Abstract
Background
Radioembolization (RE) with 90yttrium (90Y) resin microspheres generally employs a sandwich technique with separate sequential administration of contrast medium (CM), followed by vehicle (e.g., glucose 5% [G5] solution), then 90Y resin microspheres (in G5), then G5, and then CM again to avoid contact of CM and microspheres under fluoroscopic guidance. This study evaluates the visualization quality and safety of a modified sandwich technique with a 50/50-mixture of CM (Imeron 300) and G5 for administration of 90Y resin microspheres.
Materials and Methods
A retrospective analysis of 81 RE procedures in patients with primary or secondary liver tumors was performed. The quality of angiographic visualization of the hepatic vessels was assessed before the first injection and immediately before the whole dose has been injected. Visualization and flow rate were graded on a 5-point scale: 1 = very good to 5 = not visible/no antegrade flow. Univariate logistic regression models and multiple linear regression models were used to evaluate the prognostic variables associated with visualization and flow scores.
Results
Visualization quality was inversely related to flow rate, the lower the flow rate the better the grade of the visualization. Visualization quality was also inversely related to body-mass-index (BMI). Performing RE with the 50/50-CM/G5 mixture resulted in a mean injection time for 1 GBq of 15 min. No clinically significant adverse events, including radiation-induced liver disease were reported.
Conclusion
RE with a 50/50-mixture of CM and G5 for administration of 90Y resin microspheres in a modified sandwich technique is a safe administration alternative and provides good visualization of hepatic vessels, which is inversely dependent on flow rate and BMI. Injection time was reduced compared with our experience with the standard sandwich technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver-directed treatments using minimally invasive techniques are increasingly used to treat primary and metastatic liver cancers, conditions with an increasing incidence [1, 2]. The dual blood supply of the liver enables selective treatment of hepatic malignancies, which mainly receive blood via the hepatic artery, with therapeutic agents delivered in the blood supply from the hepatic artery, with minimal effects on normal liver parenchyma, which receives its blood mostly via the portal circulation [3]. Radioembolization (RE) [also referred to as selective internal radiation therapy (SIRT)] with 90yttrium (90Y) resin microspheres (SIR-Spheres®, Sirtex Medical, Sydney, Australia) is a ‘blood flow-directed’ therapy, in which the microspheres are infused by intra-arterial catheterization into branches of the hepatic artery. They then lodge within the terminal arteries (median diameter 32.5 microns) close to the liver tumors, without passing through the capillary bed (≤ 7–10 microns diameter) thus avoiding systemic effects. Consequently, RE can deliver a high dose of radiation safely and effectively to primary or secondary liver tumors while largely sparing normal liver tissue [4,5,6,7,8,9].
As 90Y is bound to the resin microspheres through an ion exchange mechanism, sterile water for injection (WFI) has been the standard vehicle for RE with 90Y resin microspheres. Although the published safety and efficacy profiles of RE with 90Y resin microspheres with WFI as the vehicle for the delivery are consistently favorable, it has recently been proposed that vehicles other than WFI might optimize delivery of the microspheres. Glucose 5% solution (G5) has been evaluated as vehicle for the injection of 90Y resin microspheres. The hypothesis is that the isotonic nature of G5 may prevent arterial endothelial injury and consequent vasoconstriction, and so, may reduce the periprocedural need for analgesia. Furthermore, replacement of WFI may reduce the incidence of stasis, which has been reported in over 35% of patients receiving RE for relatively hypovascular hepatic metastases of colon cancer in a salvage setting [10, 11]. Another recent study has reported stasis in 17% of patients receiving RE using WFI [12]. Recently published studies have shown that replacing WFI with G5 significantly reduced the need for periprocedural analgesia and that slow and pulsatile administration of the 90Y resin microspheres was associated with a low rate of stasis [13, 14].
