Abstract
Introduction
Transarterial chemoembolisation (TACE) is the most widely used locoregional treatment for patients with an unresectable hepatocellular carcinoma (HCC). Transarterial radioembolisation (TARE) with yttrium-90 containing microspheres is an emerging interventional treatment that could be complementary or an alternative to TACE.
Aim
To evaluate the safety and efficacy of TARE in patients with HCC who are refractory to TACE with drug-eluting beads (DEB-TACE).
Methods
We identified all patients who received TARE for HCC following one or more sessions of DEB-TACE in the period 2007–2016. Grade ≥3 adverse events were graded according to Common Terminology Criteria for Adverse events. Response on MRI was determined on MRI by modified RECIST. Overall survival was estimated using the Kaplan–Meier method and was determined from the first TACE and from the TARE procedure.
Results
A total of 30 patients were included. Patients had a mean of 1.7 TACE procedures (range 1–4) prior to TARE. Grade 3 adverse events following TARE included: fatigue (20%), bilirubin increase (10%), cholecystitis (3.3%) and a gastric ulcer (3.3%). Response on MRI was achieved in 36.7%. Three patients (10%) were downstaged within the Milan criteria and received liver transplantation. The median overall survival after first TACE was 32.3 months (17.2–42.1 95% CI). The median overall survival after TARE was 14.8 months (8.33–26.5 95% CI).
Conclusion
TARE is safe and can be effective in patients with an intermediate or advanced stage HCC who are refractory to TACE. This treatment strategy has the potential to downstage to liver transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transarterial chemoembolisation (TACE) is the most widely used locoregional treatment for patients with an unresectable HCC. TACE can delay disease progression and has also been shown to downstage HCC tumours that do not meet the Milan criteria [1]. In recent years, TACE with drug-eluting beads (DEB-TACE) had become a widely used alternative to conventional TACE (cTACE) with at least an equal efficacy and toxicity profile [2, 3]. Transarterial radioembolisation (TARE) is an emerging interventional treatment that could be an alternative to TACE [4] and is a viable alternative to sorafenib in patients with a portal vein thrombosis [5]. Our hypothesis is that TARE can also be a valuable treatment option in patients with an intermediate and advanced stage HCC who are refractory to TACE.
In our institution, patients with an unresectable HCC and preserved liver function receive DEB-TACE as first-line treatment. If patients are refractory to TACE, the intra-arterial therapy can be switched to TARE. The aim of the study is to evaluate the safety and effectiveness of TARE in patients with HCC who are refractory to DEB-TACE.
Materials and Methods
Study Design
This study was approved by the institution’s ethics committee. All patients treated at the interventional radiology department of our hospital are prospectively registered in a database. A retrospective study was conducted by querying this database to identify all patients who received one or more sessions of DEB-TACE prior to TARE for HCC in the period 2007–2016. The diagnosis of HCC was made by means of biopsy or accepted radiological findings as described by the European Association for Study of the Liver (EASL) [6].
All included patients underwent baseline assessment prior to TARE, including laboratory tests to evaluate liver function and α-fetoprotein level. The HCC aetiology, Child–Pugh classification, Barcelona Clinic Liver Cancer (BCLC) classification, model of end-stage liver disease (MELD) score, data on the number of prior DEB-TACE treatments and other prior treatments for HCC were collected from the hospital’s patient information system. Tumour staging was determined based on the imaging examinations prior to the mapping angiogram.
Transarterial Chemoembolisation with Drug-Eluting Beads (DEB-TACE)
All TACE procedures were performed by or under the supervision of one expert interventional radiologist. Details of the TACE procedures in our hospital have been described previously [7]. Briefly, TACE was performed using doxorubicin loaded superabsorbent polymer (SAP) microspheres (HepaSphere Microspheres, Merit Medical Systems, South Jordan, Utah, USA) in a dedicated interventional angiography suite. One vial of 25-mg dry microspheres with a nominal dry diameter of 50–100 µm was mixed with the prescribed dose of doxorubicin. The standard doxorubicin dose was 50–75 mg/m2, reduced to 25 mg/m2 in case of elevated bilirubin or cytopenia. Depending on the number and distribution of the HCC lesions, the drug-eluting beads were infused via superselective infusion of the feeding artery, lobar infusion or bilobar infusion. The treatment endpoint was either the delivery of the full calculated dose or sluggish flow in the feeding artery.
