Abstract
Purpose
To evaluate whether response based on contrast-enhanced magnetic resonance imaging (MRI) and apparent diffusion coefficient (ADC) change at diffusion-weighted MRI after transarterial radioembolization (TARE) can predict survival, in patients with prior transarterial chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma (HCC).
Methods
We identified all patients who received DEB-TACE prior to TARE for HCC between 2007 and 2016. Response on MRI was determined by modified RECIST (mRECIST) and ADC change relative to pre-TARE imaging (ADCratio). Kaplan–Meier and log-rank tests were used to correlate the response/disease and treatment variables to overall survival. Multivariable Cox regression models were used to correct for confounders.
Results
A total of 29 consecutive patients were included. Univariable analysis showed that response determined by mRECIST was a nonsignificant predictor of survival (p = 0.057), and response determined by ADCratio was a significant predictor of survival (p = 0.011). Number of prior DEB-TACE procedures (p = 0.037), female gender (p < 0.001) and BCLC C (p = 0.03) were related to worse survival. The number of prior DEB-TACE procedure was significantly higher in non-responders determined by ADCratio (p = 0.028). Multivariable analyses showed that response based on ADCratio was an independent predictor of survival (p = 0.041).
Conclusion
ADCratio following TARE is an independent predictor for survival in patients who previously underwent DEB-TACE for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The choice of treatment for hepatocellular carcinoma (HCC) depends mainly on the extent of disease and the preserved liver function [1]. Transarterial chemoembolization (TACE) is the most widely used locoregional treatment for patients with unresectable HCC. In the recent years, TACE with drug-eluting beads (DEB-TACE) has become a widely used alternative to conventional TACE (cTACE) with at least an equal efficacy and toxicity profile [2, 3]. Transarterial radioembolization (TARE) is an emerging interventional treatment that could be complementary or an alternative to TACE [4] and could be an alternative to kinase inhibitors like sorafenib in patients with a portal vein thrombosis or used as a salvage treatment following chemoembolization [5, 6].
Intra-arterial therapies, like TACE and TARE, cause acute tumor necrosis. Therefore, anatomic changes lag behind functional changes and traditional imaging response criteria based on tumor size, like World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) are unreliable when evaluating response to intra-arterial therapies. Imaging response criteria looking at changes in tumor enhancement after TACE, like modified RECIST (mRECIST) and European Association for Study of the Liver (EASL), have shown superior association with survival compared to RECIST [7,8,9]. Diffusion-weighted (DWI) magnetic resonance imaging (MRI) measures microscopic mobility of free water molecules to assess the degree of cellularity of tissues and can be quantified by apparent diffusion coefficient (ADC) [10]. There are positive data on the potential use of DWI in treatment response assessment of intra-arterial therapies in patients with HCC [11, 12]. However, it is unknown whether DWI and contrast-enhanced MRI can be used as response parameter in patients with one or more prior intra-arterial therapies for HCC.
In our institution patients with unresectable HCC and a preserved liver function receive DEB-TACE as first-line treatment. When patients are refractory (unresponsive) to TACE, the intra-arterial therapy can be switched to TARE (Figs. 1, 2) [13].
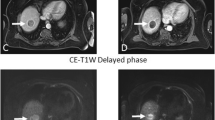
MRI before and after TARE in a patient without treatment response. Patient with multifocal HCC treated with TARE after DEB-TACE. Pretreatment MRI (A–C) showing HCC in the left lobe with peripheral nodular enhancement at arterial phase (white arrows in A), hyperintensity at b1000 DWI (ROI in B) and ADC of 0.00137 mm2/s (ROI in C). Post-treatment MRI (D–F) shows loss of peripheral contrast enhancement at arterial phase (white arrows in A), persistent hyperintensity at b1000 DWI (ROI in B) corresponding to an ADC of 0.00145 mm2/sec (ROI in C). The percentage of ADC change was 6% indicating lack of response by DWI
MRI before and after TARE in a patient with partial treatment response. Patient with multifocal HCC previously unsuccessfully treated with DEB-TACE. MRI before TARE (A–C) shows a nodular lesion in liver segment 5, heterogeneously hypervascular in the arterial phase (white arrows in A) with hyperintensity at b1000 DWI (white arrows in B) and an ADC of 0.00127 mm2/s (white arrows in C). After TARE the MRI shows a partial treatment response (D–F), expressed by less hyperenhancement in the arterial phase (white arrows in D), less hyperintensity at b1000 DWI (white arrows in E) and a higher ADC of 0.00145 mm2/s with an ADC increase of 14.2% indicative of partial treatment response
The aim of the study is to evaluate whether response determined on contrast-enhanced MRI (mRECIST) or by ADC change at DWI MRI after TARE are a predictor for overall survival, in patients who have previously undergone DEB-TACE for unresectable HCC. Our hypothesis is that ADC change a DWI MRI is the best response parameter in this patient population.