A standard technique for administering 90Y resin microspheres is the so-called sandwich technique to avoid contact between 90Y resin microspheres and the contrast medium. This is a pattern of injection of contrast medium, followed by vehicle (e.g., G5), then 90Y resin microspheres (in G5), then G5, and then contrast medium again. The sequence is repeated until the full dose of 90Y resin microspheres has been administered (or stasis is reached). Although it is known that the image contrast on fluoroscopy during administration is much better using undiluted ‘pure’ contrast medium instead of a mixture, an important disadvantage is the lack of visible real-time flow control as neither 90Y resin microspheres nor G5 are radiopaque.
In this study, we evaluate a modified pattern of injection using a 50/50 mixture of G5 and contrast medium (Imeron 300), with an injection sequence of Imeron 300/G5 followed by 90Y resin microspheres (in G5) followed by Imeron 300/G5. The aim of our study was to evaluate the visualization quality of the hepatic vessels during RE with 90Y resin microspheres in correlation with the flow rate, using this modified sandwich technique.
Materials and Methods
Ethical Considerations
The study was approved by the Institutional Review Board, and all patients provided written informed consent.
Patient Selection
The decision to treat patients with RE with 90Y resin microspheres was based on published criteria [15] and the consensus of the multidisciplinary tumor board at our center.
Between May 2015 and January 2016, 81 RE procedures were performed using a 50/50 mixture of G5 and contrast medium (Imeron 300) delivered in a single syringe as the vehicle for the administration of 90Y resin microspheres instead of the standard sandwich technique with G5 as the administration vehicle.
Study Endpoint
The study endpoint was the end of dose administration due to continuos antegrade flow. If stasis or retrograde flow developed, administration was stopped for a short time until the antegrade flow had resumed. In cases of persistent stasis or retrograde flow, RE was ended.
Angiography Specifications
All RE was performed with an Artis Zeego (2. Generation/Software VC21B) angiography machine and an Axiom Artis dTA (Software VB25E) C-arm angiography system. Fluoroscopy was performed at a setting of 7.5 frames per second (fps). A pretest within ten patients showed no significant differences regarding the image quality between the two angiography facilities. Therefore, flow analysis at the work-up was compared to flow analysis before the first treatment in the same patients at the two different angiography facilities.
Dosimetry
Dose calculations were based on the partition model, using exact liver and tumor volumes calculated by dedicated software application (OncoTREAT, MeVis, Bremen, Germany) [16].
RE Procedure
Arteriography was performed via transfemoral catheterization. The presence of angiographically occult afferent extrahepatic arteries and the magnitude of hepato-pulmonary shunting were evaluated by hepatic arterial injection of approximately 100 MBq of [99mtechnetium] Macro-Aggregated Albumin ([99mTc]-MAA) in the left and right (and sometimes segment IV) hepatic artery separately according to the planned catheter position(s) for RE. Instead of systematic coil embolization during the [99mTc]-MAA angiography, where possible the catheter was placed at least 2 cm distal from the gastroduodenal artery (GDA) (or other artery supplying blood to the gut) in order to prevent the nontarget distribution of microspheres [17].
A single syringe containing a 50/50 mixture of contrast medium and G5 was connected to the B line. For the injection of pure G5, a second syringe was connected to the D line (Fig. 1). 90Y resin microspheres (in G5 via the D line) and the 50/50 G5 and Imeron 300 mixture (via B line to minimize contact time between the spheres and the diluted contrast medium) were injected alternately under intermittent fluoroscopic visualization to ensure an appropriate antegrade flow and to avoid stasis or retrograde flow. A slow and pulsatile injection technique and regular fluoroscopic control were employed to ensure a good antegrade flow and low risk of stasis [13].