Patients who were refractory to TACE were defined as those showing progression or stable disease according to modified RECIST [8] or ADC ratio [9]. This was visualised using contrast-enhanced and diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) 1–3 months after DEB-TACE. The TACE-refractory status of individual patients was determined by a multidisciplinary team including a medical, surgical and radiation oncologist, a diagnostic and interventional radiologist and a hepatologist.
In our institution, patients with a HCC within the ‘Milan criteria’ (solitary tumour ≤5 cm and up to three nodules ≤3 cm [6, 10]) are eligible for OLT. Pre-OLT intra-arterial therapies can be used for downstaging in patients with a single HCC up to 7 cm or multiple HCC’s measuring 2–5 cm and no more than 4 lesions.
Transarterial Radioembolisation (TARE)
Patients’ eligibility for TARE was determined in a multidisciplinary team and was based on patient approval, performance status, disease course, response to prior procedures and vessel patency. All TARE procedures were performed by or under the supervision of one expert interventional radiologist. The interventional radiological technique for TARE was performed according to previously published guidelines and technical angiographic reviews [11, 12]. In summary, during the angiographic work-up coil embolisation of extrahepatic vessel was performed at the discretion of the interventional radiologist. At the end of the angiographic work-up, technetium-99 m-labelled macroaggregated albumin was injected in the target vessel and a planar scintigraphy to determine the lung shunt fraction (LSF) and a single-photon emission computed tomography (SPECT) were performed. The TARE procedure was performed between 2 and 5 weeks after the angiographic work-up with the use of resin yttrium-90 containing microspheres (90Y-microspheres; SIR spheres®, Sirtex Inc, Cosgrove, Australia). Depending on the work-up results and the number and distribution of the HCC lesions, the resin microspheres are infused via superselective infusion of the feeding artery, lobar infusion or bilobar infusion. Bilobar TARE procedures were performed in one or two sessions, depending on the patient’s liver function. The use of an anti-reflux catheter (Surefire Inc., Westminster, CO, USA) was performed at the discretion of the interventional radiologist for retrograde protection or dose redistribution [13]. Activity calculation was performed using the body surface area (BSA) method or by partition model if possible, and by taking into account the liver volume and presence or absence of cirrhosis [13]. These values were further reduced if necessary so that the calculated lung dose would be not more than 30 Gy. The procedures were performed under local anaesthesia. Anti-emetics and morphine derivatives were given when required. Bremsstrahlung imaging was performed on the day of the 90Y-microsphere infusion or the next day. Since the publication of Gil-Alzugaray et al. [14], patients were given ursodeoxycholic acid for 2 months starting on the day of the TARE at a dose of 300 mg twice a day orally, in addition to methyl-prednisolone 8 mg once a day for 1 month and 4 mg once a day for the second month, to prevent radioembolisation-induced liver disease.
Follow-Up and Response Assessment
Baseline and post-procedural follow-up imaging was conducted with MRI as published earlier [9]. Response on imaging was assessed by an expert abdominal radiologist. The radiologist was blinded to patients’ clinical and biochemical information. A maximum of two target lesions were chosen for response assessment. Response measured by using modified RECIST (mRECIST) was based on the arterial phase enhancing area of the tumour by measuring the index tumour(s) and categorising change in tumour size enhancement into four categories [complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD)].
Clinical follow-up based on physical examination and laboratory tests took place 1 month after the procedure and every 3 months thereafter. Adverse events grade ≥3 were documented and graded according to Common Terminology Criteria for Adverse events [15]. Survival was determined from the first TACE and from the TARE procedure.
Statistical Analysis
Summary statistics are presented as means and ranges for continuous variables and as frequencies and percentages for categorical variables. Overall survival is estimated using the Kaplan–Meier method, and 95% confidence intervals (95% CI) were computed. Analysis was carried out using SAS software (version 9.4 of the SAS System for Windows).
Results
Patient Characteristics
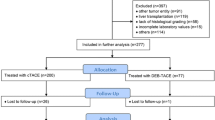
During the study period, 173 patients underwent DEB-TACE for HCC at our institution. Of these, 32 were considered refractory to TACE by lack of objective response. One patient was not able to receive a TARE procedure because of an LSF of more than 20%. Another patient had a significant worsening of his liver function after the mapping angiogram and was therefore an inadequate candidate for TARE. Eventually, 30 patients with a DEB-TACE-refractory HCC received a TARE procedure.