Materials and Methods
Study Design
This study was approved by the ethics committee of the hospital. All patients treated at the interventional radiology suite are prospectively registered in a database. A retrospective study was conducted by querying this database to identify all patients who received one or more sessions of DEB-TACE prior to TARE for HCC in the period 2007–2016. The diagnosis of HCC was confirmed by means of the accepted radiologic findings as described by the EASL [14]. Patients without prior or post-TARE MRI were excluded from analysis.
All included patients underwent baseline assessment prior to TARE that included laboratory tests to evaluate the liver function and α-fetoprotein level. The HCC etiology, Child–Pugh classification, Barcelona clinical liver cancer (BCLC) classification, model of end-stage liver disease (MELD) score, data on the amount of prior DEB-TACE treatments and other prior treatments for HCC were collected form the hospital’s patient information system. The tumor staging was determined on the MRI examinations prior to the mapping angiogram.
Transarterial Chemoembolization (TACE)
Details of the TACE procedures have been described previously [15]. Briefly, TACE is performed with doxorubicin loaded superabsorbent polymer (SAP) microspheres (HepaSphere Microspheres Merit Medical Systems, South Jordan, Utah) in a dedicated interventional angiography suite. One vial of 25-mg dry microspheres with a nominal dry diameter of 50–100 µm was mixed with the prescribed dose of doxorubicin. The standard doxorubicin dose was 50–75 mg/m2, reduced to 25 mg/m2 in case of elevated bilirubin or cytopenia. Depending on the number and distribution of the HCC lesions, the drug-eluting beads were infused via superselective infusion of the feeding artery, lobar infusion or bilobar infusion. The treatment end point was either the delivery of the full calculated dose or sluggish flow in the feeding artery. Patients who were refractory to TACE were defined as those showing no response or progressive disease according to modified RECIST [8] or ADC ratio [9] at 1 month post-treatment MRI.
Transarterial Radioembolization (TARE)
Patient eligibility for TARE following DEB-TACE was determined in a multidisciplinary team and was based on patient approval, performance status, disease course, hepatic artery vessel patency and response to prior procedures. The interventional radiologic technique for TARE was performed according to previously published guidelines and technical angiographic reviews [16, 17]. In summary, the TARE procedure was performed with the use of resin Yttrium-90 microspheres (SIR spheres, Sirtex Inc, Cosgrove, Australia), after a positive angiographic work-up. Depending on the work-up results and the number and distribution of the HCC lesions, the resin microspheres are infused via superselective infusion of the feeding artery, lobar infusion or bilobar infusion. Bilobar TARE procedures were performed in one or two sessions, depending on the patient’s liver function.
Response Assessment
Baseline and post-procedural MRI was mainly conducted with 3T MRI (Ingenia, Philips, Best, The Netherlands) and to a lesser extent with a 1.5 T MRI (SonataVision, Siemens Erlangen, Germany; Ingenia, Philips, Best, The Netherlands) with a combined six-channel body and spine phased-array coil. The protocol consisted of a precontrast T2, precontrast T1, precontrast in/out phase, precontast DWI and a post-contrast T1 summarized in Table 1. Each single patient received baseline and follow-up scan at the same field strength in order to avoid interpretation error in response assessment.
