Abstract
Purpose
In patients with symptomatic osteoarthritis knee (OAK), cryoneurolysis (CRYO) and cooled radiofrequency ablation (C-RFA) are reported to be effective and safe; however, they have not been compared directly. The objective of this study is to compare CRYO and C-RFA of the genicular nerve (GN) in terms of efficacy and safety profile in patients with Kellgren and Lawrence (KL) grade ≥ 3 OAK.
Methods
This single-centric, assessor-blinded, randomized, parallel-group, non-inferiority study will include 80 patients with KL grade ≥ 3 OAK. The patients with ≥ 50% pain relief on diagnostic block of three GNs will be randomized to one of the two groups, i.e., CRYO (n = 40) or C-RFA (n = 40). The three target GNs for the interventions will include: superior medial, superior lateral, and inferior medial. The primary outcome will be efficacy of CRYO or C-RFA at 2, 12, and 24 weeks post-procedure based on the 11-point Numerical Pain Rating Scale. The secondary outcomes will be functional improvement based on 12-item Oxford Knee Score and safety of both the procedures. The study is registered in the Clinical Trials Registry—India.
Conclusion
CRYO and C-RFA provide pain relief and improve functional outcome by preventing transmission of pain signals, though by distinct mechanisms. While C-RFA is an established treatment modality, recent evidence supports CRYO in patients with OAK. This study intends to demonstrate non-inferiority of CRYO against C-RFA, thereby supporting the use of CRYO as an additional treatment modality in patients with KL grade ≥ 3 OAK.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis knee (OAK) has a global prevalence of 22.9%, and it accounts for about 60% burden of osteoarthritis [1]. Its management is primarily aimed at pain relief, improved knee function, and enhanced QoL. Presently, the conservative management, including lifestyle changes, physiotherapy, analgesics, steroidal and non-steroidal anti-inflammatory drugs (NSAIDs), intraarticular (IA) injections (NSAIDs, corticosteroid, hyaluronic acid, polynucleotides, oxygen-ozone therapy, platelet-rich plasma, and mesenchymal stem cells), and genicular artery embolization, is focused on symptomatic relief [2, 3]. However, the relief is short-lived, and there are safety concerns, including adverse effects on gastrointestinal, cardiovascular, and renal systems with NSAIDs. Moreover, they are effective only in Kellgren and Lawrence (KL) grade 0–2 OAK, do not change the natural history or disease progression [4], and some of the aforementioned treatments are chondrite-destructive [5].
Genicular nerve radiofrequency ablation (GN-RFA) is a minimally invasive procedure, and the consensus panel of the Society of Interventional Radiology Research suggests that GN-RFA provides durable improvement in chronic knee pain relative to conservative therapy, or IA injections of glucocorticoids or hyaluronic acid derivatives [3]. A recent meta-analysis concluded that RFA is efficacious and safe for pain relief and enhancing knee function in patients with OAK [6]. Compared to conventional RFA and pulsed RFA (p-RFA), cooled RFA (C-RFA) produces a larger local neuronal lesion to increase the chances of effective denervation [7]. However, C-RFA effectively reduces pain by ≥ 50% in 33% to 65% patients with OAK [8, 9]. Thus, a substantial proportion of the patients still suffer from pain following C-RFA of GNs.
In patients with KL grade ≥ 3 OAK, another promising modality for pain relief and improved knee function is cryoneurolysis (CRYO). It acts by developing Wallerian degeneration of the target percutaneous peripheral nerve through application of cold temperatures (− 20 °C to − 100 °C) resulting in disruption of nerve function while maintaining its structure [10]. The greatest advantage of CRYO is the organized nerve regeneration when Wallerian degeneration is achieved [11]. Thus, CRYO produces only temporary denervation, while C-RFA results in complete denervation. CRYO is reported to provide significant pain relief for up to 150 days with no serious adverse events [12].
Though both C-RFA and CRYO are safe and efficacious, none of the available studies have directly compared C-RFA with CRYO. We hypothesize that CRYO can create a more significant lesion, thus may be more efficacious than C-RFA. The primary objective of the study is to compare the efficacy of CRYO and C-RFA of the GNs in terms of pain relief in patients with KL grade ≥ 3 OAK. The secondary objectives are to compare CRYO and C-RFA in terms of the functional improvement and safety profile.
