Abstract
Purpose
To reveal the mechanism of dense accumulation of lipiodol emulsion (LE) in hepatocellular carcinoma (HCC) during selective balloon-occluded transarterial chemoembolization (B-TACE).
Methods
Balloon-occluded arterial stump pressure (BOASP) at the embolization portion was measured during selective B-TACE for 43 nodules in 42 patients. Fluoroscopy and digital subtraction angiography were prospectively observed during selective B-TACE to note whether dense LE accumulation in HCC occurred. The LE concentration ratio of HCC to embolized liver parenchyma (LECHL ratio) was also calculated for each treatment on the basis of the computed tomographic scan obtained immediately after selective B-TACE. The relationships between degree of LE accumulation and the BOASP, as well as the LECHL ratio, were analyzed.
Results
Arterial flow beyond the catheter tip was maintained even after balloon inflation. In 39 of 43 treatments, LE inflow into the nontumorous liver parenchyma ceased immediately after LE droplets were filled in arteries of the nontumorous liver parenchyma while LE inflow into the HCC nodule continued (group 1). More dense LE accumulation in HCC nodule was obtained in these 39 treatments. In four treatments, LE inflow both into the nontumorous liver parenchyma and into the HCC nodule continued, and no dense LE accumulation in HCC nodule was observed (group 2). In these four treatments, thick anastomotic vessels with collateral artery were noted. The BOASP in group 1 was (mean ± SD) 33.8 ± 12.8 mmHg (range 13–64 mmHg) and that in group 2 was 92.3 ± 7.4 mmHg (range 83–100 mmHg). There was a statistically significant difference in BOASP between groups (p = 0.00004, Welch’s t test). The LECHL ratio in group 1 was 18.3 ± 13.9 (range 2.9–54.2) and that in group 2 was 2.6 ± 1.1 (range 1.7–4.2). There was a statistically significant difference in the LECHL ratio between the groups (p = 0.000034, Welch’s t test).
Conclusion
Selective B-TACE induced dense LE accumulation in HCC nodules in 39 (91 %) of 43 treatments in which BOASP was 64 mmHg or less.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transarterial chemoembolization is the most used treatment for hepatocellular carcinoma (HCC) that is not resectable or that cannot be treated with percutaneous interventions, with proven improvement in survival in selected patients [1, 2]. Since March 2008, we have used a 3F microballoon catheter in selective transarterial chemoembolization treatment for HCC to prevent proximal migration and leakage of embolization materials [3]. In our experience of selective balloon-occluded transarterial chemoembolization (B-TACE), we have noticed dense lipiodol emulsion (LE) accumulation in HCC nodule. Because dense LE accumulation in HCC increases accumulation of anticancer drugs, it is important to reveal the mechanism for this dense LE accumulation. We published a study to explain this phenomenon as a preliminary report [3]; however, the number of cases in this previous study was so small that statistical analysis could not be carried out.
The purpose of the present study was to measure balloon-occluded arterial stump pressure (BOASP) at the embolization portion in more cases, to analyze the relationship between BOASP and the degree of LE accumulation, and to disclose the mechanism for this dense LE accumulation in HCC nodule by selective B-TACE.
Materials and Methods
Patients
This clinical study was approved by the ethics committee of our hospital. Written informed consent was obtained from the patients. Selective B-TACE was indicated for patients with a limited number of HCC nodules when percutaneous radiofrequency ablation therapy was not indicated. In our hospital, contraindications to radiofrequency ablation are the: (1) size of the lesion larger than 3 cm, (2) lesions adjacent to the gallbladder, stomach, common hepatic duct, and colon, and (3) lesions of difficult location for radiofrequency ablation needle placement. Between January 2009 and February 2012, selective B-TACE was performed, and BOASP was measured in 43 nodule treatments of 42 patients (Table 1). Excluded were nontargeted B-TACE for multiple HCC nodules, repeated selective B-TACE for the region previously treated by TACE, and selective B-TACE for HCC nodules with arterioportal shunt. The diagnosis of HCC was made by dynamic computed tomography (CT) findings in addition to high serum levels of tumor markers. All patients underwent two-phase dynamic CT before selective B-TACE, and all nodules showed high attenuation at arterial-phase CT and low attenuation at delayed-phase CT. Elevation of serum α-fetoprotein level (>200 ng/mL) and/or elevation of serum protein induced in vitamin K absence 2 level (>40 μg/L) were observed in 29 patients (Table 1).
