Abstract
Background
To evaluate the course of pro- and anti-inflammatory cytokines after 90Y-radioembolization (RE) of liver malignancies and to identify prognosticators for liver-related adverse events and survival.
Methods
In 34 consecutive patients with secondary or primary liver tumors scheduled for RE, the following cytokines were measured prior to and 2 h, 3 days, and 6 weeks after RE: interleukin (IL) -1, IL-2, IL-4, IL-6, IL-8, tumor necrosis factor alpha (TNF-α), and interferon-γ. Liver function impairment was defined as an elevation of liver-related laboratory values as graded by CTCAE ≥ 2 and/or serum bilirubin ≥30 µmol/l and/or development of ascites at 6-week follow-up.
Results
Significant changes over time were seen in IL-1 (increase from 0.4 pg/ml (±0.7) at baseline to 1.1 pg/ml (±1.4) 3 days after RE (p = 0.02)), and in IL-6 (increase from 16.8 pg/ml (±21.8) at baseline to 54.6 pg/ml (±78.2) 3 days after RE (p = 0.003)). Baseline values of IL-6 and IL-8 were independently associated with liver function impairment at follow-up as well as decreased survival with an optimal cutoff at 6.53 and 60.8 pg/ml, respectively.
Conclusion
Expected changes in pro- and anti-inflammatory cytokines after RE were shown. Furthermore, baseline values of IL-6 and IL-8 were associated with later liver dysfunction and survival. We hypothesize that these biomarkers are potential prognosticators and might help in patient selection for RE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
90Y-radioembolization (RE) delivers radionuclide-embedded resin or glass microspheres to liver tumors via the hepatic artery. Upon arterial injection, the microspheres embolize in the tumor vasculature to a greater extent as compared to the normal hepatic parenchyma [1]. RE has shown promising tumor control rates in patients with both primary and secondary liver malignancies. While prospective randomized data are still scarce, diligent analyses of large phase II single-arm cohorts have suggested prognostic benefit, specifically in patients with advanced hepatocellular carcinoma (HCC) and in patients with chemorefractory liver metastases from colorectal cancer [2–12].
However, RE is technically demanding and potentially associated with—among others—liver-related complications (i.e., radioembolization-induced liver disease (REILD)) as outlined in the following. Mechanisms and predisposing factors associated with REILD are still not fully understood [13, 14]. In short, a history of chemotherapy and high applied 90Y activity as well as treatment of the whole liver in one session and subsequent chemotherapy in pretreated patients within 2 months after RE are reported to be independently associated with REILD in patients with liver metastases. Patients with HCC are at risk of REILD in case of cirrhosis, low liver volume, and increased bilirubin at baseline [13–15]. In this high-risk group, the incidence of REILD is up to 22.7% (rate of severe REILD of up to 13.3%), whereas patients not demonstrating these characteristics are at low risk of REILD (about 5.5% (severe form in 2.2%)) [13]. The paramount influence of previous damage to the liver parenchyma is demonstrated by the low incidence of REILD (1.2%) in the SIRFLOX study, where the treatment was conducted as a first-line treatment (even though it was applied in a whole-liver setting and was directly followed by chemotherapy) [12].
The pathological hallmark of REILD is a veno-occlusive disease (VOD). Although REILD sometimes resolves under symptomatic treatment, it may progress into a chronical stage with ongoing severe liver impairment with decreased quality of life, restricted possibilities for further tumor therapies and, not uncommonly, reduced survival [14].
To date, no protective agents for the prevention of radiation- or radioembolization-induced liver disease have been proven effective, although some drugs are believed to possibly act protectively (such as low molecular weight heparin, ursodesoxycholic acid, pentoxifylline, and steroids) [16–19]. Thus, in addition to further optimizing the RE procedure, reliable markers are needed to identify patients at risk prior to RE and to individualize RE treatment. Recently, a pre-RE scoring system (the so-called TuCK score) for patients with chemorefractory colorectal liver metastases was published by Damm et al., including tumor load, Karnofsky index, and tumor markers (as identified by a multivariate analysis). The TuCK score was shown to correlate significantly with survival, helping to identify patients who will most likely not benefit from the RE and may unnecessarily be exposed to the risk of treatment-associated morbidity [20].
Cytokines have received increasing attention as potential diagnostic and prognostic markers for preexisting tissue damage (liver inflammation) and cancer involvement [21–27]. In addition to the synthesis of pro-inflammatory cytokines (especially interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α)) by resident tissue macrophages (Kupffer cells) in the liver induced by the tumor infiltrates, it is proven that a variety of cancer cells themselves produce cytokines (especially IL-6) [28, 29]. These cytokines might stimulate further inflammation by attraction of inflammatory cells and induce apoptosis of hepatocytes and non-parenchymal cells in a paracrine manner. In several studies, a correlation of pretreatment interleukin levels (especially IL-6), stage of malignant disease (e.g., presence of liver metastases), and survival was shown [21, 22, 24, 26, 27, 30].
Further on, in vivo and in vitro experiments demonstrated a cytokine response after radiotherapy of the liver, with Kupffer cells and sinusoidal endothelial cells in the liver showing an immediate response to radiation consisting of release of TNF-α, IL-6, and IL-1 with following cell–cell interactions and pro-apoptotic and radiosensitizing effects on hepatocytes [31]. Predominantly, this effect has to be rated as a response of the liver parenchyma to the radiation, not of the tumor. However, only two clinical studies reported about cytokine levels after RE treatment [32, 33]. Both studies were able to show a significant increase in pro-inflammatory IL-6 after RE. In both studies, the IL-6 increase in early follow-up was not associated with later toxicities [32, 33].
