Abstract
Purpose
The purpose of this project was to test the hypothesis that temporary hepatic artery balloon occlusion would favorably alter the distribution of particle emboli within the targeted and nontargeted downstream vascular compartments.
Materials and Methods
Five Yorkshire pigs underwent transfemoral placement of balloon microcatheters into selected segmental hepatic arteries. A collection catheter was surgically introduced into a downstream hepatic artery branch. Blood pressures at the femoral artery sheath and the collection catheter were obtained with the microcatheter balloon deflated and inflated. Identical quantities of calibrated 250- and 400-µm microspheres were injected via the balloon microcatheter when inflated, then deflated. Each animal underwent up to four paired microsphere embolizations. Microspheres collected from the intrahepatic collection catheter were counted manually by light microscopy.
Results
Inflation of the balloon microcatheter in the segmental hepatic artery resulted in a consistent and significant decrease in blood pressure (mean: 30 mmHg; range 23–43 mmHg; p < 0.05) in the downstream vascular compartment. The number of microspheres selectively delivered to the targeted intrahepatic collection catheter was significantly greater when the balloon microcatheter was inflated rather than deflated in all 20 paired embolic deliveries (by 2.4-fold, mean; p = 0.0002), despite delivery of the same total number of microspheres.
Conclusion
Balloon occlusion significantly reduces blood pressure in the downstream vascular compartment, resulting in increased delivery of emboli to a targeted intrahepatic arterial collection catheter relative to other portions of the embolized vascular compartment, likely due to blood flowing into this compartment from neighboring hepatic and extrahepatic arteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-reflux devices have been used to reduce the risk of nontarget embolization when performing transarterial chemoembolization (TACE) or Yttrium-90 transarterial radioembolization (TARE) to treat liver cancers [1,2,3,4,5]. For example, when the Surefire Infusion System (Surefire Medical; Westminster, CO) (SIS) is deployed within the hepatic arteries, the mean arterial blood pressure within that downstream vascular compartment is reduced (by ~20 mmHg) relative to the mean systemic arterial blood pressure [6,7,8,9]. The SIS has a deployable cone-shaped anti-reflux tip that creates a dynamic occlusion and prevents retrograde blood flow during cardiac diastole. Pasciak and coworkers demonstrated that when injecting Technetium-99m macroaggregated albumin microparticles into hepatic arteries through an anti-reflux microcatheter, microparticle deposition in the hepatic tumors was increased relative to the adjacent nontumorous liver when compared to identical delivery through an end hole microcatheter [10]. The hypothesis of the current study was that temporary hepatic artery occlusion would cause reduction in the blood pressure within the downstream vascular compartment that in turn would result in increased delivery of embolic particles to a specific intravascular target located within the same downstream vascular compartment. This hypothesis was tested using a hepatic artery temporary occlusion balloon delivery microcatheter and a surgically implanted downstream target intrahepatic arterial collection catheter in a porcine model.
Materials and Methods
Animals
Five Yorkshire pigs weighing ~60 kg were used. The subjects were sedated with ketamine (27 mg/kg), xylazine (2 mg/kg) and atropine (0.02 mg/kg) and then underwent endotracheal intubation and induction under general anesthesia using intravenous propofol maintained with inhalational isoflurane. The animals were positioned supine on the angiographic table and the abdomen and groins prepared and draped in a standard surgical fashion. Medications used during the procedure were heparin (50 U/kg/h) to prevent intraprocedural thrombosis and verapamil (5 mg intra-arterial boluses/h as needed) to treat vasospasm if noted on hepatic arteriography. Angiography was performed using a portable C-arm fluoroscopic unit (OEC 7600, GE Healthcare; Little Chalfont, UK) with digital subtraction angiographic (DSA) capability. The institutional animal care and use committee approved of this experiment.