Measurement of Blood Pressure and the Microballoon Catheter System
Blood pressure was measured with a pressure transducer (BD DTXplus; BD Bioscience, Franklin Lakes, NJ). A 4F hook-shaped sheath (MS-kit; Medikit, Tokyo, Japan) was placed in the celiac, common hepatic, or superior mesenteric artery. The 3F microballoon catheter (Attendant; Terumo, Tokyo, Japan) was placed at the aimed portion via the sheath to perform selective B-TACE and BOASP was measured (Fig. 1). It was already verified that mean arterial pressure could be measured via the thin tube space of the microballoon catheter [3]. The maximum diameter of the balloon was 5 mm, and the microballoon catheter was introduced over a 0.014-inch guide wire (Chikai; Asahi Intec, Aichi, Japan, or Labyrinth; Piolax, Kanagawa, Japan). The location of microballoon catheter tip was caudate lobe artery (A1, n = 2); left lateral superior (A2, n = 1), left lateral inferior (A3, n = 3), left medial (A4, n = 7), right anterior inferior (A5, n = 3), right posterior inferior (A6, n = 8), right posterior superior (A7, n = 5), and right anterior superior (A8, n = 7) subsegment artery; and anterior (n = 3) and posterior (n = 4) segment artery.
Chemoembolization Procedures Using the Microballoon Catheter System
Ten milligrams of doxorubicin hydrochloride (Adriacin injection; Kyowa Hakko, Tokyo, Japan) and 2 mg of mitomycin C (mitomycin injection; Kyowa Hakko) was combined with 1–2 mL of iopamidol (Oypalomin 300; Fuji Pharm, Tokyo, Japan). The mixture of these solutions and 5–10 mL of iodized oil (Lipiodol 480 injection; Guerbet, Tokyo, Japan) was emulsified by vigorous pumping 10 times between two syringes that were interconnected with a three-way stopcock. Porous gelatin particles (Gelpart; Nihonkayaku, Tokyo, Japan) 1 mm in diameter were filled in a syringe with 1.5 mL of iopamidol. The particles were fragmented by vigorous pumping 10 times between two syringes that were interconnected with a partially opened three-way stopcock. The shape of fragmented porous gelatin particles by this method has been revealed as nonspherical, and the size was approximately 200 μm in length at microscopic observation (unpublished data). The balloon was inflated to a diameter 5–10 % larger than that of the occluded artery. Transarterial LE infusion was performed immediately after pressure measurement. LE infusion was continued under balloon occlusion until the HCC nodule was filled with LE or portal venous branches were beginning to be filled with LE. Then the fragmented porous gelatin particles were infused until the arterial branches beyond the catheter tip were filled with them.
Fluoroscopy and digital subtraction angiography (DSA) during B-TACE procedures were observed by one or two interventional radiologists, who decided whether limitation of LE inflow into nontumorous liver parenchyma and dense LE accumulation in HCC nodule were present, and whether or not anastomotic vessels with collateral artery were present.
LE Accumulations in HCC and Liver Parenchyma of the Embolized Region
Observation of LE flow pattern on DSA/fluoroscopy was relatively subjective, and this analysis was aimed for objective evaluation of dense LE accumulation in HCC. LECHL ratio was evaluated. Plain CT of the liver was obtained immediately after selective B-TACE. The CT values of the HCC nodule, the liver parenchyma of the embolized region, and the liver parenchyma of the nonembolized region were measured. To measure the CT value of the HCC, the axial slice of maximum size depiction of HCC was selected, and the entire HCC nodule was considered the region of interest. To evaluate the CT value of liver parenchyma, regions of interest were placed avoiding vascular structure at three portions or more, and measured CT values were averaged. The difference of the CT value between HCC and liver parenchyma of nonembolized region, and that between liver parenchyma of embolized region and liver parenchyma of nonembolized one were calculated for the evaluation of LE accumulations in HCC and liver parenchyma of embolized region. Finally, the LECHL ratio was calculated for each treatment.
