Abstract
The purpose of this study was to evaluate the efficacy of percutaneous endovascular techniques for the treatment of hepatic artery stenosis (HAS) occurring after liver transplantation (LT) in adult and pediatrics patients. From February 2003 to March 2009, 25 patients (15 adults and 10 children) whose developed HAS after LT were referred to our interventional radiology unit. Technical success was achieved in 96% (24 of 25) of patients. Percutaneous transluminal angioplasty (PTA) was performed in 13 patients (7 children), and stenting was performed in 11 patients (2 children). After the procedure, all patients were followed-up with liver function tests, Doppler ultrasound, and/or computed tomography. Mean follow-up was 15.8 months (range 5 days to 58 months). Acute hepatic artery thrombosis occurred immediately after stent deployment in 2 patients and was successfully treated with local thrombolysis. One patient developed severe HA spasm, which reverted after 24 h. After the procedure, mean trans-stenotic pressure gradient decreased from 30.5 to 6.2 mmHg. Kaplan–Meyer curve of HA primary patency was 77% at 1 and 2 years. During the follow-up period, 5 patients (20%) had recurrent stenosis, and 2 patients (8.3%) had late thrombosis. Two of 7 patients with stenosis/thrombosis underwent surgical revascularization (n = 1) and liver retransplantation (n = 1). Six (25%) patients died during follow-up, but overall mortality was not significantly different when comparing patients having patent hepatic arteries with those having recurrent stenosis/thrombosis. There were no significant differences in recurrent stenosis/thrombosis and mortality comparing patients treated by PTA versus stenting and comparing adult versus pediatric status. Percutaneous interventional treatment of HAS in LT recipients is safe and effective and decreases the need for surgical revascularization and liver retransplantation. However, the beneficial effects for survival are not clear, probably because the clinical complexity of many of these cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hepatic artery (HA) plays an important physiologic role after liver transplantation (LT) by providing blood supply for both the liver parenchyma and bile duct system. Hepatic artery stenosis (HAS) is one of the most insidious vascular complications occurring after LT. It can cause deterioration of liver function, biliary damage, and sepsis due to graft ischemia and possible progression in hepatic artery thrombosis (HAT). The most common complication seen in patients with HAS on cholangiography is nonanastomotic biliary stricture, which is seen in ≤49% of patients [1], whereas ischemic cholangitis and secondary biliary cast syndrome are the most common complications severely impacting graft and patient survival in patients with progression to HAT.
HAS occurs in approximately 5% of adult patients and 11–20% of pediatric patients who undergo LT [2–4]. HAS usually occurs at or near the anastomosis site because of operative technique or vascular clamp injury. The main risk factors include complex vascular reconstruction and small caliber of donor and recipient vessels.
Although surgical revision has traditionally been the therapy of choice for HAS, endovascular techniques, including percutaneous transluminal angioplasty (PTA) and stenting, have been suggested as alternative therapeutic options [4–15]. However, there is no consensus regarding the optimal treatment of HAS. The purpose of our study was to analyze the efficacy of percutaneous endovascular techniques (PTA or stenting) in 25 consecutive LT recipients (15 adults and 10 children) with HAS referred to our interventional radiology unit from February 2003 to March 2009.
Materials and Methods
No financial support was provided for this study. Informed consent was not specifically required, although written informed consent was obtained from each patient or from the parent (in the case of paediatric patients) for every diagnostic and interventional radiology procedure performed after a full explanation of the purpose and risks of the procedures was provided. The internal Institutional Research Review Board and Ethical Committee reviewed and approved this retrospective study.
In a single transplant centre, from February 2003 to March 2009, 404 LTs were performed in adult patients, and 99 LTs were performed in pediatric patients. In adult patients, Doppler studies were performed daily for the first 7 postoperative days, at 2 weeks, and during follow-up when any abnormalities were detected on liver function tests. In pediatric patients, Doppler studies were performed daily for the first 7 postoperative days, every 2 days during the second postoperative week, every 2 month for 6 months, every 3 months for 1 year, and then every year thereafter. All patients with pathologic Doppler ultrasound (US) intrahepatic resistive index <0.5 and systolic ascending time >0.8 ms, with alteration of liver function tests not explained by other US imaging findings and without biopsy-proven evidence of rejection, underwent multidetector computed tomography (MDCT). If MDCT confirmed arterial stenosis, the patients were referred to the interventional radiology unit, and an arteriogram was performed to confirm the diagnosis and possibly treat the stenosis. Twenty-five LT recipients with HAS constituted our study group (Table 1). A review of the patients’ charts, radiology imaging studies, and interventional procedures was performed.