Response on imaging was assessed by an abdominal radiologist (V.V., with 13 years of experience). The radiologist was blinded to patients’ clinical and biochemical information. A maximum of two target lesions was chosen for response assessment. For all response assessment criteria, in addition to measurements of target lesions, the possible disappearance, persistence, growth or unequivocal appearance of nontarget lesions was taken into consideration to assess overall response.
Response by the modified RECIST (mRECIST) was based on the change in arterial enhancement of the HCC. Complete remission (CR) was defined as disappearance of any intra-tumoral enhancement; partial remission (PR) as at least a 30% decrease in the sum of the unidimensional diameters of the enhancing area; progressive disease (PD) as at least a 20% increase in the sum; and stable disease as any response that did not qualify for inclusion in the other categories [18].
DWI MRI was performed with 4 b values (b0, b300, b600, b1000 s/mm2). The mean ADC was determined with a whole lesion region of interest (ROI) prior to TARE and on the first post-procedural MRI. The ADCratio was calculated as the difference of the mean pre- and post-treatment ADC (ADCpre–ADCpost) divided by the mean pretreatment ADC (ADCpre). ADCratio response was determined based a previously described threshold of the least responding lesions and was defined as follows: mean ADC increase of more than 90% was considered as complete response (CR); ADC increase between 13.6 and 90% was defined as partial response; an ADC increase between 0 and 13.6% was considered as stable disease; and a decrease in mean ADC indicated as disease progression [19].
Standard Reference
Clinical follow-up took place 1 month after the procedure and every 3 months thereafter as of which point an upper abdominal MR imaging and combined chest and abdominal CT were included as routine follow-up examinations.
Overall survival was determined since the first TARE procedure.
Statistical Analysis
Summary statistics are presented as means and standard deviation (SD) for continuous variables and as frequencies and percentages for categorical variables. For the purpose of this study, patients were divided into two groups based on their response on MRI determined by the mRECIST criteria and ADCratio: responders (CR and PR) and non-responders (SD and PD). Overall survival was estimated using the Kaplan–Meier method, and the log-rank test was used for assessing differences between responders and non-responders. Univariable Cox regression models were used for analyses of the association between disease/treatment characteristics and survival. An exact log-rank test was used in case of small group sizes. Comparison of responders and non-responders on disease/treatment characteristics was performed using the Fischer exact test for categorical variables or Mann–Whitney U test for continuous variables. Multivariable Cox regression models were used for analyses of the effect of response on survival, correcting for potential confounders.
All tests are two-sided; a 5% significance level was assumed for all tests. Analyses were performed using SAS software (Version 9.4 of the SAS System for Windows).
Results
Patient and Treatment Characteristics
We included 29 consecutive patients with unresectable HCC who received a TARE procedure following one or more DEB-TACE treatments and for whom a pre- and post-procedural MRI was available. The pre-TARE patient and treatment characteristics are listed in Table 2. The mean patient age was 65.5 years, most patients were male (82.8%) and alcoholic (31.0%) and non-alcoholic steatohepatitis (24.1%), and hepatitis B and C viruses (27.6%) were the most common underlying liver diseases. Six patients had no sign of cirrhosis and a normal liver function. In 23 patients there was evidence of cirrhosis (79.3%), which was well compensated (Child–Pugh A) in most of the patients. Fifteen patients (51.7%) had an intermediate-stage HCC (BCLC B), and 14 patients (48.3%) had an advanced stage disease. The BCLC C status was because of: portal vein invasion (n = 3), performance status of > 2 (n = 8) and extrahepatic disease (n = 3). These latter patients had a limited amount of extrahepatic disease and received systemic therapy (kinase inhibitor) prior or post-locoregional therapy. Most patients had more than three HCC’s (75.9%), and the largest tumor size was 4.6 ± 2.1 cm. Most patients had more than tree liver lesions (75.9%) and bilobar disease (65.5%). Three patients with extrahepatic disease received TARE in combination with systemic therapy. Patients had a mean of 1.7 DEB-TACE procedures prior to TARE, and almost half of the patients (48.3) received a treatment prior to DEB-TACE including resection, ablation, kinase inhibitor, conventional TACE and transplantation. Post-TARE, three patients were treated with a kinase inhibitor. Three other patients were successfully downstage to disease extent within the Milan criteria and received a liver transplantation.