Material and Methods
Trial Design
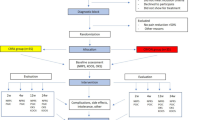
This study protocol describes the single-centric, assessor-blinded, randomized, parallel-group, non-inferiority study (Fig. 1).
Study Settings
The study will be conducted at the Department of Pain Medicine, Daradia—The Pain Clinic, Kolkata, West Bengal, India, under Datta Meghe University of Higher Education and Research (DMIHER). The study has been approved by the Institutional Ethics Committee of DMIHER, and written informed consent will be obtained prior to patient enrollment. The study is registered in the Clinical Trials Registry—India (CTRI/2023/06/053646).
Eligibility Criteria
Inclusion Criteria
Patients aged 50–80 years of either sex will be enrolled if they have
-
1.
Primary OAK-associated chronic pain for more than two years diagnosed by NICE clinical criteria [13],
-
2.
Radiological diagnosis of OAK and grade ≥ 3 based on the Kellgren and Lawrence classification [14],
-
3.
Mean pain score of ≥ 5 based on Numerical Pain Rating Scale (NPRS),
-
4.
Reduction of pain by > 50% following diagnostic GN block,
-
5.
Failed conservative management,
-
6.
Unfit or not willing for total knee replacement (TKR), and
-
7.
Written and oral understanding of Hindi.
Exclusion Criteria
Patients fulfilling any of these criteria will be excluded:
-
1.
Bilateral OAK,
-
2.
Active inflammatory arthritis, such as rheumatoid arthritis or spondyloarthritis,
-
3.
Post-traumatic knee pain, such as meniscal injury,
-
4.
Patients with prior exposure to CRYO or C-RFA,
-
5.
IA injection of platelet-rich plasma, corticosteroids, or hyaluronic acid in the past 3 months,
-
6.
Body mass index > 40 kg/m2,
-
7.
Gross mechanical deformity of the knee joint,
-
8.
Uncontrolled systemic disorders (diabetes, heart disease, cancer, etc.),
-
9.
Major psychiatric illness,
-
10.
Presence of local or systemic infection,
-
11.
Bleeding diathesis, and
-
12.
Any known contraindication of cryoneurolysis, such as cryoglobulinemia.
Following the diagnosis of OAK, the patients will visit the Department of Pain Medicine for initial clinical assessment and diagnostic block of the GNs. The enrolled patients will be informed about the proposed treatment options (CRYO or C-RFA) and randomized to either of the two treatment groups.
Randomization, Concealment, and Blinding
A total of 80 patients with ≥ 50% pain relief on diagnostic block will be allocated in 1:1 ratio and randomly distributed to any of the two groups (C-RFA: 40; CRYO: 40) by odd–even registration number of the patient. Prior to each procedure, a researcher not part of the study will seal patient details and the randomized treatment in an opaque envelope and hand over it to the designated staff nurse. The patients, relatives, investigators, nurses, and all relevant personnel would be blinded to the treatment. Following patient placement, target knee preparation, induction of conscious sedation, and readiness of pain physicians to initiate the procedure, the designated staff nurse will open the envelope. Both generators of CRYO or C-RFA would be available to the pain physicians. Once the envelope is opened, the procedure will be unblinded for all participants.
Outcome evaluation with NPRS and Oxford Knee Score (OKS) will be performed, by a researcher, not part of the baseline evaluation and blinded to the procedure, at 2, 12, and 24 weeks. Moreover, data analyst will be also blinded to the procedure. However, if a patient suffers any severe adverse event (AE), including hematoma, intense pain, and nerve damage, unblinding will be performed.
Initial Assessment
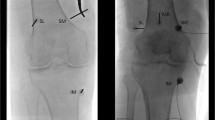
The baseline assessment will include the demographic details (age, sex, and body mass index), clinical characteristics (including comorbidities, duration of pain, use of analgesics, and prior interventions of the knee joint), radiological classification with standing antero-posterior and lateral knee joint X-rays, clinical evaluation using the NPRS, and the OKS. The NPRS is a 11-point scale, ranging from 0 to 10, with 0 suggesting no pain and 10 suggesting worst imaginable pain. The OKS is a 12-item patient-reported outcome score, especially designed to evaluate function and pain following TKR [15].
