Abstract
Adjuvant embolization of varices may reduce rebleeding in patients with a transjugular intrahepatic portosystemic shunt (TIPS). The aim of this study was to investigate the efficacy and the risks of adjuvant variceal embolization at TIPS implantation using bucrylate.
Patients and Methods
The retrospective study evaluated 104 of 237 cirrhotic patients with TIPS for variceal bleeding who received adjuvant bucrylate embolization. For TIPS creation, bare stents were used in 35 patients (33.7%) and covered stents in 69 patients (66.3%) patients. Isolated gastric varices were seen in 10 patients (9.6%).
Results
Six patients (5.8%) rebled during a median follow-up time of 26 months (1–57 months). Rebleeding occurred in 14% (5/35) of patients with a bare stent but only in 1.4% (1/69) of patients with a covered stent. The 1- and 2-year rebleeding rates of all patients were 0.9 and 2.9% and of patients receiving a bare stent were 2.9 and 8.6%, respectively. Bucrylate migration was seen in 13 patients (12.5%). In 9 of these patients (8.7%), asymptomatic lung embolization occurred. This was rare in patients with esophageal varices (3.1%) but frequent (60%) in patients with isolated gastric varices and a spontaneous splenorenal shunt.
Conclusions
Our results suggest that adjuvant embolization using bucrylate is effective and delays variceal rebleeding. The general use of covered stents, however, alleviates the utility of adjuvant bucrylate embolization which may be restricted to patients with a high risk of rebleeding indicated by large varices, active, acute or recent variceal bleeding and advanced cirrhosis. Bucrylate should not be used in isolated gastric varices because it bears a high risk of migration into the lungs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variceal bleeding is a major indication for the implantation of the transjugular intrahepatic portosystemic shunt (TIPS). The TIPS treatment is recommended when medical treatment failed or failure can be expected due to a high risk of early rebleeding in patients with large varices, active, acute or recent variceal bleeding and advanced cirrhosis [1]. During the intervention, a splenoportography is routinely performed to verify a correct puncture site of an intrahepatic branch of the portal vein and to detect varices with the aim of their occlusion if deemed necessary.
Several previous studies showed an advantage of adjuvant variceal embolization [2]. For TIPS creation, these studies included bare stents [3,4,5] and/or covered stents [6,7,8]. Two of the studies used coils; 4 studies used coils with or without bucrylate [5], or 100% ethanol [7], or gelatin sponge [4], or various sclerosing agents [3]. Overall, embolization reduced rebleeding rates significantly from 24 to 15% [2]. In the light of these findings it seems to be surprising that present guidelines do neither mention nor recommend the use of adjuvant variceal embolization [1].
Bucrylate (isobutyl cyanoacrylate) is a liquid tissue adhesive for transcatheter embolization. It is used in radiological and endoscopic interventions to occlude vessels and to stop bleedings. In comparison with sponges, coils and plugs, bucrylate is inexpensive and achieves immediate, irreversible and complete occlusion of the vessel irrespective of coagulation deficiencies [9]. With respect to side effects, bucrylate may have an increased risk of migration which may result in lung embolization.
In contrast to previous studies on adjuvant embolization predominantly using coils, our study describes the efficacy and complications of adjuvant bucrylate embolization.
Patients and Methods
The study was performed in accordance with the Declaration of Helsinki, and it has been approved by our local ethics committee (no. EK 428/14). A total of 237 patients with cirrhosis admitted to our hospital for acute variceal bleeding or for prophylaxis of variceal rebleeding between January 1, 2004, and January 26, 2017, received a TIPS treatment. Of these patients 104 (44%) received adjuvant bucrylate embolization at the time of TIPS implantation and were included in this study. The outcome measurements of our study were the rebleeding rates 1 and 2 years after treatment and the acute adverse events of the embolization. Demographical, clinical and technical variables were obtained from medical records and the reports of the TIPS intervention.