Angiography
Arterial cut down was performed to place an 8-Fr introducer sheath (Avanti+®, Cordis Corporation; Fremont, CA) into the right common femoral artery. A 6-Fr Cobra-1 guide catheter (Mach 1®; Boston Scientific, Natick, MA) was navigated into the celiac artery. The gap between the outer 8-Fr sheath and the inner coaxial 6-Fr guide catheter was intended to allow transduction of systemic arterial blood pressure. Undiluted nonionic contrast material (Optiray™ 320, Mallinckrodt Pharmaceuticals; Raleigh, NC) was hand injected with anteroposterior (AP) DSA acquisition. Over a guidewire, the guide catheter was negotiated into the proper hepatic artery. Hepatic arteriography was performed with hand injection of contrast material and AP DSA acquisition to identify a target segmental or subsegmental hepatic artery for placement of the occlusion balloon delivery microcatheter and the downstream collection catheter. The target artery was selected among segmental or subsegmental arteries with origins beyond branches to the stomach and duodenum and with one or more nearby proximal hepatic arterial branches. The left lobe of the liver was favored for ease of subsequent operative identification. A 2.9-Fr microcatheter with a compliant occlusion balloon (Sniper®, Embolx, Inc; Sunnyvale, CA) was coaxially guided through the 6-Fr catheter and into the artery supplying the targeted hepatic artery, and an exchange length 0.016 inch microguidewire (Fathom Guidewire®, Boston Scientific) was advanced peripherally into the target hepatic artery to guide subsequent surgical access.
Laparotomy
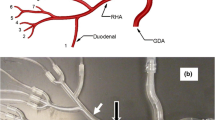
A midline abdominal incision was made for open laparotomy. The hepatic artery containing the microguidewire was identified using fluoroscopy and palpation. The microguidewire was exposed and pulled out approximately 50 cm. A 6-Fr coaxial introducer system (Accustick II®, Boston Scientific) was advanced retrograde over the exposed microguidewire into the targeted hepatic artery, and the inner stiffener and dilator were removed. The introducer catheter was secured to the liver capsule with suture and topical cyanoacrylate liquid adhesive (Dermabond, Ethicon; Somerville, NJ). This catheter was used to collect the embolic material during the experiment and was designed to simulate a structure with a separate isolated vascular compartment. The 2.9-Fr microcatheter, mounted with a compliant temporary occlusion balloon (diameter range 0–5 mm, nominal inflation volume of 0.1 ml), was used to deliver embolic particles to the targeted intrahepatic arterial collection catheter with the balloon either inflated to simulate pressure-directed delivery or deflated to simulate conventional end hole catheter delivery. A diagram of the catheter setup is provided in Fig. 1A, B.
Hepatic angiography before and after balloon occlusion. A Schematic of placement of the occlusion balloon delivery microcatheter (orange) within the feeding hepatic artery and the intrahepatic collection catheter (green) in the targeted downstream hepatic artery. B Anticipated changes in the direction of blood flow in hepatic artery branches located between the two catheters when the balloon (blue) is inflated. C Hepatic angiography in anteroposterior projection through the delivery microcatheter with the balloon deflated opacifies multiple segmental hepatic arteries. D Hepatic angiography in the same projection with the occlusion balloon inflated opacifies few arteries other than the target artery, likely due to reversed flow of unopacified blood in nontarget branches
Pressure Monitoring and Regulation
Systemic arterial blood pressure (BP) was monitored via the sidearm of the femoral artery sheath with an added stopcock to enable use of a pressure transducer (MLT0699, AD Instruments; Colorado Springs, CO) for data recording. The intrahepatic arterial collection catheter was connected via a three-way stopcock to (1) outflow tubing for particle collection and (2) tubing to an additional pressure transducer for outflow BP measurement. If the outflow tubing was open to atmospheric pressure for sample collection, then the target hepatic artery would have a much lower blood pressure than the other hepatic arteries within the downstream vascular compartment which would reflect transmitted right atrial pressure and tissue interstitial pressure. The result would be nonphysiologic sumping of blood and emboli into the low pressure circuit. In addition, large volumes of blood would be lost that may have threatened pig viability. In order to both prevent exsanguination during particle collection and to create a more realistic resistance to outflow, a roller clamp (Item 33046, Qosina; Ronkonkoma, NY) was inserted external to the pressure transducer and used to control blood outflow. When the roller clamp was wide open, the measured outflow blood pressure was consistently less than 3 mmHg and blood loss was substantial. Roller clamp resistance was increased by approximately 20–30 mmHg and adjusted such that exiting blood flow rates from the collection catheter were visibly approximately equal for both balloon deflated and balloon inflated states. In spite of these efforts, the visual estimates of collected blood volumes when the balloon was deflated were approximately twice that of when the balloon was inflated.