Patients
There were 15 adult patients (10 male and 5 female; mean age 48 years [range 19–68]). Indications for LT included hepatocellular carcinoma (HCC) in 7 patients; hepatitis C virus (HCV) cirrhosis in 2 patients; and fulminant hepatitis, metabolic disease (MD), primary sclerosing cholangitis (PSD), cryptogenetic cirrhosis (CC), drug-induced hepatitis, and sickle cell disease (SCD) in 1 patient each. One patient had a history of previous LT for HAT. Thirteen patients underwent whole-liver transplantation (WLT); 1 patient underwent extended right graft split-liver transplantation (ERSLT); and 1 patient underwent right lobe liver transplantation from a living-related liver transplant (LRLT). In 14 patients, end-to-end arterial anastomosis was performed, and 1 patient underwent arterial bypass with a donor iliac artery conduit to the recipient infrarenal aorta. Hepatic artery anomalies requiring bench reconstruction were detected in 3 grafts.
There were 10 pediatric patients (6 male and 4 female; mean age 6.4 years [range 9 months to 13 years]). Indications for LT included biliary atresia (BA) in 3 patients and HCC, fulminant hepatitis, Langerhans’ cell histiocytosis (LCH), Alagille syndrome (AS), Wilson’s disease (WD), drug-induced hepatitis, and Caroli disease (CD) in 1 patient each. Two recipients had a history of previous LT for HAS.
Four patients underwent ERSLT; 4 patients underwent left lateral segment liver transplantation (LLSLT); and 2 patients underwent WLT. End-to-end arterial anastomosis was performed in 8 patients, and 2 patients underwent arterial bypass; in particular, a donor iliac artery conduit was interposed between the right branch of the ERSL and the recipient celiac trunk in one patient and between the graft HA and the infrarenal aorta in one patient. One patient had a previous history of surgical reconstruction for HAT. The time between interventional procedures and LT was 53.6 days (range 7–168).
Technique
All of the procedures were performed in the angiographic suite (Advantax; GE Healthcare, Fairfield, USA), after the patients were fasted overnight, by three radiologists having 6, 9, and 19 years of experience, respectively, in abdominal interventional radiology. The procedures were performed with adult patients under monitored anesthesia care, consisting of spontaneous respiration and local anesthesia, and with 2 of 10 pediatric patients under monitored anesthesia care, which consisted of spontaneous respiration; the other 8 pediatric patients received general anesthesia. A warming blanket, which was placed under the patient, was used in all pediatric cases to avoid hypothermia. Noninvasive monitoring of arterial pressure (by way of automatic sphygmomanometer), heart rate (derived from continuous electrocardiogram), and oxygen saturation (by way of pulse oximeter) was performed.
All pediatric femoral access procedures were performed with standard percutaneous technique, i.e., vessel palpation, using a 21-gauge needle (Boston Scientific, Cork, Ireland) and a floppy-tipped nitinol wire (Cope Mandril Wire; Cook, Bjaewerskov, Denmark), whereas an 18-gauge needle (Boston Scientific) with a standard 0.038-inch wire (Newton Cerebral Wire; Cook) was used in adult patients. All patients underwent selective hepatic arteriogram with use of digital subtraction imaging, by way of the transfemoral route, using 4F Cobra 2 (C2) (Angiodynamics, Queensbury, NY) catheters in pediatric patients and 5F C2 or SOS (Angiodynamics) catheters in adult patients. Diagnosis of HAS was considered if there was a decrease in arterial diameter by at least 50%. A coaxial microcatheter (TurboTraker; Boston Scientific) was then advanced in the HA just before stenosis. Arterial pressure before stenosis was measured by linking the microcatheter to a pressure-transducer set (Edwards Lifesciences, Unterscleissheim, Germany), which was linked to a multichannel recorder (Solar 8000; GE Medical Systems, GE Healthcare). The stenosis was then crossed using a hydrophilic wire (Radiofocus Guide Wire with Gold Coil; Terumo, Tokyo, Japan), and the microcatheter was advanced through the stenosis. Arterial pressure was measured again, and the trans-stenotic pressure gradient (TPG) measurement was obtained.
