Abstract
Objectives
To assess the efficacy of transjugular intrahepatic portosystemic shunt (TIPS) with and without adjunctive embolisation in managing cardiofundal varices bleeding.
Methods
The retrospective study comprised 82 patients (54 men; mean age 53.9 years; mean Model of End-stage Liver Disease score 9.3) with cardiofundal varices bleeding who underwent TIPS creation from 2011 to 2015. Variceal rebleeding, the outflow tracts of varices, overt hepatic encephalopathy (HE) and post-procedure varices patency were assessed.
Results
Gastrorenal shunt was present in 92.7% of patients (n = 76). Embolisation was performed in 67.1% of patients (n = 55). The 1- and 2-year variceal rebleeding rates in the TIPS combined with embolisation group were significantly lower than those in the TIPS alone group (3.8% and 13.4% vs 13.0% and 28.0%, respectively; p = 0.041). No significant differences between the two groups were found in the cardiofundal varices patency, overt HE or survival (p > 0.05).
Conclusions
The results suggest that TIPS combined with embolisation can reduce the risk of variceal rebleeding for patients with cardiofundal varices.
Key Points
• TIPS combined with embolisation reduces the risk of rebleeding in treating cardiofundal varices.
• TIPS combined with embolisation could not completely occlude cardiofundal varices.
• TIPS combined with embolisation could not prevent the development of hepatic encephalopathy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) has been established as an effective treatment for variceal bleeding and refractory ascites [1, 2]. The aim of TIPS is to decrease the portosystemic gradient (PSG) to a target threshold (usually 12 mmHg) and adjunctive embolisation of varices will be performed usually when haemodynamic success is not achieved [2,3,4]. However, recent studies demonstrated that TIPS combined with variceal embolisation could lower the rate of rebleeding compared with TIPS alone [5,6,7]. Hitherto, a consensus has not been reached on whether adjunctive embolisation is necessary during TIPS creation.
Cardiofundal varices are located in the fundus of the stomach, including gastro-oesophageal varices type 2 (GOV-2) and isolated gastric varices type 1 (IGV-1) [8, 9]. It has been reported that the mean PSG in patients with gastric variceal haemorrhage was lower than that in patients with oesophageal variceal haemorrhage, even less than 12 mmHg [10,11,12]. Thus, the necessity of concomitant embolisation of the collaterals feeding the GVs during the TIPS procedure is recognised and accepted worldwide [8, 13]. However, only two retrospective studies with small sample sizes have analysed the efficacy of TIPS with or without embolisation for gastric variceal bleeding [7, 14]. In those studies, all types of GVs were incorporated, and cardiofundal varices were in the minority. Therefore, the present retrospective study aims to compare TIPS combined with embolisation to TIPS alone in terms of cardiofundal variceal bleeding.
Material and methods
Patient population
Written informed consent for the procedure was obtained from each patient in this study. Between December 2011 and June 2015, 91 patients with bleeding from cardiofundal varices were admitted to the Department of Gastroenterology. The inclusion criteria were as follows: (1) diagnosis of liver cirrhosis through biopsy or typical cross-sectional imaging; (2) historical evidence of haemorrhage from the varices refractory to medical or endoscopic therapy; (3) the presence of GOV-2 or IGV-1 confirmed through gastroscopy and (4) a successful TIPS creation. Patients in whom the outcome could not be assessed (lost before 1 month of follow-up) were excluded from the study. Patients were classified according to whether they received the embolisation (TIPS combined with embolisation group) or not (TIPS alone group). Disposition of the patients is shown as a flow chart in Fig. 1.
Procedure
The TIPS procedure was performed using a previously described standard process [15]. A standard TIPS set (RUPS-100; Cook Medical) was used for TIPS creation in each patient. A portal venography was performed and a PSG was calculated. After the parenchymal tract was dilated using an 8 × 60-mm balloon catheter (Powerflex pro; Cordis), an expanded-polytetrafluoroethylene-covered stent (e-PTFE, Fluency; C.R. Bard, Inc.) was implanted. The completion shunt venography was re-performed and PSG was re-measured.