Medications
For 10 days before the screening/baseline visiting, patients will be instructed to stop taking any analgesics, vitamins, chondroprotective medications, and other alternative treatments for OAK. During the follow-up period, patients will not be allowed to receive any additional treatment for OAK, including viscosupplementation, steroid injections, or analgesics, other than rescue therapy. Use of rescue medication will be recorded. Occurrence of serious local complications from either C-RFA or CRYO will result in study discontinuity; however, such patients will be included in the analysis. The patients will be assured that further treatment would be continued if the pain is not relieved. They will be given a WhatsApp number to report to the investigators at any time. Moreover, if pain relief is inadequate in either group and the patient is willing to undergo TKR, then such patient will be discontinued.
Concomitant Care and Interventions
Patients will not be allowed to take rescue therapy, acetaminophen (maximum 3 g/day), or etoricoxib (120 mg/day) as rescue therapy without permission. Rescue analgesics would be given if the pain score exceeds 5 on an 11-point NPRS or when patients request it.
Protocols for Unexpected Outcomes
A multidisciplinary team of doctors comprising anesthesiologists, critical care specialists, psychiatrists, and internal medicine specialists’ will be formed and will be informed in case of any unexpected outcome.
Diagnostic Genicular Nerve Block
All the procedures will be performed by an experienced pain physicians with more than five years of experience. In a sterile operating room, patients will be positioned supine with the knee supported with a bolster and flexed at about 30°. During the diagnostic block, three branches of GN, superior lateral (SLGN), superior medial (SMGN), and inferior medial (IMGN) will be targeted. With genicular arteries as the landmarks, three GNs will be targeted with ultrasound (US) [16], and 2% lidocaine (2 ml) (Neon Laboratories Ltd, Mumbai, India) will be injected.
The patient will be monitored for one hour, and the pain will be measured with NPRS, while executing ambulation, squatting, and other activities that usually elicit pain in the hour preceding the clinical examination. Patients with ≥ 50% pain relief will proceed to the next phase, while those with < 50% pain relief will be excluded from the study.
Interventional Procedure
In the operating room, patients will be positioned supine with their knees flexed to around 30° with the support of a bolster. A single antibiotic dosage (third-generation cephalosporin) will be administered to prevent infection after the target knee has been draped and sanitized according to the standard procedure.
Throughout the procedure, the patients would be monitored and administered conscious sedation with midazolam (1–2 mg IV) and/or fentanyl (25–100 mcg IV) and supplemental oxygen. Based on the commonly reported procedures [17,18,19], US will be used to locate the nerves. The technique described by Lash et al. [20] would be used to spot the GNs and perform accurate ablation.
C-RFA Group
Under US guidance, 2–3 mL of 1% lidocaine will be used at each target site to anesthetize the skin and subcutaneous tissues prior to inserting the cannula. Subsequently, a 50–150-mm 17G introducer needle will be positioned to ablate the nerves. Prior to ablation, 1 mL of 2% lidocaine will be given through the introducer needles. Following introducer needle placement, an 18-G, internally cooled 4-mm active-tip RFA electrode (COOLIEF, Avanos Medical Inc., GA, USA) will be inserted into the introducer needle, and the position will be confirmed with US. The introducer will be connected to COOLIEF* Cooled RF Advanced Generator. Motor nerve activity will be excluded by testing at 2 Hz and 1 mA. The C-RFA probes will be advanced, and ablation will be completed with lesion settings at 60 °C for 2.5 min.
CRYO Group
Under US guidance, 2–3 mL of 1% lidocaine will be used to anesthetize the skin and subcutaneous tissues prior to inserting the probe. As described above, an 18G CRYO probe (Metrum Cryoflex, Poland) will be inserted in the proximity of the three target points. Metrum CRYO-S Painless (Metrum Cryoflex, Poland) will be used, which employs carbon dioxide and achieves a temperature of − 78 °C. A single freeze cycle will be used during the treatment; 3 min of freezing and 1 min of active thawing will be used following each freezing cycle.