Patient characteristics prior to TARE are listed in Table 1. The mean patient age was 64 years (range 39–82), most patients were male (83.3%), and alcoholic (30.0%) and non-alcoholic steatohepatitis (23.3%) were the most common underlying liver diseases. In 25 patients, there was evidence of cirrhosis (83.3%), which was well compensated (Child–Pugh A) in 78.3%. A Barcelona Clinic Liver Cancer (BCLC) Stage B and C was assigned to 15 (50%) patients each. Most patients had more than three liver lesions (73.3%) and bilobar disease (62.3%). The largest tumour dimension ranged from 1.8 to 9.0 cm (mean 4.1). Prior to TARE, extrahepatic disease and portal vein invasion were each present in 10% of the patients.
Treatment Characteristics
Patients had a mean of 1.7 DEB-TACE procedures (range 1–4) prior to TARE. Some patients already received another treatment prior to DEB-TACE. These treatments included resection, ablation, sorafenib, conventional TACE and transplantation (Table 2). TARE was performed at a mean of 6.5 months following the last DEB-TACE procedure (range 2.8–27.4, median 4.5).
Table 3 shows the treatment characteristics of the TARE procedure. The most common reasons for switching to TARE were progression (60%) or non-response (36.7%) despite DEB-TACE. Prophylactic embolisation of non-target vessels was carried out in 63.3% of the gastroduodenal arteries, 30% of the right gastric arteries and in other arteries in 30% of cases. An anti-reflux catheter was used in 23.3% of the patients. About half of the patients received a whole-liver treatment (56.7%) because of bilobar disease; in five of these patients, the treatment was performed in two sessions because of reduced liver function. The remaining patients received a lobar or segmental infusion. An activity reduction was necessary in three patients (10%) because of a high LSF. The mean infused activity was 1307 MBq (35,324 mCi).
Clinical Follow-Up
Short-term follow-up (3 months) was available for all 30 patients. Imaging follow-up was available for 29 patients. Long-term follow-up ranged from 7 to 64 months after first DEB-TACE and 3 to 43 months after TARE. No grade 4 or 5 adverse events occurred within 3 months after the TARE procedure. The most common clinical grade 3 adverse events were fatigue (20%) and pain (6.7%). One patient developed a cholecystitis after preventive coil embolisation of the cystic artery during the angiographic work-up. A gastric ulcer occurred in one patient. In this patient, coil embolisation of the gastroduodenal artery was performed during the angiographic work-up but the right gastric artery was not found. The most common biochemical grade 3 adverse event was a bilirubin increase (Table 4). Complications occurred more often after whole-liver treatment (n = 11, 64.7%), compared to lobar (n = 4, 36.4%) and segmental treatment (n = 0, 0.0%). MRI follow-up was performed at a mean of 2.9 (standard deviation 1.0, median 3, range 1–6) months following TARE. Response rates included partial response (n = 11, 36.7%) (Fig. 1a–e), stable disease (n = 8, 26.7%) and progression of disease (n = 10, 33.3%).
A 62-year-old man with liver cirrhosis and multifocal HCC. A Completion selective angiography of the common hepatic artery after DEB-TACE (HepaSphere and doxorubicin) reveals distal occlusion of the second, third and end branches of both the right (arrow) and left (arrowheads) hepatic artery. B Contrast-enhanced MRI 2 months after initial DEB-TACE shows progression and persistent contrast enhancement of the largest HCC lesion in the left liver lobe (white arrowheads). C Selective angiography of the right hepatic artery (black arrow) and D left hepatic artery (black arrowhead) before 90Y-radioembolisation shows recanalisation and neoangiogenesis (white small arrows) in and close to the previously embolised distal hepatic artery vessels. E Contrast-enhanced MRI 3 months after 90Y-radioembolisation reveals partial response with >50% necrosis of the lesion. Note also the peritumoral post-radioembolisation oedema around the target lesion
Three patients (10%) were successfully downstaged for disease extent within the Milan criteria and received a liver transplantation (Fig. 2).