Embolisation of the GV was performed following TIPS creation at the discretion of the primary operators depending on the numbers and sizes of varices and the degree of angiographic filling following TIPS creation. Embolisation of afferent veins was necessarily performed for acute variceal bleeding. Selective catheterisation of the left, posterior or short gastric vein was performed using an angled catheter (Cobra; Terumo Medical Corporation). Subsequently, proximal embolisation was commonly performed using the materials according to the diameter of the afferent veins, such as a metallic coil (MReye; Cook Medical), alpha-cyanoacrylate (EC adhesive; Bai Yun Medical Adhesive Co.) and a vascular plug device (Amplatzer Vascular Plug; St. Jude Medical). Distal embolisation of submucosal components of gastric varices was not performed with a microcatheter. Finally, a portal venography was performed to assess the degree of embolisation. Low molecular weight heparin was prescribed for 3 days to prevent the development of an acute thrombus.
Imaging assessment
TIPS radiologic images or contrast-enhanced computed tomography (CECT) images were reviewed for the presence of fundal variceal outflow, including gastrorenal shunt, gastrocaval shunt, pericardial vein and azygous vein. CECT examinations were evaluated to assess the patency of the gastric fundal varices after TIPS creation. Variceal patency was defined as enhancement of tortuous vessels abutting into the submucosal structure of the stomach, whereas variceal occlusion was defined as the complete absence of contrast-filling varices [14].
Measured outcomes and definitions
The primary outcome was rebleeding from the varices after TIPS procedure. The secondary outcomes included stent dysfunction, cardiofundal varices patency, the incidence of overt hepatic encephalopathy (HE) and survival. Haemodynamic success refers to the post-TIPS reduction of PSG below an absolute value of 12 mmHg or a relative reduction of more than 20% [3]. Stent dysfunction was defined as stent stenosis or occlusion confirmed through contrast-enhanced CT, Doppler ultrasound or shunt venography. When performed, post-TIPS shunt venography was used as the gold-standard method to determine the stent status. The variceal rebleeding was identified by endoscopic examination. HE was assessed before and during the follow-up. Overt HE refers to the more severe HE grades (West Haven grades II–IV) [16].
Statistical analysis
Data were presented as means ± standard deviations for quantitative variables and as absolute numbers for categorical variables. The study results were analysed using Pearson’s χ2 test, the Mann–Whitney U test and Student’s t test. The cumulative probabilities were estimated using the Kaplan–Meier method and were compared with the log-rank test. All tests of significance were two-sided, and p values less than 0.05 were considered to indicate a significant difference. The data processing and statistical analyses were performed with SPSS (SPSS, version 24; IBM).
Results
Patient characteristics
Nine patients (lost before 1 month of follow-up) were excluded and 82 patients with cardiofundal varices were finally included. Fifty-five patients received adjunctive embolisation during TIPS creation and 27 patients underwent TIPS creation alone. Ten (12.2%) patients were lost to follow-up with a median follow-up time of 10.5 months (range 3–29 months). No significant differences were found between the two groups in the clinical characteristics (p > 0.05, Table 1).
TIPS procedure
The 8-mm e-PTFE-covered stent was used in four patients and the 10-mm covered stent was used in the other patients (p = 0.595). The average pre-TIPS PSG was 21.4 ± 6.5 mmHg and the post-TIPS PSG was 10.2 ± 3.4 mmHg. The haemodynamic success rate was 100%. In the TIPS combined with embolisation group, the metallic coil was used in 36 (65.5%) patients, alpha-cyanoacrylate combined with coil in 18 (32.7%) patients and a vascular plug in 1 (1.8%) patient. During the embolisation process, backflow of the alpha-cyanoacrylate was observed in three patients because of very proximal embolisation. Stent implantation was given in two patients because alpha-cyanoacrylate flowed from the afferent veins back to the intrahepatic shunt. Additionally, haemothorax or haemobilia was observed in one case each.
Variceal rebleeding
During the follow-up, 13 (15.9%) patients suffered recurrent haemorrhages from the varices rupture, including 6 (10.9%) patients from the TIPS combined with embolisation group (median rebleeding time of 21.5 months) and 7 (25.9%) patients from the TIPS alone group (median rebleeding time of 9 months) (Fig. 2). A total of 96.2% and 86.6% of the patients in the TIPS combined with embolisation group and 84.0% and 72.0% of the patients in the TIPS alone group were free from rebleeding at 12 and 24 months, respectively (p = 0.041, Fig. 3). The median time to recurrent bleeding was not reached.
Kaplan–Meier analysis of cumulative free of rebleeding among the 55 patients in the TIPS combined with embolisation group and the 27 patients in the TIPS alone group. There were no significant differences between the two groups (log-rank test, p = 0.041). TIPS transjugular intrahepatic portosystemic shunt
Five patients of variceal rebleeding had evidence of shunt patency; three of these cases were from the TIPS combined with embolisation group and two cases were from the TIPS alone group, receiving medical treatments or cyanoacrylate injection.