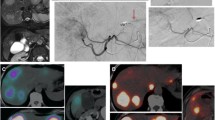
A Celiac trunc: proper hepatic artery (gray arrow), left hepatic artery (white arrow/left therapy position), right hepatic artery (black arrow/right therapy position), Gastroduodenal artery (gray arrow head), left gastric artery (white arrow head), splenic artery (black arrow head). B Right hepatic artery; therapy position: application of pure contrast agent (Imeron 300) before RE. C Right hepatic artery; therapy position: application of the 50/50 mixture of contrast agent (Imeron 300) and G5 before RE. The contrast is not as good as with pure contrast (Fig. 2B), but you can verify an antegrade flow safely. D Right hepatic artery; therapy position: application of the 50/50 mixture of contrast agent (Imeron 300) and G5 after RE. The contrast is still not as good as with pure contrast (Fig. 2B), but due to a reduction of the flow you can verify the antegrade flow even better than shown in Fig. 2C
90Y resin microspheres were administered as a lobar or sequential lobar treatment (with an interval of 4–6 weeks between procedures) depending on the distribution of tumors within the liver. In all cases, the left, right, and (if applicable) segment IV hepatic artery were catheterized and treated separately, to improve safety. RE was never performed from the common or proper hepatic artery.
Visualization and flow rate were evaluated at two defined time points:
-
before starting injection of the 90Y resin microspheres.
-
shortly before the last injection of 90Y resin microspheres.
Visualization quality and flow rate were graded from 1 = very good to 5 = not visible/no antegrade flow (Table 1) by consensus between the Interventional Radiologist and a physician from the Department of Nuclear Medicine.
Follow-Up
SPECT (or SPECT/CT) scans were performed within 24 h to confirm targeted deposition of 90Y resin microspheres (Bremsstrahlung-SPECT). Results from hematologic, liver function and blood biochemistry tests and physical examination were recorded before the first RE procedure (baseline), and at all subsequent follow-up visits (on day 1, 2, 3, after 4 weeks and 3 months). The nature and severity of any changes in liver function were recorded as well any other clinically significant adverse events (Grade 3 was graded according to the National Cancer Institute Common Toxicity Criteria Adverse Events version 3.0 (CTC v3) [18].
Statistical Analysis
Visualization and flow characteristics were scored on a 5-point integer scale (1 = very good, 5 = very bad). The association of prognostic variables on visualization and flow scores (and changes) for categorical variables was by a Chi-square general association test, and for continuous variables by analysis of variance. Univariate logistic regression models for improvement in visualization (and worsening in flow) by prognostic variables were performed, and statistically significant variables were candidates for a multivariate logistic regression model. Multiple linear regression models were constructed to evaluate the prognostic variables associated with visualization and flow scores utilizing a backwards elimination procedure. The association of visualization scores at before the first injection and before final injection was tested by the McNemar test. A p value of 0.05 was used to declare statistical significance, and SAS software (SAS Institute Inc, Cary, N.C.) was used for statistical analysis.
Results
The study included 81 patients with a mean age of 61.9 years (Table 2). The median calculated radiation dose was 750 MBq (range 300–2100 MBq), and the median administered dose was 750 MBq (range 300–1875 MBq). Mean (SD) duration of administration was 13.6 ± 5.6 min. Sixteen patients received ≥1 intra-operative medications: nine received ondansetron and 12 piritramide.
The proportion of patients in each flow and visualization grade is shown in Table 3. Stasis was observed in 5/81 patients, and visualization was rated as moderate in one of these patients and good or very good in the others. There were no observations of retrograde flow and no cases where RE was finished prematurely due to retrograde flow.
Visualization score before the first injection was higher (poorer visualization) in men than in women (p = 0.007), when BMI was higher (p = 0.001) and when no intra-procedure medication was used (p = 0.006). Poor visualization before the last injection was associated with higher BMI (p = 0.017), a higher dose of RE (p = 0.034), a higher percentage lung shunt (p = 0.014), no intra-procedure medication (p = 0.031), absence of stasis (p = 0.009) and improved flow rate (p = 0.013). Poor flow rate (higher flow rate score) before the first injection was associated with female gender (p = 0.015), the presence of stasis (p < 0.001) and non-right-sided tumor (p = 0.031), and the same factors were associated with poorer flow rate before the last injection (p = 0.026, p < 0.001 and p = 0.019, respectively).