A 5F to 7F guide catheter (Boston Scientific) was advanced at the origin of the feeding vessel over a 0.018- or a 0.014-inch stiff wire (Hi-Torque Balance Middleweight guidewire; Abbott, Diegen, Belgium, or V18 Control wire; Boston Scientific). PTA was performed using high-pressure balloon catheters with diameters ranging from 2.5 to 5 mm (Sterling or Simmetry; Boston Scientific) (Figs. 1 and 2).
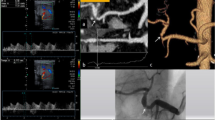
Sixty-five-year-old man after WLT for HCC. (A) DSA celiac arteriogram performed 28 days after LT shows severe HAS. (B) Because there was occurrence of severe HA spasm during selective catheterization, the procedure was suspended. (C) DSA celiac arteriogram performed 1 week later shows resolution of the spasm. (D) PTA was successfully performed with a 4-mm balloon catheter. TPG measurement decreased from 50 to 4 mmHg. There was no evidence of HAS recurrence at 19-month follow-up
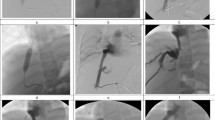
Eight-year-old boy after ERSLT for autoimmune hepatitis (AH). (A) DSA celiac arteriogram performed 51 days after LT shows severe anastomotic HAS. (B) DSA obtained after PTA shows improved luminal caliber. TPG measurement decreased from 48 to 8 mmHg. At 2-year follow-up, US shows a widely patent HA with resistive index in the normal range. Currently the patient is clinically asymptomatic and has good liver function
A covered balloon-expandable stent (Graftmaster Coronary Stent Graft System; Abbott) was implanted in five patients, and an uncovered stent (Express Vascular; Boston Scientific) was implanted in six patients (Fig. 3). Stent diameters and lengths ranged, respectively, from 4 to 7 mm and 15 to 26 mm. Different kinds of stents were chosen according to lesion size and diameter as well as the stent types available in our unit.
Forty-five-year-old man after WLT for HCV cirrhosis. (A) DSA celiac arteriogram performed 150 days after LT showed severe anastomotic HAS. (B) DSA obtained after placement of a 7 mm-diameter uncovered stent shows improved luminal caliber. TPG measurement decreased from 25 to 6 mmHg. At 1-year follow-up, US showed a widely patent HA with resistive index in the normal range. Currently the patient is clinically asymptomatic and has good liver function
Before the procedure, 0.2 mg nitroglycerine (PHT Pharma, Milano, Italy) and 2000–3000 UI heparin (100 UI/kg body weight in pediatric patients) were infused into the HA to decrease the risk of spasm or thrombosis. TPG measurement and hepatic arteriogram were repeated at the end of the procedure. The procedure was considered technically successful if a normal lumen diameter (or minimal residual stenosis), along with a significant decrease in TPG measurement, was obtained on final angiogram. Iodixanol iso-osmolar contrast medium (Visipaque 320 mgI/ml; Amersham Health, GE Healthcare) was used in all the procedures. Posttreatment antiplatelet therapy with aspirin was maintained for 1 year in adult patients and for 2 years in pediatric patients.
Follow-Up
Doppler US was performed the day after the procedure to evaluate intrahepatic and extrahepatic arterial resistive index and systolic acceleration time as baseline measurements for Doppler US follow-up. Patients were periodically followed-up with clinical evaluation and liver function tests. Doppler US was performed when any abnormalities were detected on liver function tests. Intra-hepatic resistive index <0.5 and systolic ascending time >0.8 ms were considered suggestive of HA restenosis. HAS detected by Doppler US was further investigated by MDCT and, if necessary, by hepatic arteriogram for final diagnosis. Mean follow-up was 15.8 months (range 5 days to 58 months).
Statistical Analyses
Comparison between patient groups was performed using Student t test for continuous variables and chi-square test for categoric variables, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (version 12.0; SPSS, Chicago, IL).