Of the 30 patients, 20 died during the study period, nine survived and one was lost to follow up. The median overall survival after first TACE was 32.3 months (17.2–42.1, 95% CI) (Fig. 3a). The median overall survival after TARE was 14.8 months (8.33–26.5, 95% CI) (Fig. 3b).
Discussion
TACE is the most widely used locoregional treatment for patients with an unresectable HCC. If chemoembolisation is no longer feasible due to tumour progression or intolerance of TACE, systemic therapies like sorafenib are the only treatment option according to the American and European guidelines [6, 10]. They are associated with a median overall survival varying from 8.6 to 25.4 months [16,17,18]. The current study shows that radioembolisation may be an alternative to sorafenib in patients with intermediate and advanced stage HCC who are refractory to TACE, with a good survival and safety profile.
The median overall survival of TARE in BCLC B and C patients without prior TACE treatment varies from 10.0 to 16.9 months [5, 19, 20]. Recently Johnson et al. published the results of patients who received TARE as a salvage therapy in patients after chemoembolisation using conventional TACE, DEB-TACE or a combination of these two [21]. They found a median overall survival of 8.4 months following TARE in mainly BCLC B patients with a mean of 3.7 prior TACE procedures (range 1–8). In our study population, we found a slightly longer median overall survival of 14.8 months after the TARE procedure and a median overall survival of 32.3 months after the first TACE treatment in BCLC B and C patients with a mean of 1.7 prior DEB-TACE treatments (range 1–4). The lesser amount of prior TACE procedures in our study might explain the better overall survival, as Johnson et al. [21] showed that TARE following TACE was less feasibly in patients with >4 prior TACE procedures.
The safety profile of TARE following DEB-TACE also seems comparable to either the series in treatment-naïve patients [22] or the previously mentioned study of Johnson et al. [21] with fatigue (20%) and bilirubin increase (10%) as the most common clinical and biochemical grade 3 adverse event, respectively. These complications occurred more often after treatment of the whole liver compared to lobar and segmental treatment.
Orthotopic liver transplantation (OLT) is considered the only curative therapeutic approach for patients with cirrhosis and HCC, not only for treatment of the neoplastic disease but also for resolution of the underlying liver disease. OLT is recommended for patients within the Milan criteria; however, many patients are diagnosed at an intermediate or advanced stage and are therefore not candidates for this curative therapy [23]. For selected patients, downstaging can be considered to bring patients within Milan criteria by using liver-directed therapies [24]. TACE is the most widely used downstaging therapy for multiple HCCs [25,26,27,28,29], but other therapies such as TARE [30, 31], ablation [32] or a combined approach [32, 33] are also used. The current study shows that TARE is able to downstage patients into the Milan criteria even if they are refractory to DEB-TACE.
Heterogeneity remains a major obstacle in all chemoembolisation studies, because there is no consensus concerning whether cTACE or DEB-TACE is the preferred treatment [3]. A strength of the current study is the homogenous patient population: all patients were treated with the same DEB-TACE (doxorubicin loaded SAP microspheres) and the same resin 90Y-microspheres. However, HCC has many treatment options for different stages of the disease (e.g. ablation, resection, transplantation, TACE, Sorafenib). Therefore, as in other studies, some of the included patients received one of these other treatments prior to DEB-TACE.
The main limitation of this study is its retrospective nature; although all patients treated in the institutional interventional radiology department are registered prospectively in a database, the data were collected retrospectively from the patient’s information system. Due to this retrospective nature a mild complication, for example, pain, that did not need further treatment or was successfully treated with painkillers (CTCAE 1 or 2), may not have been documented in the patient’s information system and was therefore not traceable in a retrospective manner. Further, the inclusion period of this study had a wide range from 2007 till 2016. In this period, diagnostic and treatment protocols might have been changed due to new insights and developments.
Conclusion
TARE following DEB-TACE is a safe and efficient treatment strategy in patients with HCC, with the potential to downstage to liver transplantation. TARE should be considered as an alternative to systemic therapies such as sorafenib in patients with an intermediate or advanced stage HCC who are unresponsive to (multiple) TACE treatments. Future randomised controlled trials are needed to compare sorafenib with TARE in HCC patients who are refractory to TACE.
References
Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11–22. doi:10.1016/S1470-2045(11)70175-9.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi:10.1007/s00270-009-9711-7.
Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(6):571–7. doi:10.1016/j.dld.2016.02.005.
