with a flow-diverter stent. J Neurosurg. 2015;123(6):1540–5. https://doi.org/10.3171/2014.11.JNS141603.
Pezzella FR, Vadalà R. Anterior choroidal artery territory infarction. Front Neurol Neurosci. 2012;30:123–7. https://doi.org/10.1159/000333608.
Raz E, Shapiro M, Becske T, Zumofen DW, Tanweer O, Potts MB, Riina HA, Nelson PK. Anterior choroidal artery patency and clinical follow-up after coverage with the pipeline embolization device. AJNR Am J Neuroradiol. 2015;36(5):937–42. https://doi.org/10.3174/ajnr.A4217.
Sheen JJ, Suh DC. Low-angled microcatheter approach for coil embolization of the anterior choroidal artery aneurysm. Neuroradiology. 2017;59(10):1053–6. https://doi.org/10.1007/s00234-017-1879-y.
Srinivasan VM, Ghali MGZ, Cherian J, Mokin M, Puri AS, Grandhi R, Chen SR, Johnson JN, Kan P. Flow diversion for anterior choroidal artery (AChA) aneurysms: a multi-institutional experience. J Neurointerv Surg. 2017. pii: neurintsurg-2017-013466; https://doi.org/10.1136/neurintsurg-2017-013466.
Tanriover N, Kucukyuruk B, Ulu MO, Isler C, Sam B, Abuzayed B, Uzan M, Ak H, Tuzgen S. Microsurgical anatomy of the cisternal anterior choroidal artery with special emphasis on the preoptic and postoptic subdivisions. J Neurosurg. 2014;120(5):1217–28. https://doi.org/10.3171/2014.1.JNS131325.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
AlMatter, M., Aguilar Pérez, M., Henkes, H. (2020). Anterior Choroidal Artery Aneurysm: Incidental Anterior Choroidal Artery Aneurysm, Treated by Flow Diversion Using a p64 with Preservation of the Anterior Choroidal Artery. In: Henkes, H., Lylyk, P., Ganslandt, O. (eds) The Aneurysm Casebook. Springer, Cham. https://doi.org/10.1007/978-3-319-77827-3_44
Download citation
DOI: https://doi.org/10.1007/978-3-319-77827-3_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77826-6
Online ISBN: 978-3-319-77827-3
eBook Packages: MedicineReference Module Medicine