Results
Anastomotic stenosis was detected in all patients by angiography, and 2 patients had tandem lesions (both anastomotic and distal to the anastomosis). Three patients (1 pediatric) had long stenoses (>1 cm). PTA was performed in 13 patients (8 pediatric), whereas stenting was performed in 12 patients (2 pediatric). Two stents were deployed in 3 adult patients in cases of tandem stenotic lesions (n = 2) and in case of nonperfect position of the first stent (n = 1). Technical success was achieved in 24 of 25 (96%) cases. In 1 pediatric patient with severe anastomotic stricture, we were not able to advance a microcatheter thought the stenotic segment. Mean TPG measurement decreased from 30.5 to 6.2 mmHg (P < 0.05).
Complications
Four procedure-related immediate complications developed (16%). In particular, one small iatrogenic pseudoaneurysm spontaneously resolved in a pediatric patient; one severe HA spasm spontaneously resolved in an adult patient (Fig. 1); and two acute HA thromboses, which developed immediately after stent deployment, were successfully treated with local thrombolysis in one adult and one pediatric patient.
The pediatric patient who developed the pseudoaneurysm was 9 years old at the time of LT. The procedure was performed 16 days after LT for progressive worsening of Doppler resistive index and liver function tests. We were able to cross the stenosis with a wire, but it was impossible to pass a microcatheter thought the stenotic segment. We suspended the procedure. One day later we performed angiography to try PTA again, but the arteriogram showed a small iatrogenic pseudoaneurysm at the anastomosis. The aneurysm was also evaluable with US. Two days later the aneurysm was no longer evaluable on US. We did not perform angiography again because the patient’s liver function tests slowly improved. MDCT performed 2 months later showed no signs of pseudoaneurysm and mild to moderate narrowing of the anastomosis. At 4-years follow-up, the patient is in good condition. His resistive index is within normal limits (0.60 to 0.65), and there is no biliary tree impairment.
The pediatric patient who developed acute HA thrombosis was 1 year old at the time of LT. Local thrombolysis was performed 19 days after LT. The patient had a long stenosis on angiography. A covered stent was used after failure of PTA. An acute thrombosis was detected after stent deployment. A 2000-U bolus dose of heparin was administered, followed by intrahepatic boluses totaling 10 mg tissue plasminogen activator (tPA) and 10 mg tPA with slow infusion (30 min) into the hepatic artery. Final arteriogram showed good intrahepatic flow, but the patient developed biliary strictures, one intrahepatic and one at the anastomosis, 1 month later. The strictures were successfully treated with biliary catheter placement and balloon dilatations. Currently the patient is in good general conditions at 1-year follow-up (9 months without a biliary catheter), and his resistive index on Doppler US is within normal limits.
In the adult patient acute HA thrombosis, local throbolysis was performed 7 days after LT. Doppler US resistive index worsened daily. After MDCT, in accord with the transplantation team, we decided to attempt an endovascular procedure to avoid thrombosis. The patient had tandem stenoses on angiography. Two covered stents were deployed. An acute thrombosis was detected after stent deployment. A 5000-U bolus dose of heparin was administered, followed by intrahepatic boluses totaling 20 mg tPA and 10 mg tPA with slow infusion (30 min) from the hepatic artery. Final arteriogram showed good intrahepatic flow, but the patient developed intrahepatic biliary strictures 7 months later. Currently the patient is in good general condition with two endoscopic biliary stents.
Overall HA primary patency was 70.8% (17 of 24 patients). There were no significant differences in HA primary patency between adult versus pediatric status (66.7% vs. 77.7%) or between patients treated with PTA versus those treated with stenting (69.2% vs. 72.7%). Kaplan–Meyer curve of HA primary patency showed 77% at 1 and 2 years (Fig. 4).
Seven patients developed delayed complications during follow-up, in particular, late thrombosis (n = 2) or restenosis (n = 5). Two late stent thromboses developed in two adult recipients, with two stents in both, at 4 and 5 months after the procedure. In both patients, the diagnosis was suspected on Doppler US and confirmed on MDCT. One patient developed sufficient arterial collaterals and is currently in good general conditions. The second patient was retransplanted 2 months after diagnosis of thrombosis and is currently in good general condition. Five patients, two pediatric and three adult, developed restenosis.
In one pediatric patient, restenosis was suspected 2 days after the procedure on Doppler US. The patient died 9 days after PTA from gastrointestinal (GI) bleeding unrelated to the procedure. The second pediatric patient developed Doppler US–suspected stenosis 3 years after PTA, but because of the patient’s stable clinical condition, i.e., no alteration in liver function tests, no further treatment was performed.