Lobo L, Yakoub D, Picado O, Ripat C, Pendola F, Sharma R, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2016;39(11):1580–8. doi:10.1007/s00270-016-1426-y.
Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826–37. doi:10.1002/hep.26014.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. doi:10.1002/hep.24199.
Dekervel J, van Malenstein H, Vandecaveye V, Nevens F, van Pelt J, Heye S, et al. Transcatheter arterial chemoembolization with doxorubicin-eluting superabsorbent polymer microspheres in the treatment of hepatocellular carcinoma: midterm follow-up. J Vasc Interv Radiol. 2014;25(2):248–55. doi:10.1016/j.jvir.2013.10.017.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132.
Vandecaveye V, Michielsen K, De Keyzer F, Laleman W, Komuta M, Op de Beeck K, et al. Chemoembolization for hepatocellular carcinoma: 1-month response determined with apparent diffusion coefficient is an independent predictor of outcome. Radiology. 2014;270(3):747–57. doi:10.1148/radiol.13130591.
European Association for The Study of The L, European Organisation for R, Treatment of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi:10.1016/j.jhep.2011.12.001.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. doi:10.1016/j.ijrobp.2006.11.060.
Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17(10):1571–93. doi:10.1097/01.RVI.0000236744.34720.73.
Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26(5):660–9. doi:10.1016/j.jvir.2015.01.029.
Gil-Alzugaray B, Chopitea A, Inarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57(3):1078–87. doi:10.1002/hep.26191.
Institute. NC. Common Terminology Criteria for Adverse Events (CTCAE). Version 4. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 27 Oct 2016.
Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330–41. doi:10.1159/000365993.
Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49(5):932–40. doi:10.1007/s00535-013-0853-7.
Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–62. doi:10.1159/000367743.
Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–78. doi:10.1002/hep.24451.
Hilgard P, Hamami M, Fouly AE, Scherag A, Muller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52(5):1741–9. doi:10.1002/hep.23944.
Johnson GE, Monsky WL, Valji K, Hippe DS, Padia SA. Yttrium-90 radioembolization as a salvage treatment following chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2016;27(8):1123–9. doi:10.1016/j.jvir.2016.03.046.
Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20(9):1121–30. doi:10.1016/j.jvir.2009.05.030 (quiz 31).
Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–53. doi:10.1038/ajg.2014.11.
Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22(1):7–17. doi:10.3350/cmh.2016.22.1.7.
San Miguel C, Muffak K, Triguero J, Becerra A, Villegas T, Nogueras F, et al. Role of transarterial chemoembolization to downstage hepatocellular carcinoma within the milan criteria. Transplant Proc. 2015;47(9):2631–3. doi:10.1016/j.transproceed.2015.10.008.
Green TJ, Rochon PJ, Chang S, Ray CE Jr, Winston H, Ruef R, et al. Downstaging disease in patients with hepatocellular carcinoma outside of Milan criteria: strategies using drug-eluting bead chemoembolization. J Vasc Interv Radiol. 2013;24(11):1613–22. doi:10.1016/j.jvir.2013.07.024.
De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, et al. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9(5):1158–68. doi:10.1111/j.1600-6143.2009.02576.x.
Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248(4):617–25. doi:10.1097/SLA.0b013e31818a07d4.
Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260–7. doi:10.1002/lt.20837.
Pracht M, Edeline J, Lenoir L, Latournerie M, Mesbah H, Audrain O, et al. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649. doi:10.1155/2013/827649.
Inarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D’Avola D, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38(7):594–601. doi:10.1016/j.ejso.2012.02.189.
Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–77. doi:10.1002/hep.27752.
Barakat O, Wood RP, Ozaki CF, Ankoma-Sey V, Galati J, Skolkin M, et al. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl. 2010;16(3):289–99. doi:10.1002/lt.21994.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GM and CMD are consultants to SIRTEX, the manufacturer of the 90Y-microspheres. The other authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Formal Consent
Formal consent is not required for this type of study. This article does not contain any studies in animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Klompenhouwer, E.G., Dresen, R.C., Verslype, C. et al. Safety and Efficacy of Transarterial Radioembolisation in Patients with Intermediate or Advanced Stage Hepatocellular Carcinoma Refractory to Chemoembolisation. Cardiovasc Intervent Radiol 40, 1882–1890 (2017). https://doi.org/10.1007/s00270-017-1739-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1739-5