Response on MRI
MRI to determine response was performed at a mean of 2.9 (SD 1.0, median 3.0, range 1–6) months following TARE, and results are shown in Table 3. The response rate (CR and PR) was 36.7% determined by mRECIST and 62.1% determined by ADCratio.
Survival Analysis
Responders determined by mRECIST had a nonsignificantly better survival (median 26.5 months) compared to non-responders (median 11.5 months) (p = 0.057, Fig. 3A). Responders determined by ADCratio had a significantly better survival (median 26.5 months) compared to non-responders (median 8.9 months) (p = 0.011, Fig. 3B).
Survival analysis showed that patients with a female gender (p < 0.001) and BCLC C (p = 0.03) and more prior TACE procedures (p = 0.037) had a significantly worse survival. A higher AFP level had a trend toward association with worse survival (p = 0.072). None of the other pretreatment characteristics showed a significant association with survival (Table 2).
Table 4 demonstrates the pretreatment characteristics associated with survival. No association was found between these characteristics and response determined by mRECIST. The number of prior TACE procedure was significantly higher in non-responders determined by ADCratio (p = 0.028).
Multivariable analysis demonstrated that ADCratio showed a significant association with survival (p = 0.041), independent of the amount of prior TACE procedures (Fig. 4).
Discussion
In this study we determined the correlation between treatment response based on post-contrast and DWI MRI following TARE in patients who previously received DEB-TACE for HCC. We found that response after TARE determined by mRECIST had a trend toward a better survival (p = 0.057). However, response determined by ADCratio at DWI MRI was a significant and independent predictor of survival (p = 0.041).
A change in ADC value of DWI MRI can be a useful quantitative imaging marker to predict early treatment response of intra-arterial therapies in HCC. In 2006, Kamel et al. [20] first demonstrated the feasibility of DWI MRI to monitor HCC response and showed an increase in ADC at 4–6 weeks after cTACE. A year later they showed that hepatic tumors had an increase in ADC, without a statistically significant change in tumor size early after treatment with yttrium-90-labeled microspheres (Y90) [21]. Later, other studies confirmed that ADCratio was a useful tool to determine response following Y90 [22, 23] and cTACE [24,25,26,27]. Recently, a study of Kokabi et al. [28] showed that an ADCratio immediately post-DEB-TACE was an accurate predictor of prolonged survival in unresectable HCC. The results of the current study are concordant with the previously published data. In addition, the current study focused on the response of TARE in a patient population with prior DEB-TACE for HCC in contrast to a mainly treatment naïve population as described in other series. The current study shows that the predictive value of ADCratio following TARE persists despite prior TACE.
The multivariable analyses showed that a higher number of prior TACE procedures were observed for non-responders according to ADCratio and were associated with worse survival. Hepatic artery damage (HAD) is an inherent and necessary consequence of TACE [29]. A recently published paper even suggests that the incidence and grade of HAD is higher after DEB-TACE compared to cTACE [30]. Due to this HAD vessel patency may be compromised by repeated chemoembolizations. This potential HAD following DEB-TACE might explain the decreased response following TARE in patients with more than one prior TACE procedure.
The current study showed that a 13.6% ADC increase of HCC lesions following TARE predicted prolonged survival. Earlier, Vandecaveye et al. [19] demonstrated that an ADCratio of 13.6% 1 month after TACE was an independent predictor of progression-free survival. However, the used ADCratio thresholds in patients with HCC are usually higher. Kokabi et al. showed that a 30% increase in ADC following Y90 was a reproducible early imaging biomarker predicting prolonged survival in patients in patients with portal vein thrombosis and that an ADCratio of ≥ 20% immediately post-DEB-TACE was an accurate predictor of prolonged survival in unresectable HCC [12, 28]. In a study of Bonekamp et al. [23] tumors with 25% or more increase in ADC after intra-arterial therapy were considered responders. It is difficult to compare these data due to the variability in experimental designs. We mainly used 3T MRI and 4 differences b values 0–1000 s/mm2, while others use a 1.5 T MRI and different b values [12, 28]. In addition, the delay between therapy and follow-up MRI in the current study had a mean of 2.9 months, while others varied from hours to 1 month. Future prospective clinical trials with standardized DWI MRI protocol are warranted to determine the optimal ADCratio threshold.