One adult patient with early restenosis detected on US and confirmed on MDCT underwent surgical revascularization 7 days after failed PTA and is currently in good general condition. Another adult patient developed Doppler US–suspected restenosis 3 years after PTA, but because of the patient’s stable clinical condition, no further treatment was performed. The patient is currently in good general condition.
The last patient, the same adult patient who developed early thrombosis that was treated with local thrombolysis, developed Doppler US findings suspicious of restenosis 4 weeks after deployment of two stents. Because of the previous acute thrombosis, we decided not to perform a second endovascular procedure. The patient currently has two endoscopic biliary stents.
All patients (n = 3) with 2 stents developed late complications, in particular, 1 restenosis and 2 late thromboses. One patient had acute thrombosis, which was treated with thrombolysis. Four weeks later, pathologic Doppler US findings suggested restenosis. In 1 of the 2 cases of late stent thrombosis (LST), difficult anatomy with tortuous vessels could explain 1 case, whereas coexistent severe rejection could explain the other. During follow-up, 8 of 24 (33%) treated patients (3 pediatric and 5 adult) developed biliary complications. Of note, 2 of 8 patients had intrahepatic and anastomotic strictures, whereas 6 patients had only anastomotic strictures.
Overall mortality was 25% (6 of 24 patients). Causes of death included septic multiple-organ failure (n = 3), GI bleeding (n = 1), cerebral hemorrhage (n = 1), and recurrent HCV cirrhosis (n = 1). There were no significant differences in mortality between adult and pediatric patients (20% vs. 33%, not significant [NS]) between patients treated with PTA versus stenting (30.8% vs. 18.2% [NS]) and patients with patent HAs and those with recurrent stenosis/thrombosis (29% vs. 14.3% [NS]) during follow-up.
Discussion
The results of our study underscore that percutaneous interventional treatments of HAS in adult and pediatric LT recipients are safe and effective and may decrease the need for surgical revascularization and/or liver retransplantation.
However, although PTA with or without stent placement has been reported in adult and pediatric patients with HAS after LT [4–15], few data, due to the limited number of treated patients, are available on mid-term and long-term follow-up, especially for pediatric patients, for which only small series or cases reports exist in the literature. Our report contributes to the data currently available on this topic.
Of note, only two patients developed intrahepatic biliary strictures during follow-up, and each had acute thrombosis during treatment. Six patients developed anastomotic stenosis. Considering that 9 of 24 treated patients (38%) had partial LTs and a high rate of biliary complications, the incidence of biliary complications in this cohort of treated patients was not greater than that in a similar cohort of patients with patent HAs.
One salient result of this small series is that although a high technical success rate is reported, the beneficial effects on survival are not clear, perhaps because of the clinical complexity of many of these cases, reflected by the fact that there were no significant differences in mortality between patients with patent HA and patients with recurrent stenosis/thrombosis (29% vs. 14.3% [NS]) during follow-up. Several independent complications, such as anastomotic biliary strictures, rejection, infective complications, or other vascular complications, can coexist in LT recipients with HAS. In our series, causes of death were septic multiple-organ failure, GI bleeding, cerebral hemorrhage, and recurrent HCV cirrhosis, all of which were unrelated to HAS.
Even considering the small number of patients studied, our experience supports that endovascular treatments of HAS could be of similar clinical value in children as in adults. This is illustrated by the finding that no significant differences in HA primary patency (66.7% vs. 77.7% [NS]), mortality (20% vs. 33% [NS]), or procedural complications were found between adults and pediatric patients. In our opinion, this encourages the use of endovascular procedures to treat HAS in pediatric patients.
However, the percutaneous approach in pediatric patients, especially in infants, is a challenging procedure with some differences from that in adult patients. Even “simple” femoral vascular access can be difficult in very small babies, and, occasionally, surgical exposure of the artery is required [16]. In our small series, the youngest patient was 9 months old, and we had no need to surgically expose the artery.
Pediatric vessels, especially in children weighing >15 kg, tend to occlude more easily compared with adult patients, and there is a greater occurrence of vasospasm and dissection. The overall incidence of vascular complications of arteriography in the adult population is <1%, whereas the risk may be as high as 7% to 10% in the pediatric population [16], predominantly in neonates and infants. This occurs because the caliber of vessels in young children is very small, and infant arteries tend to be more easily injured than adult arteries; they frequently develop severe spasm as a result of mechanical stimulation.