The TIPS implantation was performed as described previously [10]. All interventions were performed or supervised by the same interventionalist (M.R.). After puncture of an intrahepatic branch of the portal vein using a modified Colapinto needle (TIPS-needle, Optimed, Ettlingen, Germany), the guiding catheter (TFE 9F, 45 cm, Cook-Medical, Hamburg, Germany) was advanced into the portal vein and a pigtail catheter (5F, 90 cm, Performa®, Meritmedical, Galway, Ireland) introduced through the guiding catheter. Pressures in the portal vein and the right atrium were measured using an electronic measuring device (Infinity C500, Dräger, Lübeck, Germany). Thereafter, a splenoportography was performed. A decision for embolization was made if varices were large (i.e., diameter > 3–4 mm at origin) and/or the patient had acute or recent (within 2 weeks) bleeding before the intervention. Using a guide wire (stiff type, angled, 0.035″, 180 cm, Terumo Corporation, Tokyo, Japan) a Cobra catheter (5F, 100 cm, Cordis Corporation, Miami Lakes, USA) was then advanced through the guiding catheter into the varices and a selective angiography was performed. A mixture of bucrylate (Histoacryl, B. Braun Surgical, Rubi, Spain) and Lipiodol (Lipiodol® Ultra-Fluid, Guerbet, Roissy, France) (1:1–1:3 vol/vol) was prepared and, after having flushed the Cobra catheter with 20% glucose, 1–3 ml of bucrylate was injected (mean 1.8 ml) slowly under fluoroscopic control. Thereby, a greater dilution of bucrylate with Lipiodol (e.g., 1:3 vol/vol) was used for esophageal varices to obtain a more extended occlusion of the varices to hamper future collateral formation. The success of embolization was defined as lack of flow through the varices before the TIPS implantation. It should be emphasized that residual bucrylate may occlude the Cobra catheter. This is the reason why the guiding catheter must be advanced into the portal vein before performing embolization. To prevent reflux of bucrylate into the portal vein, embolization was performed before the TIPS implantation. After embolization, the guiding catheter was removed into the parenchymal tract close to the hepatic vein and contrast medium was injected to opacify the parenchymal tract to exclude a communication with bile ducts or branches of the hepatic artery. The parenchymal tract was then dilated using a balloon catheter (eucaPW, 6F, 8 × 40 mm, Eucatech AG, Weil am Rhein, Germany) and a Viatorr stent (Viatorr, Gore Medical, Putzbrunn, Germany) implanted and dilated to 8 or 10 mm. A final angiography and pressure measurement were performed and, finally, the sheath and the catheter removed.
Follow-up of patients included a clinical and laboratory examination and duplex sonography. Patients were seen at intervals of 3 months during the first year of follow-up and 6-monthly thereafter. Variceal rebleeding was defined as hematemesis or melena together with a drop in the hemoglobin concentration by > 2 g/L in the presence of varices and absence of other bleeding sources at endoscopy.
Statistics
Continuous variables are expressed as mean with standard deviation, whereas categorical variables are reported as frequencies and percentages unless stated otherwise. Patients were followed up until death, loss to follow-up or the end of study. Time to rebleeding was calculated using Kaplan–Meier analyses. Patients who were lost to follow-up were censored at the time of their last visit.
Statistical analyses were performed with SPSS (version 25.0, IBM, New York, USA) and GraphPad Prism (version 7, GraphPad Software, San Diego, CA, USA).
Results
Patients Characteristics
The median follow-up time was 22.0 months (range 0–154). Twenty-three patients (22.1%) who received variceal embolization and who were analyzed in terms of acute adverse events were lost to follow-up during the study period. Five patients (4, 8%) received liver transplantation during follow-up.
Table 1 shows the biomedical baseline characteristics and interventional data of the patients receiving TIPS with embolization for variceal bleeding. Clinical scores confirm advanced liver disease with a majority of patients having Child–Pugh B cirrhosis (52.9%). Most patients had esophageal varices (55.3%) or esophageal and gastric varices (36.5%), and only a minority (9.6%) had isolated gastric varices draining through a spontaneous splenorenal shunt. Patients received bare (33.7%) or covered stents according to the time of their TIPS implantation.
Efficacy of Bucrylate Embolization
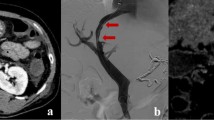
Bucrylate embolization was successfully performed in all patients leading to an abolishment of the variceal blood flow before the TIPS implantation. Figure 1 shows an example of bucrylate embolization of esophagogastric varices. The final splenoportography after TIPS placement (Fig. 1D) shows a small residual collateral which has not been embolized. Figure 2 shows the situation in a patient with isolated gastric varices draining into a spontaneous splenorenal shunt. Bucrylate embolization may be problematic since it may migrate into the lungs.