Statistical Analysis
BOASP and LECHL ratio were compared by the Welch’s t test or Student’s t test by dividing the treatments into two groups: whether limitation of LE inflow into nontumorous liver parenchyma and dense LE accumulation in HCC nodule were noted on fluoroscopy/DSA, and whether collateral artery was noted. The software for statistical analysis was Statcel 2 (OMS, Saitama, Japan).
Results
Measurement of BOASP and selective B-TACE were successfully performed in all 43 treatments. The dose of lipiodol ranged 0.5–10 mL (4.5 ± 3.5). The contrast material did not stay, but the arterial flow beyond the occluded portion was maintained on DSA and fluoroscopy in all treatments even after the balloon was inflated. At the beginning of LE infusion, pulsatile flow of the LE droplets into vessels supplying the HCC nodule and those supplying the liver parenchyma was noted in all 43 treatments. After LE droplets reached the peripheral thin vessels supplying the nontumorous liver parenchyma, two flow patterns were observed. The LE inflow into the nontumorous liver parenchyma ceased after the peripheral thin vessels were filled with LE in 39 treatments (group 1) (Figs. 2, 3) but did not cease in four treatments (group 2) (Fig. 4). In all 43 treatments, the LE droplets continued to flow into the HCC nodule. Thus, LE accumulation in the HCC nodule was dense in group 1 (Figs. 2, 3) but not in group 2 (Fig. 4). The BOASP in group 1 was 33.8 ± 12.8 mmHg (range 13–64) and that in group 2 was 92.3 ± 7.4 mmHg (range 83–100). The BOASP in group 1 was lower than that in group 2, with a statistically significant difference (p = 0.00004, Welch’s t test) (Fig. 5). The LECHL ratio was 18.3 ± 13.9 (range 2.9–54.2) in group 1 and 2.6 ± 1.1 (range 1.7–4.2) in group 2. There was a statistically significant difference between the groups (p = 0.000034, Welch’s t test) (Fig. 6).
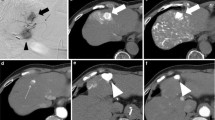
Selective B-TACE without depiction of collateral artery in the same case as that shown in Fig. 1. A The microballoon catheter was placed at A7 for selective B-TACE (arrow). Peripheral tumor feeding arteries (arrowheads) were thinner than the 3F (1 mm) shaft of the microballoon catheter (white arrow). B At the beginning of LE infusion, LE was distributed into the peripheral vessels supplying liver parenchyma (arrowheads) and those supplying HCC nodule (arrows). C The LE inflow into the liver parenchyma soon ceased (arrowheads), while that into the HCC nodule (arrows) continued, and LE was densely accumulated in HCC nodule. A LE droplet was always present at the tip of the microballoon catheter (white arrow B, C). D Finally, fragmented porous gelatin particles were embolized into arterial branches (arrows), and selective B-TACE was completed
Selective B-TACE with depiction of collateral artery. A The celiac artery was obstructed, and the microballoon catheter was placed at the posterior segment artery (arrowhead) via the superior mesenteric artery (arrow). The main feeding artery was A7 (white arrow), and thin feeding arteries originated from A6 (white arrowhead). These thin feeding arteries were thinner than the 3F (1 mm) shaft of the microballoon catheter (large arrow). B At the beginning of LE infusion, LE droplets were distributed into the peripheral vessels supplying liver parenchyma (arrowheads) and HCC nodule (arrows). The LE inflow into the liver parenchyma (arrowheads) soon ceased while that into the HCC nodule continued (arrows), and LE was densely accumulated in HCC nodule. An LE droplet present at the tip of the microcatheter during LE infusion (white arrow B, C). D The arterial branches of the posterior segment were embolized and filled with fragmented porous gelatin particles (arrows). At the end of gelatin embolization, A8 was depicted on fluoroscopy. E The microballoon catheter was placed at A8 (arrowhead), and DSA was performed via the right anterior oblique view to escape the overlap of HCC filled with LE on collateral artery. DSA revealed anastomosis vessel (arrow) between A8 and A7 (white arrow), and A8 was embolized with fragmented porous gelatin particles. F CT obtained immediately after selective B-TACE revealed dense LE accumulation in HCC (large circle). G Slight LE accumulation in liver parenchyma of the embolized region was also evident (circle)