Another salient point is the need for general anesthesia in the majority of pediatric patients. However, this limitation applies to all of the usual procedures, including endoscopy, liver biopsy, and even noninvasive imaging studies (e.g., computed tomography or magnetic resonance), required in severely ill pediatric patients. Serious adverse events during or after deep sedation or general anesthesia are rare in the hands of experienced anesthesiologists, and the risk of death is <1/100,000 cases [17].
As a technical note, routine TPG measurement, performed before and after endovascular treatment, can easily be done with no significant prolongation of the procedure and adds valuable information regarding the hemodynamic results obtained. TPG measurement has been used to evaluate stenosis and response to angioplasty of the venous system in LT recipients, but no data are available in the literature to evaluate TPG measurement in the HAs of transplanted patients. A pressure gradient >3 mmHg between the hepatic vein and the right atrium has been reported to be pathologic in transplanted children with hepatic vein stensosis [18], whereas the persistence of a pressure gradient >5 mmHg between the hepatic vein and the right atrium after several angioplasties has been reported as a failure sign and considered an indication for placement of a metallic stent [19].Using this data, we empirically considered a threshold of 10 mmHg as a significant TPG measurement in the arterial system with high pressure. TPG measurement seems helpful especially in those patients who, on final angiogram, have mild to moderate residual stenosis, and the decision to perform a new PTA or place a stent could be taken in consideration. In the investigators’ experience, a final TPG measurement <10 mmHg is considered a good technical result. However, no data are available in the literature to evaluate whether, and at what threshold, the final TPG measurement could be a predictor of HAS recurrence or progression to thrombosis. Further studies on this topic could be easily performed.
Although stenoses that occur in the immediate postoperative period are likely related to faulty surgical technique, successful endovascular procedures have been reported [13, 14]. Four of our treatments, two in pediatric patients, were performed within 2 weeks of LT. There was no bleeding from the suture line, but one recipient developed acute thrombosis after stent deployment. Nevertheless, we consider endovascular treatment of HAS more safely performed after a period of several weeks to decrease possible rupture of the suture line. In our practice, we consider early endovascular treatment only in selected cases after collegial revision with the surgical transplant team and balancing the pro and cons of eventual surgical revision for every case.
Currently there is no consensus regarding the optimal endovascular treatment of HAS. A cumulative primary patency rate of 53% to 60% at 1 year for covered coronary stents used to treat HAS have been reported [11–15]. In adult recipients undergoing PTA, a patency rate of 60% to 80% at 1 year has been reported [7, 10]. Although the superiority of vascular stenting over balloon angioplasty has been reported in patients with coronary artery stenosis after cardiac transplantation [20], no prospective randomized studies are available to evaluate the efficacy of PTA versus primary stenting or to evaluate the efficacy of covered versus noncovered stents in LT recipients with HAS.
As a rule in our pediatric patients, because long-term follow-up of stents is still unknown, PTA has always been considered the first line of treatment, and stenting is performed only in cases of PTA failure (i.e., persistent significant stenosis on angiogram and/or TPG measurement not significantly decreased). In adult patients, PTA or primary stenting is chosen by the radiologist after angiogram is performed; PTA is preferred in cases of arterial tortuosity and small arterial diameter [10].
Our study has some limitations: (1) the sample size is small and (2) it is not possible to correctly evaluate the efficacy of PTA versus primary stenting because randomization for the treatment was not performed, and thus the results of this study cannot offer much information concerning this matter. Of note, mortality was not significantly different between patients treated with PTA versus those treated with stenting (30.8% vs. 18.2% [NS]), but it is possible that a significant difference could be proven in a large group of patients.
In conclusion, percutaneous endovascular treatment of HAS after LT represents a valuable therapeutic option in adult and pediatric recipients. However, the beneficial effects for survival are not clear, probably because of the clinical complexity of many of these cases.