Stent dysfunction
Stent dysfunction, which is a common complication of TIPS, occurred in nine patients. Of these patients, 4 (7.3%) patients were from the TIPS combined with embolisation group and 5 (18.5%) patients were from the TIPS alone group (p = 0.126, Fig. 2). Six patients experienced GVs rebleeding; two cases were from the TIPS combined with embolisation group and four cases were from the TIPS alone group. Shunt patency was restored with TIPS revision in seven patients.
Imaging assessment
Seventy-six (92.7%) patients’ dominant gastric fundal varices outflow tracts were gastrorenal shunt, 4 (4.9%) gastrocaval shunt and 1 (1.2%) each was azygous vein or ascending lumbar vein. During the follow-up, 30 (36.6%) patients had CECT images at a median of 8 months (range 0–32 months). Of these 30 patients, eight were from the TIPS alone group and the others were from the TIPS combined with embolisation group (Fig. 2). The rate of cardiofundal varices patency was not significantly different between the two groups (68.2% vs 87.5%, p = 0.391, Fig. 4).
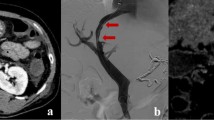
Images from a 61-year-old man with IGV-1. Pre-TIPS axial enhanced CT image (a) demonstrates submucosal components of GV (white arrow) in gastric fundus. The patient underwent TIPS creation and coil embolisation on the LGV and PGV, with PSG reduction from 19 to 9 mmHg. Variceal occlusion (white arrow) is evident on axial enhanced CT scan (b) performed 418 days after TIPS creation; black arrow designates patent shunt
Hepatic encephalopathy
During follow-up, 28 (34.1%) patients experienced overt HE; 18 (64.3%) cases were categorised as West Haven grade II, 3 (10.7%) West Haven grade III and 7 (25.0%) West Haven grade IV (Fig. 2). The 12- and 24-month probability of overt HE was 29.4% and 37.4%, respectively, in the TIPS combined with embolisation group and 33.9% and 33.9%, respectively, in the TIPS alone group (p = 0.746, Fig. 5). No significant difference in the classification of overt HE was found between the two groups (p = 0.507).
Kaplan–Meier analysis of cumulative HE among the 55 patients in the TIPS with embolisation group and the 27 patients in the TIPS alone group. There were no significant differences between the two groups (log-rank test, p = 0.746). TIPS transjugular intrahepatic portosystemic shunt, HE hepatic encephalopathy
Survival
Sixteen (19.5%) patients died during follow-up. Additionally, one patient underwent liver transplantation after 41 months. The transplant-free survival rates after 12 and 24 months were 94.5% and 82.3%, respectively, for the patients who underwent TIPS creation with embolisation and 84.7% and 84.7%, respectively, for the patients who received TIPS alone (p = 0.759, Fig. 6). The causes of death included hepatic failure (37.5%), rebleeding (25%), hepatocellular carcinoma (12.5%), hepatic encephalopathy (12.5%), multiple organ failure (6.25%) and unknown causes (6.25%) (Fig. 2).
Discussion
The present study was conducted to access the efficacy of TIPS combined with embolisation and TIPS alone in the cardiofundal varices bleeding. The results obtained suggested that TIPS combined with embolisation significantly lowered the rebleeding rate, but could not prevent the overt HE and the varices patency as compared with TIPS alone.
Recurrent variceal bleeding was lower in the TIPS combined with embolisation group than that in the TIPS alone group, a result which differs from previous studies [7, 14]. First, this inconsistency may be due to the different objectives as the previous studies incorporated all types of GVs. Gastro-oesophageal varices type 1, the most common GV subtype, is considered as a continuation of oesophageal varices, sharing similar vascular anatomy and response to treatments [13]. The common dominant feeders of cardiofundal varices are the posterior gastric vein (PGV) or the short gastric vein (SGV) [17, 18]. These veins, which are located distant from the TIPS compared with the left gastric vein (LGV), may maintain the preferential blood flow through spontaneous portosystemic shunt after TIPS creation [19, 20]. Second, a possible explanation for this discrepancy may be the MELD score because this score is positively correlated with the rebleeding risk [21]. The MELD scores reported in the previous studies were obviously higher than that in present study (15.5 and 13.5 vs 9.7).