Selective B-TACE without dense LE accumulation. A DSA of the celiac artery demonstrated an HCC nodule (white arrowheads) in the medial segment, and selective B-TACE was carried out while placing the microballoon catheter at A4 (white arrow). LE continued to flow both into the liver parenchyma and HCC nodule, and no dense LE accumulation in HCC nodule was noted. B DSA after selective B-TACE revealed anastomotic vessels for both the left (arrow) and right hepatic arteries (arrowhead). C CT obtained immediately after selective B-TACE revealed intermediate LE accumulation in HCC (arrow) and liver parenchyma of the embolized region (arrowheads)
Scatter diagrams of BOASP. The small symbols represent treatments in which dense LE accumulation in HCC was present (n = 39, group 1), and large symbols represent those in which no dense LE accumulation was present (n = 4, group 2). The symbols on the left side represent treatments in which collateral artery was observed (n = 19, group A), and those on the right side represent those in which no collateral artery was observed (n = 24, group B). There were statistically significant differences in BOASP between groups 1 and 2 (33.8 ± 12.8 mmHg vs. 92.3 ± 7.4 mmHg, p = 0.00004, Welch’s t test) and between groups A and B (51.3 ± 23.8 mmHg vs. 29.8 ± 12.7 mmHg, p = 0.001, Welch’s t test)
Scatter diagrams of the LECHL ratio. The small symbols represent treatments in which dense LE accumulation in HCC was present (n = 39, group 1), and large symbols represent those in which no dense LE accumulation was present (n = 4, group 2). The symbols on the left side represent treatments in which collateral artery was observed (n = 19, group A), and those on the right side represent those in which no collateral artery was observed (n = 24, group B). There was a statistically significant difference in LECHL ratio between groups 1 and 2 (18.3 ± 13.9 vs. 2.6 ± 1.1, p = 0.000034, Welch’s t test) but not between groups A and B (14.7 ± 15.0 vs. 18.5 ± 13.3, p = 0.38, Student’s t test)
Anastomotic vessels with collateral artery were present in 19 treatments, including all four with no dense LE accumulation in HCC nodule (group A) (Figs. 3, 4). No anastomotic vessel with collateral artery was observed in 24 of 43 treatments (group B) (Fig. 2). The BOASP in group A was 51.3 ± 23.8 (range 19–100) mmHg and that in group B was 29.8 ± 12.7 (range 13–64) mmHg (Fig. 5). There was a statistically significant difference between the groups (p = 0.001, Welch’s t test). The LECHL ratio was 14.7 ± 15.0 (range 1.7–54.2) in group A and 18.5 ± 13.3 (range 2.9–51.5) in group B (Fig. 6). There was no statistically significant difference between the groups (p = 0.38, Student’s t test).
Discussion
In 39 of 43 selective B-TACE treatments, LE stasis in the peripheral vessels supplying the liver parenchyma was observed immediately after LE infusion. In these 39 treatments, LE still continued to flow into the HCC nodule, and this is the reason why dense LE accumulation in the HCC nodule was noted. The LECHL ratio was 7 times greater in these 39 treatments compared with that in the other four treatments, in which no LE stasis in the peripheral vessels supplying the liver parenchyma was observed. Dense LE accumulation in HCC on DSA/fluoroscopy was objectively verified by this increased LECHL ratio. This phenomenon could be explained by the anastomotic vessels with collateral artery, viscosity of LE, and difference in size between the peripheral vessels supplying the liver parenchyma and those supplying the HCC nodule.