A Splenoportography (DSA) showing esophagogastric varices emerging from the left gastric vein. B Selective angiography of varices. C Selective angiography of occluded varices after injection of 1 ml bucrylate/Lipiodol. D Final splenoportography after TIPS placement and bucrylate embolization. Please note the small collateral which was not occluded and which originates from the splenic vein
A summary of the endpoints of the study is presented in Table 2. Rebleeding was observed in 7 patients. One patient rebled due to the presence of severe portal hypertensive gastropathy. Six patients had variceal rebleeding; all of them had insufficient or occluded shunts. Five patients had a bare and 1 a covered stent implanted. Thus, 5 (14%) of the 35 patients with a bare stent and only 1 patient (1.4%) of the 69 patients with a covered stent rebled. Variceal rebleeding occurred after a median of 26 months. The 1- and 2-year probability of variceal rebleeding was 0.9% and 2.9%, respectively (Fig. 3). The revision of the angiographies performed before TIPS implantation showed that, in all 6 cases with variceal rebleeding, additional smaller collaterals along the splenoportal axis have been present.
Complications of Embolization
Non-target embolization was the only complication observed in 13 patients (12.5%) (Table 2). Backward migration into the portal vein was seen in 7 patients. Since this may cause thrombotic occlusion, removal of embolic material has been performed consistently and successfully in all 7 patients. The material was detached from the wall of the portal vein using a pigtail catheter or j-guide wire and transferred into intrahepatic portal branches or into the lungs via the TIPS (3 patients). Forward migration of bucrylate was seen in 6 patients with a spontaneous splenorenal shunt who were treated for isolated gastric variceal bleeding. A small proportion of the injected bucrylate migrated through the spontaneous shunt into the lungs before occlusion could be achieved. Backward bucrylate migration into the liver did not result in deterioration of the liver test results. Migration into the lungs was clinically asymptomatic in all patients and did not cause respiratory or cardiac dysfunction. Overall, lung embolization was rare in patients treated for esophageal varices or combined esophageal and gastric varices (3.1%) but frequent in patients with isolated gastric varices (60%), all of them having a spontaneous splenorenal shunt. With the exception of 1 patient treated for acute bleeding who died 3 days after the intervention from multi-organ failure, all of the 104 patients receiving bucrylate embolization survived the index hospital stay.
Discussion
Our study shows an overall rebleeding rate with bare stents of 14%, a finding which is in the lower range when compared to earlier randomized studies without adjuvant embolization showing overall rebleeding rates in the TIPS groups of 9–40.6% [10]. Our 1- and 2-year rebleeding rates were only 2.9 and 8.6%. Previous studies of our institution with bare stents including comparable patients showed 1- and 2-year rebleeding rates of 15 and 21% although 10 and 51% of patients received adjuvant bucrylate embolization, respectively [11, 12]. In a meta-analysis published in 2008 including 12 studies performed between 1996 and 2002 with bare stents, variceal rebleeding occurred in 24% of patients during a mean follow-up time of 24 months [13]. Thus, comparing the results of the present study with historical data it can be suggested that systematic bucrylate embolization may delay rebleeding effectively. All shunts showed stenosis or occlusion at the time of rebleeding, indicating that embolization alone does not prevent rebleeding if portal hypertension returns.
In patients receiving covered stents with a much smaller risk of stenosis/occlusion, the positive effect of embolization may be limited. As shown in recent studies, 1- and 2-year rebleeding rates are 0–8% only [14,15,16,17]. However, interventionalists were free to decide for adjuvant embolization and a variable proportion of patients received embolization with coils or bucrylate in all studies. Although adjuvant embolization does not seem to be essential to further decrease rebleeding rates, embolization of large varices may be recommended for the following reasons: First, 25–40% of covered stents develop insufficient function within 2–3 years of follow-up [18,19,20], suggesting a considerable long-term risk of rebleeding. Second, large gastric varices are often combined with a very low portal pressure questioning the rationale and benefit of portal decompressive treatment alone [10]. In these cases, the reason for variceal bleeding is rather the thin wall of the large varices than the elevated portal pressure [10]. Third, it may be speculated that large collaterals compete with the shunt maybe increasing the incidence of shunt failure. Clinical long-term studies are required to show whether adjuvant embolization may also be advisable in patients receiving covered stents.