Outcomes
Primary Outcome
The primary outcome would be the efficacy of CRYO or C-RFA. It is based on the NRPS and evaluated at 2, 12, and 24 weeks post-procedure.
Secondary Outcomes
The secondary outcomes will be functional improvement based on 12-item OKS, evaluated at 2, 12, and 24 weeks post-procedure. Moreover, expected AEs (e.g., numbness, bruising, swelling, erythema, and/or inflammation) related to percutaneous nerves access and use of local anesthesia will be evaluated at each follow-up visit and recorded separately. The CIRSE classification system for complications will be used, and AEs occurring during the study will be graded from 1 to 6 [21].
Sample Size
Based on the previous literature, at 24 weeks, Davis et al. [22] reported that 74% patients had at least 50% pain relief with CRYO, and Fogarty et al. [23] reported that 62% patients had similar pain relief with RFA. Assuming a power of 90%, significance level of 5%, and non-inferiority limit of 20%, a sample size of 36 patients in each group was calculated. Considering 10% drop-out, 40 patients each would be required in both the groups. Sample size was calculated with SampSize (https://app.sampsize.org.uk).
Statistical Analyses
The data will be analyzed with SPSS (IBM, Armonk, NY, USA) version 23.0. The categorical and continuous data will be expressed in terms of frequency (percentage) and mean (standard deviation), respectively. The association between categorical and continuous data will be evaluated with Chi-square and independent sample t test, respectively. Within group, analysis will be performed with repeated measures ANOVA followed by post-hoc analysis with Bonferroni’s multiple comparison test. A two-tailed p value of < 0.05 will be regarded as statistically significant.
Discussion
Conservative therapy for varying OAK grades raises safety concerns [24], and KL grade ≥ 3 patients often undergo TKR, leading to increased morbidity and costs [25,26,27]. Non-pharmacological therapies, such as CRYO and C-RFA, aim to alleviate pain, enhance function, and improve QoL [28, 29]. With these objectives, CRYO and C-RFA have been explored and reported to have excellent safety profile and provide significant improvement in patients with OAK [8, 12, 22, 30,31,32,33,34,35].
Recently, Nygaard et al. [10] proposed a two-arm, parallel-group RCT, where 94 patients will be randomly allocated (1:1) to a CRYO + standardized education and exercise (CRYO group) or a Sham + standardized education and exercise (Sham group). The target nerves would be infrapatellar branch of the saphenous nerve and anterior femoral cutaneous nerve, and the primary outcome will be the change in NPRS at 2 weeks. Moreover, Panagopoulos et al. [36] proposed a prospective, single-blind RCT, where 70 patients will be randomly allocated to a CRYO group (n = 35) or C-RFA (n = 35). The target nerves would be SMGN, SLGN, IMGN, and medial genicular branch from vastus intermedius, and the primary outcome will be efficacy of C-RFA or CRYO at 2, 4, 12, and 24 weeks post-procedure based on NPRS. In the present study, both CRYO and C-RFA groups will be treated in similar manner, and SMGN, SLGN, and IMGN would be targeted.
Strengths and Limitations
The strengths include non-inferiority design, inclusion of sufficient number of patients to depict statistical significance of 90%, and similar target nerves for both the procedures, while the limitations include assessor-blinded nature of study, thereby leading to performance bias. Both procedures produce pain relief by different mechanisms, and this difference might decrease the risk of AEs with CRYO; it might also reduce the treatment effect, and thus long-term pain relief might differ. Moreover, though both the procedures will be US-guided, the treatment effect would rely on the experience of the pain physician to precisely spot the GNs.
Conclusion
CRYO and C-RFA prevent pain transmission in different ways. In the present study, both the procedures will cause spherical nerve lesion, target similar GNs, and evaluated with similar outcome measures. The findings of the study will result in additional treatment modality in patients with KL grade ≥ 3 OAK.
References
Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022;74(7):1172–83.