The majority of cardiofundal varices showed consistent patency despite TIPS creation and concurrent embolisation of afferent vessels, which was in accordance with the results of a previous study [14]. We speculated that it might be caused by the mechanism and location of embolisation as the metallic coil was only used in most patients to embolise the proximity of the feeders. Balloon-occluded retrograde transvenous obliteration (BRTO) has been introduced as an alternative procedure for the treatment of GVs with large gastrorenal shunt [22,23,24]. This approach is desirable as it could directly eliminate the submucosal parts of GVs. However, it is questionable whether variceal patency is a significant clinical outcome for TIPS creation. Because size of varices was directly related to the risk of bleeding. The patients should remain free of variceal rebleeding if the varices became smaller and the PSG became lower after TIPS combined with embolisation.
At the same time, there is no significant difference in overt HE between the two groups. In contrast, Shi et al [25] reported that TIPS with adjunctive embolisation using cyanoacrylate decreased the HE incidence as compared with TIPS alone. The degree of embolisation may explain this discrepancy. The efficacy of liquid embolic material appears to be superior to that of metallic coils [26, 27]. Moreover, a probable explanation may be the use of stents with different diameters. A recent study suggested that the stent diameter is a risk factor for the incidence of HE [28]. A smaller stent was implanted in the TIPS combined with embolisation group in the previous study, whereas a 10-mm-diameter stent was implanted in most patients in this study.
The use of adjunctive embolisation during the TIPS process is generally safe and well tolerated [29, 30]. The primary advantage of embolisation after stent implantation is the ability to assess the response of angiographic collateral vessel filling after TIPS creation. However, a patent shunt represents a channel for misplaced embolisation of embolic material. In this study, stent implantation was performed in two patients because of alpha-cyanoacrylate migration. Distal embolisation with a microcatheter could be helpful in the prevention of ectopic embolism.
The present study had several important limitations. First, this study represents the non-randomized, retrospective experience of a single institution. Second, embolisation was specifically performed at the discretion of the operators. Thus, the numbers of patients in the two cohorts were disproportionate. Third, the Fluency stent was implanted in all patients instead of the Viatorr stent. The Viatorr stent is superior to the Fluency during the TIPS creation [31]. However, it was not available in our country until 2016. Several recent studies suggested that TIPS using the Fluency stent is also effective in treating complications of portal hypertension [28, 32]. Finally, CECT examination was not performed in all cases after TIPS creation because it was not necessary during the follow-up.
In conclusion, TIPS combined with concurrent embolisation might be more effective than TIPS alone in reducing the risk of variceal rebleeding, despite not exerting a great influence on varices occlusion and overt HE incidence.
Abbreviations
- BRTO:
-
Balloon-occluded retrograde transvenous obliteration
- CECT:
-
Contrast-enhanced computed tomography
- GOV:
-
Gastro-oesophageal varices
- GVs:
-
Gastric varices
- HE:
-
Hepatic encephalopathy
- IGV:
-
Isolated gastric varices
- LGV:
-
Left gastric vein
- PGV:
-
Posterior gastric vein
- PSG:
-
Portosystemic pressure gradient
- SGV:
-
Short gastric vein
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
References
Rossle M (2013) TIPS: 25 years later. J Hepatol 59:1081–1093
Dariushnia SR, Haskal ZJ, Midia M et al (2016) Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 27:1–7
Krajina A, Hulek P, Fejfar T, Valek V (2012) Quality improvement guidelines for transjugular intrahepatic portosystemic shunt (TIPS). Cardiovasc Intervent Radiol 35:1295–1300
Xiao T, Chen L, Chen W et al (2011) Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol 45:643–650
Qi X, Liu L, Bai M et al (2014) Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol 29:688–696
Chen S, Li X, Wei B et al (2013) Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology 268:900–906
Gaba RC, Bui JT, Cotler SJ et al (2010) Rebleeding rates following TIPS for variceal hemorrhage in the Viatorr era: TIPS alone versus TIPS with variceal embolization. Hepatol Int 4:749–756
Garcia-Pagan JC, Barrufet M, Cardenas A, Escorsell A (2014) Management of gastric varices. Clin Gastroenterol Hepatol 12:919–928 e911; quiz e951-912
Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK (1992) Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 16:1343–1349
Tripathi D, Therapondos G, Jackson E, Redhead DN, Hayes PC (2002) The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut 51:270–274