Our data are in accordance with a meta-analysis including 6 studies showing an advantage of adjuvant embolization [2,3,4,5,6,7,8]. All studies used coils without [6, 8] or with sclerosing agents such as bucrylate [5], 100% ethanol [7], gelatin sponge [4], or various sclerosing agents [3]. A recent retrospective study by Lakhoo et al. [21] investigated the reasons for rebleedings in patients having received coil embolization. 25 of 141 patients receiving TIPS with bare as well as covered stents for variceal bleeding rebled, and their records were subjected to a root cause analysis to detect the cause of treatment failure. These authors revised the angiographies obtained at TIPS implantation and found that the most common cause of recurrent variceal bleeding was lack or insufficient variceal embolization detected in 64% of rebleeders. This interpretation presumes that proper embolization should definitely prevent rebleeding, an assumption of questionable validity. Our study confirms the findings by Lakhoo. All patients with rebleedings showed additional small collaterals which have not been embolized at TIPS implantation, but all of them had also some degree of shunt failure. The additional occlusion of these small collaterals may be technically difficult and time-consuming and may have a high risk of retrograde bucrylate migration. Its effectivity may be questioned because new small collaterals may develop rapidly when portal hypertension recurs. Based on these considerations we did not embolize small collaterals because we felt that the technical challenge, the risk and questionable success were not worth the presumable small benefit of occlusion. Thus, in contrast to Lakhoo et al. [21] we conclude that sufficient reduction in the pressure gradient (e.g., < 12 mmHg) is mandatory to prevent rebleeding while embolization of large collaterals is a supportive measure that delays rebleeding when shunt failure develops. The study by Lakhoo et al. also demonstrates that embolization using coils may be technically challenging and not always sufficiently effective [21, 22].
With respect to safety, non-target embolization occurred in 13 of our 104 patients. In 7 patients we observed backward migration of the glue into the portal vein, and in 6 patients a forward migration through a spontaneous portacaval shunt into the systemic circulation occurred. Fortunately, due to the small amount of bucrylate injected (1.8 ml), bucrylate migration was asymptomatic in all patients. In cases with backward migration into the portal vein, bucrylate was always removed from the main stem of the portal vein to prevent portal vein thrombosis. We aimed at embolizing collaterals from their portal origin to obtain complete and permanent occlusion. This may have increased the risk of backward migration of bucrylate into the portal vein. A more selective catheterization and peripheral injection of bucrylate may be recommended to reduce the risk of backward migration.
Lung embolization was rare in patients treated for esophageal varices but common in patients with isolated gastric varices draining through a spontaneous splenorenal shunt. Although patients did not develop clinical signs of lung embolization, this type of varices should not be embolized with bucrylate but with coils or plugs instead. If bucrylate is still preferred, coils or a balloon catheter placed in the proximal varices or in the left renal vein may be used to reduce the blood flow before the glue is injected. In addition, as published recently [23], migration of bucrylate into the systemic circulation may cause cerebral embolization in patients with a patent foramen ovale (PFO). In view of the fact that about 25% of individuals have PFO, bucrylate migration into the systemic circulation should be avoided strictly. In contrast to isolated gastric varices, bucrylate embolization seems to be sufficiently safe and can be recommended for adjuvant treatment of esophageal varices. This statement may also be relevant for endoscopic bucrylate embolization for bleeding from gastric varices. In a large study including 140 patients, 6 patients (4, 3%) showed migration of embolization into the lungs [24]. Due to the application of larger amounts of the agent (4.2 ml), 4 of these patients developed respiratory distress. In addition, several case reports describing respiratory failure after endoscopic bucrylate embolization have been published [25, 26]. Lung embolization after endoscopic treatment is likely as frequent as after transjugular embolization. Therefore, endoscopic bucrylate embolization of isolated gastric varices should be applied only if demanded by severe emergency bleeding. It should be performed under fluoroscopic control, and the amount of bucrylate injected should be limited to 1–2 ml.
Our study is limited by its retrospective character and the lack of a control group. Patients not receiving embolization were not considered as a control group because of an obvious selection bias provided by the interventionalist. These patients generally had no acute or active bleeding, a lower risk of rebleeding and smaller or no visible varices at angiography. Statistical methods often applied in retrospective studies such as multiple regression or propensity score matching are not appropriate to reduce the selection bias because in our study confounding variables (e.g., risk of bleeding) were complex, unknown, not quantifiable or not recorded. Comparison between groups with or without adjuvant embolization did, therefore, not seem to be meaningful.
In summary, our results with bare stents suggest that adjuvant embolization using bucrylate is effective and delays variceal rebleeding. The general use of covered stents, however, alleviates the utility of adjuvant bucrylate embolization which may be restricted to situations with a high risk of rebleeding such the presence of large varices, active, acute or recent variceal bleeding and advanced cirrhosis. Bucrylate should not be used in isolated gastric varices because it bears a high risk of migration into the lungs and a risk of cerebral infarction. In these patients, plugs or coils should be preferred.