References
Orons PD, Sheng R, Zajko AB (1995) Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. Am J Roentgenol 165:1145–1149
Moray G, Boyvat F, Sevmiş S, Karakayali F, Ayvaz I, Dalgiç A et al (2005) Vascular complications after liver transplantation in pediatric patients. Transplant Proc 37(7):3200–3202
Sieders E, Peeters PM, TenVergert EM, de Jong KP, Porte RJ, Zwaveling JH et al (2000) Early vascular complications after pediatric liver transplantation. Liver Transpl 6(3):326–332
Abbasoglu O, Levy MF, Vodapally MS, Goldstein RM, Husberg BS, Gonwa TA et al (1997) Hepatic artery stenosis after liver transplantation: incidence, presentation, treatment and long term outcome. Transplantation 63:250–255
Amesur NB, Zajko AB (2006) Interventional radiology in liver transplantation. Liver Transpl 12:330–351
Abad J, Hidalgo EG, Cantarero JM, Parga G, Fernandez R, Gomez M et al (1989) Hepatic artery anastomotic stenosis after transplantation: treatment with percutaneous transluminal angioplasty. Radiology 171(3):661–662
Saad WE, Davies MG, Sahler L, Lee DE, Patel NC, Kitanosono T et al (2005) Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol 16(6):795–805
Hasegawa T, Sasaki T, Kimura T, Okada A, Nakatsuchi Y, Sugiura T et al (2002) Successful percutaneous transluminal angioplasty for hepatic artery stenosis in an infant undergoing living-related liver transplantation. Pediatr Transplant 6(3):244–248
Boyvat F, Aytekin C, Karakayali H, Ozyer U, Sevmis S, Emiroğlu R et al (2006) Stent placement in pediatric patients with hepatic artery stenosis or thrombosis after liver transplantation. Transplant Proc 38(10):3656–3660
Kodama Y, Sakuhara Y, Abo D, Shimamura T, Furukawa H, Todo S et al (2006) Percutaneous transluminal angioplasty for hepatic artery stenosis after living donor liver transplantation. Liver Transpl 12:465–469
Denys AL, Qanadli SD, Durand F, Vilgrain V, Farges O, Belghiti J et al (2002) Feasibility and effectiveness of using coronary stents in the treatment of hepatic artery stenoses after orthotopic liver transplantation: preliminary report. Am J Roentgenol 178:1175–1179
Huang M, Shan H, Jiang Z, Li Z, Zhu K, Guan S et al (2006) The use of coronary stent in hepatic artery stenosis after orthotopic liver transplantation. Eur J Radiol 60:425–430
Orons PD, Zajko AB (1995) Angiography and interventional procedures in liver transplantation. In imaging of organ transplantation. Radiol Clin North Am 33:541–558
López-Benítez R, Schlieter M, Hallscheidt PJ, Radeleff BA, Kauffmann G, Richter GM et al (2008) Successful arterial thrombolysis and percutaneous transluminal angioplasty for early hepatic artery thrombosis after split liver transplantation in a four-month-old baby. Pediatr Transplant 12(5):606–610
Ueno T, Jones G, Martin A, Ikegami T, Sanchez EQ, Chinnakotla S et al (2006) Clinical outcomes from hepatic artery stenting in liver transplantation. Liver Transpl 12:422–427
Heran MK, Marshalleck F, Temple M, Grassi CJ, Connolly B, Towbin RB et al (2010) Joint quality improvement guidelines for pediatric arterial access and arteriography: from the Societies of Interventional Radiology and Pediatric Radiology. Pediatr Radiol 40:237–250
Guthrie EW (2006) General anesthesia in pediatric patients. US Pharm 8:HS15–HS27
Kubo T, Shibata T, Itoh K, Maetani Y, Isoda H, Hiraoka M et al (2006) Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantation. Radiology 239:285–290
Lorenz JM, Van Ha T, Funaki B, Millis M, Leef JA, Bennett A et al (2006) Percutaneous treatment of venous outflow obstruction in pediatric liver transplants. J Vasc Interv Radiol 17(11 Pt 1):1753–1761
Heublein B, Pethig K, Maas C, Wahlers T, Haverich A (1997) Coronary artery stenting in cardiac allograft vascular disease. Am Heart J 134:930–938
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maruzzelli, L., Miraglia, R., Caruso, S. et al. Percutaneous Endovascular Treatment of Hepatic Artery Stenosis in Adult and Pediatric Patients After Liver Transplantation. Cardiovasc Intervent Radiol 33, 1111–1119 (2010). https://doi.org/10.1007/s00270-010-9848-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-9848-4