In none of the cases could the hepatic arterial flow be stopped by balloon inflation on DSA/fluoroscopy. In all cases, pulsatile movement of LE droplets was also present beyond the tip of the microballoon catheter. This arterial flow maintenance could not be explained by leakage via the space between the balloon and the occluded artery because stasis of a LE droplet was observed at the tip of the microballoon catheter (Figs. 2, 3). We think that intrahepatic collateral artery such as peribiliary plexus [4], interlobar communicating arcade [5], and isolated artery [6] play an important role in maintaining arterial flow beyond the occluded portion. Observation via DSA/fluoroscopy revealed anastomotic vessels with collateral artery in 19 treatments (Figs. 3, 4). However, in 24 treatments, no anastomotic vessels with collateral artery were depicted (Fig. 2). In these cases, they might have been so thin that they could not be recognized by fluoroscopy or DSA. Thus, we assume that the mean blood pressure of the occluded artery was lower in these treatments. Another explanation for no depiction of collateral artery is overlap of LE-accumulating HCC nodule on collateral artery.
Decrease of pressure gradient between the inflow vessel and the outflow one causes a decrease of flow. During TACE treatment with a conventional microcatheter, infused LE into the liver parenchyma is often washed out to the portal venous system through the arterioportal communication [7]. Animal experiments with rats also indicated that the lipiodol injected into the hepatic artery appeared in the portal vein and subsequently passed through the sinusoids into the systemic circulation [8]. The pressure gradient from the artery to the portal vein would be an important factor to determine the LE inflow into the liver parenchyma. When limited LE inflow into the liver parenchyma was observed (group 1), mean BOASP was 33.8 mmHg. Although we did not measure the portal venous pressure, another study reported a portal venous pressure in the cirrhotic liver of approximately 21 mmHg and in active hepatitis of approximately 15 mmHg [9]. The mean pressure gradient between the occluded artery and portal vein would be approximately 13–18 mmHg in 39 treatments in which LE inflow into the liver parenchyma was limited (group 1), and this decreased pressure gradient was one of the reasons for the limited LE inflow into the liver parenchyma.
Other reasons for the limited LE inflow into the liver parenchyma are LE viscosity and size of oily droplets. Two types of LE could be prepared: water in an oil emulsion (WOE) and oil in a water emulsion. In this clinical study, the volume of lipiodol was 3–5 times greater than that of dissolved anticancer drugs with contrast material, and WOE was prepared. WOE is a plastic fluid and has a high yield stress. This yield stress conveys potential to occlude a thin tube at a low pressure gradient as a solid-like embolic material [10]. Moreover, the oily droplet size of WOE is larger than that of oil in a water emulsion [11]. Thus, WOE flow into the liver parenchyma was highly limited under the decreased arterial pressure.
Decrease of pressure gradient and high viscosity of WOE also causes disturbance of LE inflow into the HCC nodule. However, even under balloon occlusion, LE continued to flow into the HCC in all 43 treatments. The reason for this maintenance of LE inflow into the HCC nodule could be explained by thicker tumor vessels with lower flow resistance.
There is a limitation in this study. Although we measured LECHL ratio in patients treated by selective B-TACE, we did not measure LECHL ratio in patients treated by selective TACE with a conventional microcatheter. We have performed selective TACE with a conventional microcatheter until February 2008; however, plain CT was obtained 1 week after TACE during that era, and almost all targeted TACE treatments have been performed with a microballoon catheter system since March 2008 in our hospital. Thus, we could not compare LECHL ratio by selective B-TACE with that by selective TACE with a conventional microcatheter. However, in our experience in selective TACE with a conventional microcatheter, the behavior of LE was similar with that in cases of group 1 in which blood pressure of the occluded artery was more than 64 mmHg and LE continued to flow into both the HCC nodule and the nontumorous liver parenchyma.
It has been reported that iodine-131 (I-131) lipiodol ultra fluid injected into the hepatic artery concentrated in the HCC with a tumorous (T) to nontumorous (NT) activity ratio (T/NT) of 4.3 ± 3.6 [12]. If the I-131 T/NT ratio was comparable with the LECHL ratio, the LECHL ratio in group 1 was 18.3 and B-TACE might improve I-131 T/NT ratio to 18.3 when the pressure of the occluded artery was decreased to 64 mmHg or less. We consider improved LE accumulation by B-TACE could also be applied to improve the effect of radioembolization with I-131 lipiodol ultra fluid for the treatment of HCC [13, 14].