There was an inverse relationship between flow rate and quality of visualization (correlation coefficient r = −0.50 [p = 0.001] before injection of 90Y resin microspheres; r = −0.51 [p = 0.001] before last injection of 90Y resin microspheres).
Changes in visualization and flow score between the first injection and last injection of 90Y resin microspheres were minor, but flow rate did deteriorate slightly (Table 3; association between two time points for visualization score and flow rate score, p < 0.001 and p = 0.156, respectively). Univariate regression analysis showed that the worsening of flow score from before the first injection to before the last injection was associated not only with improvement in visualization score but also the presence of stasis, the presence of coiling and non-right-sided tumor. No other variables assessed had a significant association with the changes in flow score. The multivariate analyses of these factors confirmed their significant influence upon the changes in flow score: improvement in visualization score (odds ratio [OR] 0.13, 95% CI 0.02–0.83; p = 0.03), the presence of stasis (OR 0.01, 95% CI < 0.01–0.20; p = 0.002), the presence of coiling (OR 0.01, 95% CI < 0.01–0.25; p = 0.004) and right-sided tumor (OR 0.04, 95% CI < 0.01–0.65; p = 0.023).
Poor visualization before final infusion tends p = 0.071) to be associated with tumors on the right side (e.g., 17/39 44% of right-sided tumors had score of 4 or 5, vs. 7/42 17% of non-right tumors). Multiple regression models support the univariate results regarding tumor side (right vs. non-right).
Worsening in flow from first infusion to before final infusion in non-right-sided tumors (10/42 24%) versus right-sided tumors (3/42 7%) is statistically significant (p = 0.05), and confirmed in univariate logistic model (p = 0.06). Better flow (p = 0.031) at first infusion is associated with tumors on the right side (e.g., right-sided tumors with flow scores of 3 + of 6/39 16% versus non-right-sided tumors 18/42 42%). Better flow (p = 0.019) before last infusion is associated with tumors on the right side (e.g., right-sided tumors with flow scores of 3+ of 7/39 18% versus non-right-sided tumors 21/42 50%). Multivariate regression models support the univariate results regarding tumor side (right vs. non-right).
There were no significant differences in visualization between the two angiography machines used in the pre-assessment of ten patients (results not shown). It was not possible to distinguish visually between the hot phases (90Y resin microspheres in G5) and the cold phases (50/50 G5 contrast medium mixture).
No adverse events of CTCAE grade 3 or above were reported for any of the patients (including thrombocytopenia or other indicators of bone marrow toxicity), and there were no cases of radiation-induced liver disease over 3 months of follow-up.
Serum bilirubin was increased from baseline at the time of hospital discharge following RE and was still elevated at the 1 month follow-up (Table 4). The mean thrombocyte count was markedly reduced from baseline at hospital discharge but had risen at the 1 month follow-up although not to the baseline value.
Discussion
To the best of our knowledge, this is the first published study on the use of a 50/50 mixture of contrast medium (Imeron 300) and G5 as a modified sandwich technique for RE procedures with 90Y resin microspheres, alternatively to a standard sandwich procedure with undiluted contrast medium and G5 administered separately.
This is the first study to demonstrate that use of a 50/50 mixture of a contrast medium and G5 in an RE procedure for administration of 90Y resin microspheres, provides good visualization quality. It showed that visualization quality is inversely dependent on flow rate, i.e., visualization improves as the flow rate decreases. Optimal flow for RE is defined as antegrade flow without the appearance of stasis, this modified sandwich technique enabled relevant changes in flow to be detected in all patients. Visualization quality was also inversely related to increases in BMI.
Practice at our center when performing RE is to inject slowly with a pulsatile injection technique, under fluoroscopic control, to avoid retrograde flow due to too powerful and rapid injection. Use of a larger volume syringe, at least 10 ml, or preferably 20 ml, to increase injection resistance, helps in avoiding too rapid administration. In this study, performing RE with the 50/50 contrast medium G5 mixture reduced the mean injection time of 1 GBq from 20 min (from historical experience with more than 1000 patients at our center) with the standard sandwich technique, to 13.6 min. This may be attributable to easier handling of the contrast media, and vehicle in the 50/50 technique.