The BP within the downstream vascular compartment was primarily recorded through the intrahepatic arterial collection catheter. Measurements of intrahepatic BP via the tip of the balloon occlusion catheter were only taken sporadically because of the need to utilize the infusion port for particle injection and because of reduced recording fidelity due to the small lumen size (500 µm) over the length of the catheter (120 cm). A pressure transducer malfunction occurred in the first animal, so BP data were available only for four animals. Systemic arterial BP and downstream vascular compartment BP (systolic and diastolic) were recorded prior to any embolic delivery, measured with and without inflation of the delivery catheter occlusion balloon. These values were taken from the same phase of respiration.
The mean arterial BP was calculated using the formula:\( {\text{Mean}}\;{\text{BP}} = \left[ {{\text{Systolic}}\;{\text{BP}} + 2\left( {{\text{diastolic}}\;{\text{BP}}} \right)} \right]/3 \). The systemic-to-hepatic arterial pressure differential (SHAPD, mmHg) was calculated as mean systemic arterial BP (mmHg) minus mean downstream vascular compartment BP (mmHg) when the occlusion balloon was inflated. The deflation–inflation compartmental pressure differential (DICPD) (mmHg) was the mean BP of the downstream vascular compartment as measured through the intrahepatic arterial collection catheter when the balloon was deflated minus the same BP measured when the balloon was inflated.
Particle Injection
One 2-ml syringe of calibrated spherical embolic particles (Embozene, Boston Scientific), either 250 or 400 µm in diameter, had approximately 1 ml of supernatant expelled and then was reconstituted in 5-ml nonionic contrast material (Optiray™ 320, Mallinckrodt Pharmaceuticals; Raleigh, NC), yielding a 6-ml suspension. The embolic suspension was generated through agitation between a 10-ml reservoir syringe and a 1-ml delivery syringe via a three-way stopcock. Particles were delivered through the balloon occlusion microcatheter in a sequence designed to permit multiple injections in order to maximize the number of data points per animal. Initial injections were performed with the balloon occlusion microcatheter placed approximately 5–10 cm proximal (upstream) to the intrahepatic arterial collection catheter, with at least one branch hepatic artery observed in between. The study design of having at least one segmental artery between the delivery microcatheter and the targeted branch hepatic artery was to allow inflow of blood from adjacent vascular beds. It had been postulated that balloon occlusion would result in reduction in downstream compartmental blood pressure that in turn would cause blood to flow into this compartment from neighboring hepatic and extrahepatic vascular territories. Once placed, 6 ml of the 250-µm particles was injected over 15 s through the microcatheter with the balloon inflated, then repeated with the balloon deflated (except as noted). Theoretically, the first embolization could cause embolic occlusion of considerable portions of the nontarget arteries within the vascular territory and therefore favor embolic delivery to the targeted outflow collection catheter during the subsequent embolizations, regardless of the status of the occlusion balloon. In order to assess this possibility, the order of embolization was varied (see Table 2). If anatomy permitted, the balloon occlusion microcatheter was next retracted centrally past at least one additional branch hepatic artery in order to collect data involving additional vascular territories using first 250-µm and then 400-µm particles. In the first animal, there was an additional injection of 400-µm particles at the distal location.
The entire 6-ml aliquot of embolic microspheres was delivered for each test condition. Particles were collected after each delivery of 6 ml of embolic particles using a 70-µm cell strainer (Falcon 352350, Corning; Corning, NY). Particles were washed with water to lyse remaining red blood cells and to clean the contents of the filter.
Particle Sample Counting
Collected particles from each strainer were first allowed to dry and then reconstituted in 25 ml of distilled water. Particles were mixed using a magnetic stirrer to generate a homogenous suspension. A micropipette was used to collect a 250-µl sample from the 400-µm particle suspension or a 100-µl sample from the 250-µm suspension. The sample was placed on a slide, and the particles were visualized under a light microscope and counted manually. Samples were repeated three times per suspension and averaged and then normalized to the full 25-ml suspension to extrapolate the total number of particles collected. Using the known quantities of particles per ml as obtained from the manufacturer, the normalized values of collected particles were calculated. 250-µm particles are supplied as approximately 75,000 particles/ml (range 68,000–82,000 per ml); 400-µm particles are supplied as approximately 17,000 particles/ml (range 16,000–18,000 per ml).