References
De Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension report of the Baveno VI consensus workshop : stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52.
Qi X, Liu L, Bai M, Chen H, Wang J, Yang Z, et al. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol. 2014;29:688–96.
Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology. 2005;236:360–7.
Wu XJ, Cao JM, Han JM, Li JS. Long-term results of TIPS, TIPS with CVO and combined TIPS and portal azygos disconnection for the treatment of portal hypertension. Chin J Surg. 2009;47:446–9.
Xiao T, Chen L, Chen W, Xu B, Long Q, Li R, et al. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol. 2011;45:643–50.
Chen S, Li X, Wei B, Tong H, Zhang MG, Huang ZY, et al. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology. 2013;268:900–6.
Xue H, Yuan J, Chao-Li J, Palikhe M, Wang J, Shan-Lv L, Qiao W. Follow-up study of transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Dig Dis Sci. 2011;56:3350–6.
Gaba RC, Bui JT, Cotler SJ, Kallwitz ER, Mengin OT, Martinez BK, et al. Rebleeding rates following TIPS for variceal hemorrhage in the Viatorr era: TIPS alone versus TIPS with variceal embolization. Hepatol Int. 2010;4:749–56.
Dotter CT, Goldman ML, Rösch J. Instant selective arterial occlusion with isobutyl 2-cyanoacrylate. Radiology. 1975;114:227–30.
Rössle M. TIPS: 25 years later. J Hepatology. 2013;59:1081–93.
Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165–71.
Rössle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of transjugular intrahepatic portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1997;349:1043–9.
Zheng M, Chen Y, Bai J, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients. Meta-Analysis update. J clin Gastroenterol. 2008;42:507–16.
Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + ß-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581–9.
García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–9.
Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, Panther E, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149:660–8.
Rudler M, Cluzel P, Corvec TL, Benosman H, Rousseau G, Poynard T, Thabut D. Early-TIPSS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther. 2014;40:1074–80.
Perarnau J-M, Le Gouge A, Nicolas C, d` Alteroche L, Borentain P, Saliba F, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962–8.
Bureau C, Garcia-Pagan JC, Pomier-Layrargues G, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742–7.
Rössle M, Siegerstetter V, Euringer W, et al. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long-term follow-up of 100 patients. Acta Radiol. 2006;47(7):660–6.
Lakhoo J, Bui JT, Zivin SP, Lokken RP, Minocha J, Ray CE Jr, Gaba RC. Root cause analysis of rebleeding events following transjugular intrahepatic portosystemic shunt creatinon for variceal hemorrhage. J Vasc Interv Radiol. 2015;26:1444–53.
Rössle M. Root cause analysis: can it improve outcome after transjugular intrahepatic portosystemic shunt creation? J Vasc Interv Radiol. 2015;26:1453–4.
Tuennemann J, Mössner J, Hoffmeister A. Acute cerebrovascular incident as a complication of TIPS procedure. Z Gastroenterol. 2013;51:381–3.
Hwang SS, Kim HH, Park SH, Kim SE, Jung JI, Ahn BY, et al. N-butyl-2-cyanoacrylate pulmonary embolism after endoscopic injection sclerotherapy for gastric variceal bleeding. J Comput Assist Tomogr. 2001;25:16–22.
Singer AD, Fananapazir G, Maufa F, Narra S, Ascher S. Pulmonary embolism following 2-octyl-cyanoacrylate/lipiodol injection for obliteration of gastric varices: an imaging perspective. J Radiol Case Rep. 2012;6:17–22.
Witthöft T, Homann N, Dodt C, Ludwig D. Massive pulmonary embolism after endoscopic therapy for gastric variceal bleeding. Z Gastroenterol. 2004;42:383–6.
Author information
Authors and Affiliations
Contributions
MS performed interventions, designed and conducted the study and wrote the manuscript. MG, AS and LS collected the data and wrote the manuscript. LM performed interventions and was involved in documentation of data. RT designed the study and reviewed the manuscript. MR performed the interventions, designed the study, evaluated the data and wrote the manuscript. DB wrote the manuscript and collected statistics.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schultheiß, M., Giesler, M., Maruschke, L. et al. Adjuvant Transjugular Variceal Occlusion at Creation of a Transjugular Intrahepatic Portosystemic Shunt (TIPS): Efficacy and Risks of Bucrylate Embolization. Cardiovasc Intervent Radiol 42, 729–736 (2019). https://doi.org/10.1007/s00270-019-02176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02176-y