Stanley AJ, Jalan R, Ireland HM, Redhead DN, Bouchier IA, Hayes PC (1997) A comparison between gastric and oesophageal variceal haemorrhage treated with transjugular intrahepatic portosystemic stent shunt (TIPSS). Aliment Pharmacol Ther 11:171–176
Jalan R, Redhead DN, Forrest EH, Hayes PC (1995) Relationship between directly measured portal pressure gradient and variceal hemorrhage. Am J Gastroenterol 90:1994–1996
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J (2017) Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 65:310–335
Lakhoo J, Bui JT, Lokken RP, Ray CE Jr, Gaba RC (2016) Transjugular intrahepatic portosystemic shunt creation and variceal coil or plug embolization ineffectively attain gastric variceal decompression or occlusion: results of a 26-patient retrospective study. J Vasc Interv Radiol 27:1001–1011
Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li X (2015) Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology 276:286–293
Vilstrup H, Amodio P, Bajaj J et al (2014) Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 60:715–735
Saad WE (2013) Vascular anatomy and the morphologic and hemodynamic classifications of gastric varices and spontaneous portosystemic shunts relevant to the BRTO procedure. Tech Vasc Interv Radiol 16:60–100
Kiyosue H, Ibukuro K, Maruno M, Tanoue S, Hongo N, Mori H (2013) Multidetector CT anatomy of drainage routes of gastric varices: a pictorial review. Radiographics 33:87–100
Saad WE (2014) Combining transjugular intrahepatic portosystemic shunt with balloon-occluded retrograde transvenous obliteration or augmenting TIPS with variceal embolization for the management of gastric varices: an evolving middle ground? Semin Intervent Radiol 31:266–268
Sanyal AJ, Freedman AM, Luketic VA et al (1997) The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology 112:889–898
Lakhoo J, Bui JT, Zivin SP et al (2015) Root cause analysis of rebleeding events following transjugular intrahepatic portosystemic shunt creation for variceal hemorrhage. J Vasc Interv Radiol 26:1444–1453
Mukund A, Deogaonkar G, Rajesh S, Shasthry SM, Sarin SK (2017) Safety and efficacy of sodium tetradecyl sulfate and lipiodol foam in balloon-occluded retrograde transvenous obliteration (BRTO) for large porto-systemic shunts. Cardiovasc Intervent Radiol 40:1010–1016
Kobayakawa M, Kokubu S, Hirota S et al (2017) Short-term safety and efficacy of balloon-occluded retrograde transvenous obliteration using ethanolamine oleate: results of a prospective, multicenter, single-arm trial. J Vasc Interv Radiol 28:1108–1115
Luo X, Ma H, Yu J, Zhao Y, Wang X, Yang L (2017) Efficacy and safety of balloon-occluded retrograde transvenous obliteration of gastric varices with lauromacrogol foam sclerotherapy: initial experience. Abdom Radiol. https://doi.org/10.1007/s00261-017-1346-6
Shi Y, Tian X, Hu J et al (2014) Efficacy of transjugular intrahepatic portosystemic shunt with adjunctive embolotherapy with cyanoacrylate for esophageal variceal bleeding. Dig Dis Sci 59:2325–2332
Urbano J, Cabrera M, Alonso-Burgos A (2014) Sclerosis and varicocele embolization with N-butyl cyanoacrylate: experience in 41 patients. Acta Radiol 55:179–185
Sze DY, Kao JS, Frisoli JK, McCallum SW, Kennedy WA 2nd, Razavi MK (2008) Persistent and recurrent postsurgical varicoceles: venographic anatomy and treatment with N-butyl cyanoacrylate embolization. J Vasc Interv Radiol 19:539–545
Wang Q, Lv Y, Bai M et al (2017) Eight millimeter covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. https://doi.org/10.1016/j.jhep.2017.05.006
Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W (2005) Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology 236:360–367
Alkari B, Shaath NM, El-Dhuwaib Y et al (2005) Transjugular intrahepatic porto-systemic shunt and variceal embolisation in the management of bleeding stomal varices. Int J Colorectal Dis 20:457–462
Saad W, Darwish W, Davies M, Waldman D (2010) Stent-grafts for transjugular intrahepatic portosystemic shunt creation: specialized TIPS stent-graft versus generic stent-graft/bare stent combination. J Vasc Interv Radiol 21:1512–1520
Lv Y, Qi X, He C et al (2017) Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. https://doi.org/10.1136/gutjnl-2017-314634
Funding
This study has received funding by the Beijing Hope Run Special Fund of Cancer Foundation of China (Grant No. LC2015A01 to X.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xiao Li.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
No complex statistical methods were necessary for this paper.
Informed Consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical Approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Yu, J., Wang, X., Jiang, M. et al. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone and combined with embolisation for the management of cardiofundal varices: a retrospective study. Eur Radiol 29, 699–706 (2019). https://doi.org/10.1007/s00330-018-5645-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5645-2