Statistical Analysis
Paired t tests were used to compare blood pressure measurements and particle delivery counts in the targeted vascular compartment when the temporary occlusion balloon was deflated versus inflated. Analysis was carried out using GraphPad Prism version 5.0 software (La Jolla, California) and Microsoft Excel (Redmond, Washington). Statistical significance was reached at p < 0.05.
Results
The mean arterial BPs measured through the femoral artery sheath (systemic arterial BP), the intrahepatic arterial collection catheter when the balloon microcatheter was deflated, then inflated, and the calculated SHAPD and DICPD pressure gradients for each animal taken before each particle administration are presented in Table 1. A pressure transducer malfunction occurred in animal A, so BP data were available only for animals B, C, D, and E. The SHAPD and DICPD values that reflect reduction in BP in the downstream vascular compartment were significant (p = 0.006). The mean SHAPD for all animals was 45 mmHg (range 31–61 mmHg), and the mean DICPD was 30 mmHg (range 23–43 mmHg).
Hepatic arteriograms were obtained before and after initial balloon inflation in two animals. In both animals, at least five hepatic artery branches were opacified to the subcapsular periphery of the liver when the balloon was deflated (Fig. 1C). After inflation of the temporary occlusion balloon, only one or two hepatic artery branches were seen (including the target hepatic artery that contained the intrahepatic arterial collection catheter in both animals) (Fig. 1D).
The absolute number of collected and counted particles and the relative proportion of the total delivered dose of particles in the effluent sample from the intrahepatic arterial collection catheter for each delivery experiment under conditions of balloon deflation compared to balloon inflation are presented in Table 2. The mean increase in the number and proportion of particles when the balloon was inflated compared to the baseline of balloon deflated was 2.37 (range 1.35–4.54) (p = 0.0002).
Discussion
This model was meant to illustrate the blood flow dynamics and relative embolic distribution into a structure that resides within a targeted vascular territory yet has a separate outflow from the other normal hepatic arteries within the same vascular territory, potentially similar to a tumor. In accordance with the presented hypothesis, the recordings taken before and after balloon occlusion in the current study confirm a relative reduction in the BP of the downstream vascular compartment, as previously described [6,7,8,9]. The mean reduction in SHAPD was 45 mmHg, and the mean reduction in the DICPD was 30 mmHg (p = 0.006). The difference between SHAPD and DICPD (mean 15 mmHg) was interpreted as due to resistance to hepatic artery blood flow caused by the presence of the guide catheter, the balloon mounted microcatheter, and potentially unrecognized upstream hepatic artery vasospasm.
In the face of hepatic arterial occlusion without collateral flow, the downstream BP should approach a value between the BP of the portal vein and that of the right atrium, assuming there are no other sources of vascular inflow and no obstruction to outflow. The mean arterial BP of the targeted vascular compartment when the balloon was occluded was 35 ± 5 mmHg (range 24–51 mmHg), well above the expected BP of 5–10 mmHg within the normal porcine portal vein [11]. This difference indicates that vascular inflow must also occur from adjacent hepatic arteries or nearby hepaticoenteric arteries that serve as collateral conduits.
When the downstream vascular compartment blood pressure is reduced relative to systemic arterial blood pressure, blood should flow from higher pressure hepatic and extrahepatic vascular territories into the lower pressure downstream vascular compartment. Logically, this inflow of blood from adjacent vascular territories should passively flush embolic particles from the nontarget portions of the downstream vascular compartment into the targeted structure which is the collection catheter in this study. The angiographic images support this thesis. When the balloon on the delivery microcatheter was deflated, all five downstream hepatic artery branches were visualized. When the balloon was inflated, the only vessel consistently opacified was the artery supplying the surgically implanted intrahepatic arterial collection catheter. The strongest evidence was the relative counts of embolic particles. In every case of delivered embolic counts comparing the nonocclusive condition to the occlusive condition, the embolic count was greater in the occlusive condition to varying degrees. The mean increase in embolic count was 137%, for a 2.37-fold increase in embolic delivery. The range of values for increase in delivered emboli with balloon occlusion was substantial: 35–354%.