The choice of contrast medium will influence the quality of visualization in RE, and its compatibility with the 90Y resin microspheres is a critical factor in this choice [19]. Previously, it was recommended that 90Y resin microspheres should not be mixed with contrast media as clumping had been observed when microspheres were mixed the contrast media commercially available at the time of their introduction in the 1990s. The evidence for this phenomenon was largely anecdotal and inconclusive, nevertheless the use of ionic contrast media with 90Y resin microspheres is still contraindicated; there is no specific guidance on non-ionic contrast media.
Imeron 300 (Iomeprol), the X-ray contrast medium, used in this study is a tri-iodinated, non-ionic, water soluble, nephrotropic, low-osmolality formulation. Formulations of its active ingredient Iomeprol (chemical formula C17–H22–I3–N3–O8; molecular weight 777.09) yield contrast media of particularly low osmolality and viscosity. Viscosity is an important factor in determining flow rate and hence visualization quality. A study has shown that contrast medium viscosity is inversely related to opacification, and hence image quality, due to its negative impact on both flow rate and injection pressure [20]. The use of low viscosity contrast media (LVCM)—such as Imeron 300—improves flow and injection pressure, which could result in superior opacification and safety. These beneficial effects may allow for modifications in diagnostic and interventional procedural technique, resulting in improved outcomes.
The current study showed an inverse correlation between BMI and visualization quality; the higher the patient’s BMI the worse the image quality. This has previously been reported in several other studies investigating the influence of BMI on image quality in angiography [21,22,23]. One study reported that for every increase of 1 kg in bodyweight there was an 8% increase in the risk of poor image quality and that image quality was good in patients with a BMI <29 kg/m2 regardless of imaging rate [22]. Another study reported that the contrast-to-noise ratio (CNR) was significantly decreased in patients undergoing coronary CT angiography in patients with BMI ≥40 kg/m2 or 30–39.9 kg/m2, compared with those with a BMI of 25–29.9 kg/m2, and between those with BMIs ≥ 40 kg/m2 compared with a BMI of 30–39.9 kg/m2 [23]. Other factors (use of intra-procedure medication, a higher dose of RE, a higher percentage lung shunt, and absence of stasis) that influenced visualization are generally expected based upon observed visualization using the standard sandwich procedure.
The extent of the treated liver territory may also impact on visualization quality. In this study, we observed that due to smaller territory of the left lobe the flow rate is often slower and as a result visualization is better.
An important concern about possible side effects or toxicity arising from the combination of 90Y resin microspheres and Iomeprol (Imeron 300) is bone marrow suppression, due to the theoretical possibility of 90Y leaching from the resin microspheres. However, it is known that 90Y is immobilized onto the resin microsphere following its precipitation as a phosphate salt yttrium phosphate is highly insoluble with a pKa of 24.76, therefore an interaction between Iomeprol and 90Y phosphate is highly unlikely. In terms of other additive toxicities between 90Y resin microspheres and Iomeprol, the safety profiles of both products are totally different. In the case of 90Y resin microspheres, the predominant adverse effects are hepatotoxicity, and nontarget effects in the upper gastrointestinal tract and lung, whereas for non-ionic iodinated contrast media, these are mainly acute reactions (hypersensitivity or otherwise) and nephrotoxicity. Therefore, it was not envisaged that concurrent use of both would lead to additive toxicities. Thrombocytopenia was not reported as an adverse event in our study, indicating that there were no incidences of bone marrow suppression.