Srinivasan V, Ethiraj P, Agarawal S, Arun HS, Parmanantham M. Comparison of various modalities in the treatment of early knee osteoarthritis: an unsolved controversy. Cureus. 2023;15(1):e33630.
Ahmed O, Block J, Mautner K, Plancher K, Anitescu M, Isaacson A, et al. Percutaneous management of osteoarthritis in the knee: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol. 2021;32(6):919.e1-e6.
Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, et al. Platelet-rich plasma intraarticular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthrosc J arthrosc Relat Surg. 2011;27(11):1490–501.
Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr cartil / OARS, Osteoarthr Res Soc. 2010;18(4):476–99.
Liu J, Wang T, Zhu ZH. Efficacy and safety of radiofrequency treatment for improving knee pain and function in knee osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2022;17(1):21.
Rojhani S, Qureshi Z, Chhatre A. Watercooled radiofrequency provides pain relief, decreases disability, and improves quality of life in chronic knee osteoarthritis. Am J Phys Med Rehabil. 2017;96:e5–8.
Chen AF, Khalouf F, Zora K, DePalma M, Kohan L, Guirguis M, et al. Cooled radiofrequency ablation provides extended clinical utility in the management of knee osteoarthritis: 12-month results from a prospective, multi-center, randomized, cross-over trial comparing cooled radiofrequency ablation to a single hyaluronic acid injection. BMC Musculoskelet Disord. 2020;21(1):363.
Vanneste T, Belba A, Kallewaard JW, van Kuijk SMJ, Gelissen M, Emans P, et al. Comparison of cooled versus conventional radiofrequency treatment of the genicular nerves for chronic knee pain: a multicenter non-inferiority randomized pilot trial (COCOGEN trial). Reg Anesth Pain Med. 2023;48(5):197–204.
Nygaard NPB, Koch-Jensen C, Vægter HB, Wedderkopp N, Blichfeldt-Eckhardt M, Gram B. Cryoneurolysis for the management of chronic pain in patients with knee osteoarthritis; a double-blinded randomized controlled sham trial. BMC Musculoskelet Disord. 2021;22(1):228.
Filippiadis D, Efthymiou E, Tsochatzis A, Kelekis A, Prologo JD. Percutaneous cryoanalgesia for pain palliation: current status and future trends. Diagn Interv Imaging. 2021;102(5):273–8.
Radnovich R, Scott D, Patel AT, Olson R, Dasa V, Segal N, et al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthr Cartil. 2017;25(8):1247–56.
National Clinical Guideline Centre (UK). osteoarthritis: care and management in adults. National Institute for Health and Care Excellence (UK). 2014.
Kellgren JH, Lawrence JS. Radiological assessment of Osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Jt Surg Br. 1998;80:63–9.
Wong J, Bremer N, Weyker PD, Webb CA. Ultrasound-guided genicular nerve thermal radiofrequency ablation for chronic knee pain. Case Rep Anesthesiol. 2016;2016:8292450.
Choi WJ, Hwang SJ, Song JG, Leem JG, Kang YU, Park PH, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152(3):481–7.
McCormick ZL, Korn M, Reddy R, Marcolina A, Dayanim D, Mattie R, et al. Cooled radiofrequency ablation of the genicular nerves for chronic pain due to knee osteoarthritis: six-month outcomes. Pain Med. 2017;18(9):1631–41.
Conger A, Cushman DM, Walker K, Petersen R, Walega DR, Kendall R, et al. A novel technical protocol for improved capture of the genicular nerves by radiofrequency ablation. Pain Med. 2019;20(11):2208–12.
Lash D, Frantz E, Hurdle MF. Ultrasound-guided cooled radiofrequency ablation of the genicular nerves: a technique paper. Pain Manag. 2020;10(3):147–57.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–6.
Davis T, Loudermilk E, DePalma M, Hunter C, Lindley D, Patel N, et al. Prospective, multicenter, randomized, crossover clinical trial comparing the safety and effectiveness of cooled radiofrequency ablation with corticosteroid injection in the management of knee pain from osteoarthritis. Reg Anesth Pain Med. 2018;43(1):84–91.