In the work of Irie and colleagues, the transluminal blood pressure of the downstream vascular compartment after balloon inflation was termed the balloon-occluded arterial stump pressure (BOASP) [12]. In the 91% of treatments with a BOASP less than 64 mmHg, the emulsified ethiodized oil of the balloon-occluded transarterial chemoembolization (B-TACE) was fluoroscopically observed to flush out of the nontumorous liver but continue to opacify the hepatocellular carcinomas (HCCs). In the 9% of treatments with a BOASP greater than 64 mmHg, the ethiodized oil was observed to indiscriminately fill both tumor and nontumorous blood vessels. On noncontrast abdominal CT scans obtained immediately following the B-TACE, the ethiodized oil mean density ratio of the tumor to the nontumorous liver within the same treated segment was 18.3 in the cohort with a BOASP of less than 64 mmHg compared to 2.6 in the group with a BOASP greater than 65 mmHg. One potential explanation for this phenomenon is that when the BOASP is less than 64 mmHg, reconstitution of the downstream segmental hepatic artery via small caliber hepatic artery-to-hepatic artery connections from neighboring hepatic segments is occurring sufficiently to flush the B-TACE agents out of the nontumorous liver. Potentially, the tumors are separate vascular compartments that do not exhibit the same intersegmental arterial connectivity and therefore continue to be filled with embolic agents.
There are several limitations to this study. This model does not include a tumor; thus, conclusions cannot be directly extrapolated to embolic delivery into tumors, which have widely ranging degrees of vascularity and varying interstitial pressures. No porcine hepatic tumor model is widely accepted and available to the best of the investigators’ knowledge. The literature relevant to microvascular blood pressure measurements within tumors is sparse. What limited data that are available suggest that compared to microvascular blood pressure in neighboring nontumorous tissue, the intratumorous blood pressure on the arterial side is approximately the same, but that on the venous side is lower, possibly due to increased intratumoral resistance to blood flow [13, 14]. The conclusions from this study are therefore limited to the hemodynamic effects of inflow of blood from surrounding neighboring liver and extrahepatic structures. Another limitation is the unknown effect of the roller clamp system on the intrahepatic arterial collection catheter outflow tubing that was instituted to prevent abnormal sumping of blood flow into the outflow system and exsanguination during particle collection. Although the volume of blood issuing from the collection catheter outflow during particle recovery was not measured, the operators’ impression was that the volume of discarded blood was greater for collections with the delivery catheter balloon deflated than when inflated, which suggests that the roller clamp likely did not enhance particle delivery to the outflow collection catheter during conditions of balloon inflation. The estimated outflow resistance of this system (20–30 mmHg) is approximately the same as the tumoral arterial microvascular pressures (17–45 mmHg) and higher than tumoral venous microvascular pressures (7.5–11 mmHg) reported by Jain et al. [13]. Angiographic evaluation of the effect of balloon deflation and inflation was limited to two pigs. The interpretation of changes in blood flow direction was based on the absence of opacification of hepatic arteries not containing the collection catheter rather than direct observation of compartmental artery opacification from extracompartmental adjacent hepatic segments or extrahepatic structures. Subsequent studies could be designed to directly investigate this postulated hemodynamic phenomenon. The pig model is not identical to humans. Although the pigs have segmental hepatic anatomy similar to humans, one major difference is the greater degree of separation of hepatic segments from each other. If anything, this phenomenon would be expected to understate the phenomenon expected in humans since the degree of direct intersegmental interface is substantially less, thus likely limiting the degree of arterial interconnectivity between adjacent hepatic segments. The effect of performing multiple sequential tests of embolic delivery on subsequent tests in the same animal is unknown. This study design was used to provide as many data points per animal as feasible. Observations from this study suggest that the effect of multiple embolizations was not substantial, since the relative increase in the proportion of microspheres collected through the collection catheter when the delivery catheter balloon was inflated occurred with both the 250- and 400-µm microspheres, whether collected from the first or the subsequent collections, and whether embolization with balloon deflated preceded that with balloon inflated or vice versa.
In summary, temporary hepatic arterial occlusion during embolization results in downstream blood pressure reduction in the targeted vascular territory that in turn causes emboli to be redirected to varying degrees into the targeted intrahepatic arterial collection catheter that has an outflow that is isolated from the other portions of the downstream vascular compartment. The angiographic observations suggest that this redirection is due to inflow of blood from neighboring hepatic segments or nearby extrahepatic structures. This phenomenon may result in less nontarget embolization of nontumorous liver and potentially greater delivery into the targeted tumor when using a temporary balloon occlusion microcatheter.