Only one previous study is dealing with a modification of the administration of resin spheres in general [12] but this study differs from ours in several points. Chao et al. investigated the use of a 50/50 mixture of contrast medium (Isovue Bracco Diagnostics Inc, Monroe Township, NJ) and saline in a modified delivery technique for RE [12]. They injected the 50/50 contrast medium/saline mixture via the B and D line. This is a procedure we cannot recommend hence saline should not be used with resin spheres at all and additionally the contact time with any contrast agent should be as short as possible (theoretical possibility of 90Y leaching). Therefore, we injected the 50/50 contrast medium/G5 mixture only via B line for the radioembolization procedures.
A potential benefit of our modification of the sandwich technique is that there may be a reduction of up to 50% in the amount of contrast medium administered, compared with the standard sandwich technique. Patients undergoing RE may have reduced renal function due to their primary disease, comorbidities, previous treatments or diagnostic procedures, and contrast medium-induced nephropathy is an important clinical problem after intravascular administration of iodinated contrast media [24,25,26]. A reduction of the amount of contrast medium administered may reduce the risk of nephropathy.
As the visualization is worse in patients with a high flow rate, it is important not to increase the injection rate during the procedure as it is not possible to distinguish between the hot (spheres in G5) and cold (50/50 contrast medium phase/G5) phase.
There are important limitations to this study; firstly it is a non-randomized, retrospective, and conducted at a single center. Secondly, the treatment cohort was a heterogeneous population. These factors limit the ability to generalize the findings to specific tumor types, although our study represents a typical RE cohort. Further prospective studies would be valuable to compare some technical parameters in order to optimize administration of 90Y resin microspheres.
Conclusion
A 50/50 mixture of contrast medium and G5 for administration RE with 90Y resin microspheres in a modified sandwich technique provides good visualization of hepatic vessels, which is inversely dependent on flow rate and BMI. The technique is a viable alternative to the standard sandwich technique for RE administration. Visualization can be optimized by a slow pulsatile injection technique, under fluoroscopic guidance to maintain a low flow rate.
References
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27–34.
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30.
Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist. 2003;8(6):553–66.
Jakobs TF, Hoffmann RT, Tatsch K, Trumm C, Reiser MF. Therapy response of liver tumors after selective internal radiation therapy. Der Radiologe. 2008;48(9):839–49. doi:10.1007/s00117-008-1730-x.
Jakobs TF, Hoffmann RT, Tatsch K, Trumm C, Reiser MF, Helmberger TK. Developments and perspectives in radioablative techniques. Der Radiologe. 2007;47(12):1083–8. doi:10.1007/s00117-007-1572-y.
Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE Jr, Loehr SP, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65(2):412–25. doi:10.1016/j.ijrobp.2005.12.051.
Lin M, Shon IH, Wilson R, D’Amours SK, Schlaphoff G, Lin P. Treatment response in liver metastases following 90Y SIR-spheres: an evaluation with PET. Hepatogastroenterology. 2007;54(75):910–2.
Hoffmann RT, Paprottka PM, Schon A, Bamberg F, Haug A, Durr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–16. doi:10.1007/s00270-011-0142-x.
Sommer WH, Ceelen F, Garcia-Albeniz X, Paprottka PM, Auernhammer CJ, Armbruster M, et al. Defining predictors for long progression-free survival after radioembolisation of hepatic metastases of neuroendocrine origin. Eur Radiol. 2013;23(11):3094–103. doi:10.1007/s00330-013-2925-8.
Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Petre EN, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin colorectal cancer. 2014;13(1):27–36. doi:10.1016/j.clcc.2013.11.010.
Sofocleous CT, Violari EG, Sotirchos VS, Shady W, Gonen M, Pandit-Taskar N, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin colorectal cancer. 2015;14(4):296–305. doi:10.1016/j.clcc.2015.06.003.
Chao C, Stavropoulos SW, Mondschein JI, Dagli M, Sudheendra D, Nadolski G, et al. Effect of Substituting 50% isovue for sterile water as the delivery medium for SIR-Spheres: improved dose delivery and decreased incidence of stasis. Clin Nucl Med. 2017;42(3):176–9. doi:10.1097/RLU.0000000000001532.