Fogarty AE, Burnham T, Kuo K, Tate Q, Sperry BP, Cheney C, et al. The effectiveness of Fluoroscopically guided genicular nerve radiofrequency ablation for the treatment of chronic knee pain due to osteoarthritis: a systematic review. Am J Phys Med Rehabil. 2022;101(5):482–92.
Charlesworth J, Fitzpatrick J, Perera NKP, Orchard J. Osteoarthritis- a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet Disord. 2019;20:151.
Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–21.
Healy WL, Della Valle CJ, Iorio R, Berend KR, Cushner FD, Dalury DF, et al. Complications of total knee arthroplasty: standardized list and definitions of the knee society. Clin Orthop Relat Res. 2013;471(1):215–20.
Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a bundled payment care improvement initiative. J Arthroplasty. 2016;31(9):1862–5.
Ferreira RM, Torres RT, Duarte JA, Gonçalves RS. Non-pharmacological and non-surgical interventions for knee osteoarthritis: a systematic review and meta-analysis. Acta Reumatol Port. 2019;44(3):173–217.
Migliorini F, Driessen A, Quack V, Sippel N, Cooper B, Mansy YE, et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg. 2021;141(9):1473–90.
Davis T, Loudermilk E, DePalma M, Hunter C, Lindley DA, Patel N, et al. Twelve-month analgesia and rescue, by cooled radiofrequency ablation treatment of osteoarthritic knee pain: results from a prospective, multicenter, randomized, cross-over trial. Reg Anesth Pain Med. 2019;44(4):499–506.
Hunter C, Davis T, Loudermilk E, Kapural L, DePalma M. Cooled radiofrequency ablation treatment of the genicular nerves in the treatment of osteoarthritic knee pain: 18- and 24-month results. Pain Pract. 2020;20(3):238–46.
Lyman J, Khalouf F, Zora K, DePalma M, Loudermilk E, Guiguis M, et al. Cooled radiofrequency ablation of genicular nerves provides 24-month durability in the management of osteoarthritic knee pain: outcomes from a prospective, multicenter, randomized trial. Pain Pract. 2022;22(6):571–81.
Kapural L, Minerali A, Sanders M, Matea M, Dua S. Cooled radiofrequency ablation provides prolonged pain relief compared to traditional radiofrequency ablation: a real-world, large retrospective clinical comparison from a single practice. J Pain Res. 2022;15:2577–86.
Chou SH, Shen PC, Lu CC, Liu ZM, Tien YC, Huang PJ, et al. Comparison of efficacy among three radiofrequency ablation techniques for treating knee osteoarthritis: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(14):7424.
Mihalko WM, Kerkhof AL, Ford MC, Crockarell JR Jr, Harkess JW, Guyton JL. Cryoneurolysis before total knee arthroplasty in patients with severe osteoarthritis for reduction of postoperative pain and opioid use in a single-center randomized controlled trial. J Arthroplasty. 2021;36(5):1590–8.
Panagopoulos A, Tsiplakos P, Katsanos K, Antzoulas P, Lakoumentas J. Cooled radiofrequency ablation versus cryoneurolysis of the genicular nerves for the symptomatic pain management in knee osteoarthritis: a study protocol of a prospective, randomized, single-blinded clinical trial. J Orthop Surg Res. 2023;18(1):295.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Clinical Trial Registration
This study was registered in the Clinical Trials Registry—India (CTRI/2023/06/053646).
Consent for Publication
For this protocol, consent for publication is not required.
Ethical Approval
This study was approved by the Institutional Ethics Committee of Datta Meghe Institute of Higher Education and Research, Sawangi, Wardha, Maharashtra, India (DMIHER(DU)/IEC/2023/549).
Human and Animal Participants
All procedures performed in studies involving human participants will be in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent will be obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, G., Singam, A., Chakole, V. et al. Efficacy and Safety of Cryoablation Compared with Cooled Radiofrequency Ablation of Genicular Nerves in Advanced Osteoarthritis of the Knee: A Study Protocol of Single-Centric, Assessor-Blinded, Randomized, Parallel-Group, Non-inferiority Study. Cardiovasc Intervent Radiol 47, 508–514 (2024). https://doi.org/10.1007/s00270-024-03703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-024-03703-2