Belgium is a low endemic area for HCC, with a surprisingly high proportion of non-cirrhotic patients with HCC up to 40% [31]. In the current population there was no evidence of cirrhosis in more than 20% of the patients (n = 6). Three of these patients had a primary HCC, of which two had a proven malignant degenerated adenoma. One patient had a glycogen storage disease as etiology for his HCC, and two patients had a NASH without signs of cirrhosis.
The main limitation of this study is its retrospective nature and the relative small study population. Although all patients treated in the interventional radiology suite are registered prospectively in a database, the data were retrospectively collected from the patient’s information system. Due to this retrospective nature, the post-treatment imaging was, for example, not performed on exact fixed times. Most patients received their imaging at 3 months’ post-TARE; however, there were outliners to 1 and 6 months. Further, baseline and post-procedural MRI was mainly conducted with 3T MRI, however also to a lesser extent with a 1.5 T MRI. It is not clear if this might have influenced the results. A prospectively performed study with a consistent MRI protocol and follow-up has to confirm the current results.
Conclusion
In this study, change in ADC following TARE remains an independent predictor of survival in patients with prior DEB-TACE for HCC.
References
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. https://doi.org/10.1007/s00270-009-9711-7.
Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(6):571–7. https://doi.org/10.1016/j.dld.2016.02.005.
Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Interv Radiol. 2016;39(11):1580–8. https://doi.org/10.1007/s00270-016-1426-y.
Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826–37. https://doi.org/10.1002/hep.26014.
Johnson GE, Monsky WL, Valji K, Hippe DS, Padia SA. Yttrium-90 radioembolization as a salvage treatment following chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2016;27(8):1123–9. https://doi.org/10.1016/j.jvir.2016.03.046.
Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55(6):1309–16. https://doi.org/10.1016/j.jhep.2011.03.007.
Prajapati HJ, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, et al. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol. 2013;24(4):965–73. https://doi.org/10.1093/annonc/mds605.
Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262(2):708–18. https://doi.org/10.1148/radiol.11110282.
Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31(6):1773–91. https://doi.org/10.1148/rg.316115515.
Guo Y, Yaghmai V, Salem R, Lewandowski RJ, Nikolaidis P, Larson AC, et al. Imaging tumor response following liver-directed intra-arterial therapy. Abdom Imaging. 2013;38(6):1286–99. https://doi.org/10.1007/s00261-013-0017-5.
Kokabi N, Camacho JC, Xing M, Qiu D, Kitajima H, Mittal PK, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39(5):969–78. https://doi.org/10.1007/s00261-014-0127-8.
Klompenhouwer EG, Dresen RC, Verslype C, Laenen A, De Hertogh G, Deroose CM, et al. Safety and efficacy of transarterial radioembolisation in patients with intermediate or advanced stage hepatocellular carcinoma refractory to chemoembolisation. Cardiovasc Interv Radiol. 2017. https://doi.org/10.1007/s00270-017-1739-5.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. https://doi.org/10.1002/hep.24199.
Dekervel J, van Malenstein H, Vandecaveye V, Nevens F, van Pelt J, Heye S, et al. Transcatheter arterial chemoembolization with doxorubicin-eluting superabsorbent polymer microspheres in the treatment of hepatocellular carcinoma: midterm follow-up. J Vasc Interv Radiol. 2014;25(2):248–255 e1. https://doi.org/10.1016/j.jvir.2013.10.017.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. https://doi.org/10.1016/j.ijrobp.2006.11.060.
Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17(10):1571–93. https://doi.org/10.1097/01.RVI.0000236744.34720.73.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. https://doi.org/10.1055/s-0030-1247132.
Vandecaveye V, Michielsen K, De Keyzer F, Laleman W, Komuta M, Op de beeck K, et al. Chemoembolization for hepatocellular carcinoma: 1-month response determined with apparent diffusion coefficient is an independent predictor of outcome. Radiology. 2014;270(3):747–57. https://doi.org/10.1148/radiol.13130591.
Kamel IR, Bluemke DA, Eng J, Liapi E, Messersmith W, Reyes DK, et al. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17(3):505–12. https://doi.org/10.1097/01.RVI.0000200052.02183.92.
Kamel IR, Reyes DK, Liapi E, Bluemke DA, Geschwind JF. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18(1 Pt 1):49–56. https://doi.org/10.1016/j.jvir.2006.10.005.
Rhee TK, Naik NK, Deng J, Atassi B, Mulcahy MF, Kulik LM, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19(8):1180–6. https://doi.org/10.1016/j.jvir.2008.05.002.
Bonekamp S, Halappa VG, Geschwind JF, Li Z, Corona-Villalobos CP, Reyes D, et al. Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part II. Response stratification using volumetric functional criteria after intraarterial therapy. Radiology. 2013;268(2):431–9. https://doi.org/10.1148/radiol.13121637.
Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, et al. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants–initial experience. Radiology. 2006;239(2):448–56. https://doi.org/10.1148/radiol.2392042202.
Chung JC, Naik NK, Lewandowski RJ, Deng J, Mulcahy MF, Kulik LM, et al. Diffusion-weighted magnetic resonance imaging to predict response of hepatocellular carcinoma to chemoembolization. World J Gastroenterol. 2010;16(25):3161–7.
Sahin H, Harman M, Cinar C, Bozkaya H, Parildar M, Elmas N. Evaluation of treatment response of chemoembolization in hepatocellular carcinoma with diffusion-weighted imaging on 3.0-T MR imaging. J Vasc Interv Radiol. 2012;23(2):241–7. https://doi.org/10.1016/j.jvir.2011.08.030.
Dong S, Ye XD, Yuan Z, Xu LC, Xiao XS. Relationship of apparent diffusion coefficient to survival for patients with unresectable primary hepatocellular carcinoma after chemoembolization. Eur J Radiol. 2012;81(3):472–7. https://doi.org/10.1016/j.ejrad.2010.12.081.
Kokabi N, Camacho JC, Xing M, Edalat F, Mittal PK, Kim HS. Immediate post-doxorubicin drug-eluting beads chemoembolization Mr Apparent diffusion coefficient quantification predicts response in unresectable hepatocellular carcinoma: a pilot study. J Magn Reson Imaging. 2015;42(4):981–9. https://doi.org/10.1002/jmri.24845.
Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008;26(4):206–12. https://doi.org/10.1007/s11604-007-0216-5.
Lee S, Kim KM, Lee SJ, Lee KH, Lee DY, Kim MD, et al. Hepatic arterial damage after transarterial chemoembolization for the treatment of hepatocellular carcinoma: comparison of drug-eluting bead and conventional chemoembolization in a retrospective controlled study. Acta Radiol. 2016. https://doi.org/10.1177/0284185116648501.
Van Roey G, Fevery J, Van Steenbergen W. Hepatocellular carcinoma in Belgium: clinical and virological characteristics of 154 consecutive cirrhotic and non-cirrhotic patients. Eur J Gastroenterol Hepatol. 2000;12(1):61–6.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study (retrospective) formal consent is not required.
Informed Consent
This is a retrospective study.
Rights and permissions
About this article
Cite this article
Klompenhouwer, E.G., Dresen, R.C., Verslype, C. et al. Transarterial Radioembolization Following Chemoembolization for Unresectable Hepatocellular Carcinoma: Response Based on Apparent Diffusion Coefficient Change is an Independent Predictor for Survival. Cardiovasc Intervent Radiol 41, 1716–1726 (2018). https://doi.org/10.1007/s00270-018-1991-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1991-3