Paprottka KJ, Lehner S, Fendler WP, Ilhan H, Rominger A, Sommer W, et al. Reduced peri-procedural analgesia following replacement of water for injection (WFI) with glucose 5% (G5) solution as the infusion medium for 90 yttrium resin microspheres. J Nucl Med Off Publ Soc Nucl Med. 2016. doi:10.2967/jnumed.115.170779.
Ahmadzadehfar H, Meyer C, Pieper CC, Bundschuh R, Muckle M, Gartner F, et al. Evaluation of the delivered activity of yttrium-90 resin microspheres using sterile water and 5% glucose during administration. EJNMMI Res. 2015;5(1):54. doi:10.1186/s13550-015-0133-z.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68(1):13–23. doi:10.1016/j.ijrobp.2006.11.060.
Ho S, Lau WY, Leung TW, Chan M, Ngar YK, Johnson PJ, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23(8):947–52.
Paprottka PM, Paprottka KJ, Walter A, Haug AR, Trumm CG, Lehner S, et al. Safety of radioembolization with (90)yttrium resin microspheres depending on coiling or no-coiling of aberrant/high-risk vessels. Cardiovasc Intervent Radiol. 2015;38(4):946–56. doi:10.1007/s00270-015-1128-x.
Institute NC. Common terminology criteria for adverse events v3.0. 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Eloy R, Corot C, Belleville J. Contrast media for angiography: physicochemical properties, pharmacokinetics and biocompatibility. Clin Mater. 1991;7(2):89–197.
Voeltz MD, Nelson MA, McDaniel MC, Manoukian SV. The important properties of contrast media: focus on viscosity. J Invasive Cardiol. 2007;19(3):1A–9A.
Rodriguez-Olivares R, El Faquir N, Rahhab Z, Maugenest AM, Van Mieghem NM, Schultz C, et al. Determinants of image quality of rotational angiography for on-line assessment of frame geometry after transcatheter aortic valve implantation. Int J Cardiovasc Imaging. 2016;32(7):1021–9. doi:10.1007/s10554-016-0889-x.
Schultz CJ, van Mieghem NM, van der Boon RM, Dharampal AS, Lauritsch G, Rossi A, et al. Effect of body mass index on the image quality of rotational angiography without rapid pacing for planning of transcatheter aortic valve implantation: a comparison with multislice computed tomography. Eur Heart J Cardiovasc Imaging. 2014;15(2):133–41. doi:10.1093/ehjci/jet037.
Mangold S, Wichmann JL, Schoepf UJ, Litwin SE, Canstein C, Varga-Szemes A, et al. Coronary CT angiography in obese patients using 3rd generation dual-source CT: effect of body mass index on image quality. Eur Radiol. 2016;26(9):2937–46. doi:10.1007/s00330-015-4161-x.
Karaman A, Diyarbakir B, Durur-Subasi I, Kose D, Ozbek-Bilgin A, Topcu A, et al. A novel approach to contrast-induced nephrotoxicity: the melatonergic agent agomelatine. Br J Radiol. 1061;2016(89):20150716. doi:10.1259/bjr.20150716.
Gul I, Zungur M, Tastan A, Okur FF, Damar E, Uyar S, et al. The importance of contrast volume/glomerular filtration rate ratio in contrast-induced nephropathy patients after transcatheter aortic valve implantation. Cardiorenal Med. 2015;5(1):31–9. doi:10.1159/000369943.
Yamamoto M, Hayashida K, Mouillet G, Chevalier B, Meguro K, Watanabe Y, et al. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6(5):479–86. doi:10.1016/j.jcin.2013.02.007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Paprottka, K.J., Todica, A., Ilhan, H. et al. Evaluation of Visualization Using a 50/50 (Contrast Media/Glucose 5% Solution) Technique for Radioembolization as an Alternative to a Standard Sandwich Technique. Cardiovasc Intervent Radiol 40, 1740–1747 (2017). https://doi.org/10.1007/s00270-017-1712-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1712-3