Measurement of BOASP was useful to predict degree of LE accumulation in HCC. Advancement of the microballoon catheter beyond a thick anastomotic vessel might be a reasonable option to achieve dense LE accumulation when BOASP was more than 64 mmHg. For this purpose, it is desirable to improve the microballoon catheter shaft to make it more flexible and thinner.
The original porous gelatin particles were calibrated to be 1 mm in diameter and were crushed into approximately 200-μm fragments for use as embolization material. This is because the diameter of the peripheral tumor vessels is less than that of the 3F (1 mm) microballoon catheter shaft on DSA (Figs. 2A, 3A), and the use of the original porous gelatin particles would cause proximal embolization. It was also reported that the gelatin block was cut into 500-μm cubes and the cubes were crushed into 200-μm fragments by pumping between two syringes interconnected with a three-way stopcock, and the fragments were used for ultraselective TACE as embolization material [15]. However, the best size of gelatin particles (fragments) for TACE is still unknown.
In conclusion, dense LE accumulation in the HCC nodule could be achieved by B-TACE when BOASP was 64 mmHg or less.
References
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37:429–442
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171
Irie T, Kuramochi M, Takahashi N (2009) Improved accumulation of lipiodol under balloon-occluded transarterial chemoembolization (B-TACE) for hepatocellular carcinoma: measurement of blood pressure at the embolized artery before and after balloon inflation. IVR 26:49–54
Cho KJ, Lunderquist A (1983) The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology 147:357–364
Tohma T, Cho A, Okazumi S et al (2005) Communicating arcade between the right and left hepatic arteries: evaluation with CT and angiography during temporary balloon occlusion of the right or left hepatic artery. Radiology 237:361–365
Ekataksin W (2000) The isolated artery: an intrahepatic arterial pathway that can bypass the lobular parenchyma in mammalian livers. Hepatology 31:269–279
Nakamura H, Hashimoto T, Oi H, Sawada S (1988) Iodized oil in the portal vein after arterial embolization. Radiology 167:415–417
Kan Z, Ivancev K, Hägerstrand I et al (1989) In vivo microscopy of the liver after injection of lipiodol into the hepatic artery and portal vein in the Rat. Acta Radiol 30:419–425
Moriyasu F, Nishida O, Ban N et al (1986) “Congestion index” of the portal vein. AJR Am J Roentgenol 146:735–739
Demachi H, Matsui O, Abo H, Tatsu H (2000) Simulation model based on non-newtonian fluid mechanics applied to the evaluation of the embolic effect of emulsions of iodized oil and anticancer drug. Cardiovasc Intervent Radiol 23:285–290
de Baere T, Zhang X, Aubert B et al (1996) Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology 201:731–735
Raoul JL, Bourguet P, Bretagne JF et al (1988) Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology 168:541–545
Lau WY, Lai ECH, Leung TWT, Yu SCH (2008) Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial—update on 5-year and 10-year survival. Ann Surg 247:43–48
Chua TC, Chu F, Butler SP et al (2010) Intra-arterial iodine-131-lipiodol for unresectable hepatocellular carcinoma. Cancer 116:4069–4077
Miyayama S, Matsui O, Yamashiro M et al (2007) Ultraselective transcatheter arterial chemoembolization with a 2-F tip microcatheter for small hepatocellular carcinomas:relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 18:365–376
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 1595 kb)
Supplementary material 2 (MOV 4609 kb)
Rights and permissions
About this article
Cite this article
Irie, T., Kuramochi, M. & Takahashi, N. Dense Accumulation of Lipiodol Emulsion in Hepatocellular Carcinoma Nodule during Selective Balloon-occluded Transarterial Chemoembolization: Measurement of Balloon-occluded Arterial Stump Pressure. Cardiovasc Intervent Radiol 36, 706–713 (2013). https://doi.org/10.1007/s00270-012-0476-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0476-z