References
Van den Hoven AF, Prince JF, Samin M, Arepally A, Zonneberg BA, Lam MGEH, van den Bosch MAAJ. Posttreatment PET-CT-confirmed intrahepatic radioembolization performed without coil embolization, by using the anti-reflux surefire infusion system. Cardiovasc Intervent Radiol. 2014;37:523–8.
Fischman AM, Ward TJ, Patel RS, Arepally A, Kim E, Nowakowski FS, Lookstein RA. Prospective, randomized study of coil embolization versus surefire infusion system during yttrium-90 radioembolization with resin microspheres. J Vasc Interv Radiol. 2014;25:1709–16.
Matsumoto T, Endo J, Hashida K, et al. Balloon-occluded transarterial chemoembolization using a 1.8-French tip coaxial microballoon catheter for hepatocellular carcinoma: technical and safety considerations. Minim Invas Ther. 2015;24:94–100.
Morshedi MM, Bauman M, Rose SC, Kikolski SG. Yttrium-90 resin microsphere radioembolization using an antireflux catheter: an alternative to traditional coil embolization for nontarget protection. Cardiovasc Intervent Radiol. 2015;38:381–8.
Maruyama M, Yoshizako T, Nakamura T, Nakamura M, Yoshida R, Kitagaki H. Initial experience with balloon-occluded trans-catheter arterial chemoembolization (B-TACE) for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2016;39:359–66.
Rose SC, Kikolski SG, Chomas JE. Downstream hepatic arterial blood pressure changes caused by deployment of the surefire antireflux expandable tip. Cardiovasc Intervent Radiol. 2013;36:1262–9.
Rose SC, Kikolski SG, Morshedi MM, Narsinh KH. Feasibility of intraprocedural transluminal hepatic and femoral artery blood pressure measurements as an alternative chemoembolization endpoint when using anti-reflux devices during lobar chemoembolization. Am J Roentgenol. 2015;205(1):196–202.
Xu Z, Jernigan S, Kleinstreuer C, Buckner GD. Solid tumor embolotherapy in hepatic arteries with an anti-reflux catheter system. Ann Biomed Eng. 2016;44:1036–46.
Rose SC, Narsinh KH, Newton IG. Quantification of blood pressure changes in the vascular compartment when using an anti-reflux catheter during chemoembolization versus radioembolization: a retrospective case series. J Vasc Interv Radiol. 2017;28:103–10.
Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26:660–9.
Schulman AR, Thompson CC, Ryou M. EUS-guided portal pressure measurement using a digital pressure wire with real-time remote display: a novel, minimally invasive technique for direct measurement in an animal model. Gastrointest Endosc. 2016;83:817–20.
Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36:706–13.
Jain RK. Determinants of tumor blood flow: a review. Can Res. 1988;48:2641–58.
Peters W, Teixeira M, Intaglietta N, Gross JF. Microcirculatory studies in rat mammary carcinoma. 1. transparent chamber method, development of microvasculature and pressures in tumor vessels. J Natl Cancer Inst. 1980;65:631–42.
Acknowledgements
We wish to express our gratitude to Laura Anderson and Olivia Hernandez for their valued assistance in preparing this manuscript and to Isabel G. Newton, M.D. Ph.D., for her insightful editorial input. This study was funded by the NSF 1417279, A Novel Occlusion Balloon Microcatheter for Improved Delivery of Embolization Therapy to Tumors of the Liver, Embolx, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Steven C. Rose has disclosed his role as a consultant, minor stockholder, scientific advisory board member, and proctor for several medically affiliated companies. Gregory D. Halstead has disclosed his role as an employee with equity stock at a medical company. Kazim H. Narsinh has nothing to disclose. All applicable institutional and/or national guidelines for the care and use of animals were followed. Informed consent does not apply to this study.
Rights and permissions
About this article
Cite this article
Rose, S.C., Halstead, G.D. & Narsinh, K.H. Pressure-Directed Embolization of Hepatic Arteries in a Porcine Model Using a Temporary Occlusion Balloon Microcatheter: Proof of Concept. Cardiovasc Intervent Radiol 40, 1769–1776 (2017). https://doi.org/10.1007/s00270-017-1753-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1753-7