Analyses of the association of baseline cytokine levels, treatment-associated toxicities, and survival after RE are not available yet.
The objective of the current analysis was to verify the reported data on the change in cytokine levels after RE and to determine whether baseline values or early changes in selected pro- and anti-inflammatory cytokines (IL-1, IL-2, IL-4, IL-6, IL-8, TNF-α, and interferon-γ) may be useful to predict (treatment-related and/or tumor inherent) liver function impairment and/or survival in patients treated with RE.
Materials and Methods
Study Design
During a 14-month period, 60 consecutive patients with secondary or primary liver tumors scheduled for RE as salvage treatment at our institution were included in this exploratory study. Serum levels of distinct cytokines (see section laboratory analyses) were determined in these patients out of routinely drawn blood samples prior to, directly after, 3 days after, and 6 weeks after RE.
The aim of this exploratory study was to evaluate (1) the course of selected cytokines after RE and the potential of these cytokines (pre-RE level or early after RE level) for early prediction of liver function impairment and/or (2) survival. On the basis of these data, a confirmative study will be planned.
The study was approved by the institutional review board, and all patients gave written consent for data evaluation.
Patient Selection and Eligibility Criteria and Patient Characteristics
Of the initially included patients, only patients with complete laboratory work-up (see section laboratory analyses) [2 patients excluded] and magnetic resonance imaging (MRI) work-up (see section MRI) [17 patients excluded] were included. Patients treated incompletely [2 patients] as well as patients undergoing other additional liver-targeted local therapies (radiofrequency ablation, local radiotherapy) [1 patient] or chemotherapy [2 patients] in follow-up period were excluded. Additionally, patients with an infection (fever, elevated C-reactive protein, and leukocytes) [2 patients] in the observatory period were excluded. Out of 60 consecutive patients scheduled for a RE of liver malignancies, 34 patients (mean age 63.3 years, 28 men, 6 women) fulfilled the eligibility criteria of the final analysis (Table 1).
Radioembolization
The conduction of RE was performed as described elsewhere [15]. In brief, prior to RE all patients underwent a work-up including clinical status, laboratory, and imaging (CT and MRI of the liver). Further on, a work-up angiography of the vascular supply of the liver was performed using a transfemoral approach. Vessels at risk to lead to an extrahepatic deposition of the microspheres were identified (e.g., gastroduodenal artery, right gastric artery, and other arteries) and embolized, if necessary. This was followed by 99mTc-MAA injection (150 MBq, Tc-99m-LyoMAA, Covidien, Neustadt/Donau, Germany) at the planned treatment positions. 99mTc-MAA Lung shunt was quantified in planar imaging, and 99mTc-MAA distribution in the upper abdomen was visualized by a SPECT (single-photon emission computed tomography) scan (E.CAM 180, Siemens, Erlangen, Germany) including image fusion to CT or MRI from the day before. If an extrahepatic accumulation was visible, a re-angiography was conducted to identify and to embolize or to bridge the according vessel.
Activity of the 90Y resin microspheres (SIR-Spheres®, Sirtex Medical, Lane Cove, Australia) for RE was calculated according to BSA (body surface area) method with activity reduction according to the extent of the lung shunt. In the therapeutic angiography for the RE, the microspheres were injected at the previously defined treatment position(s). The treatment was done in an in-patient fashion, and patients were typically discharged 3 days after the procedure. In order to ameliorate effects of the radiation to liver parenchyma, all patients received low-dose steroids and ursodesoxycholic acid for 6 weeks [13].
The mean applied activity of 90Y was 1.32 GBq (±0.57). In 4 patients, the whole liver was treated in one session; in 21 patients, the treatment of the whole liver was performed in two sessions (sequential one lobe per session) 4–6 weeks apart; and in 9 patients, only one liver lobe was treated. The mean applied activity in these groups was whole liver: 1.7 GBq, sequential: 1.42 GBq, unilobar: 0.9 GBq.
Laboratory Analyses
Serum levels of the following cytokines were measured 1 day prior to and 2 h, 3 days, and 6 weeks after the RE procedure: interleukin (IL) -1, IL-2, IL-4, IL-6, IL-8, tumor necrosis factor alpha (TNF-α) and interferon-γ. One day before and 3 days and 6 weeks after completed RE, the following liver-related and inflammation-related laboratory parameters were determined: alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin, alkaline phosphatase [12], albumin, gamma-glutamyltransferase (GGT), glutamate dehydrogenase (GLDH), prothrombin time (INR), cholinesterase (CHE), leukocytes, C-reactive protein (CRP). Deviations from normal values for ALT, AST, total bilirubin, ALP, and albumin were graded according to the common terminology criteria for adverse events (CTCAE), version 4.02.
Magnetic Resonance Imaging
On the day of admission for 99mTc-MAA work-up and 6 weeks after completed RE, an MRI scan (Achieva 1.5T, Philips, Best, the Netherlands) of the liver was performed routinely. The protocol consists of a three-dimensional (3D) T1-weighted gradient recalled echo sequence (time to echo/time to repetition (TE/TR) 2/4 ms, flip angle (FA) 10°, slice thickness 3 mm) before, directly after, and 20 min after application of the hepatocyte-specific contrast media Gd-EOB-DTPA (Gadoxetic acid, Primovist©, Bayer Healthcare, Germany; dosage: 0.025 mmol/kg/bodyweight) and a T2-weighted turbo spin echo sequence (TE/TR 90/2100 ms, FA 90°, slice thickness 6 mm) with fat saturation. Two sequences were utilized for this study: the 3D T1 GRE sequence 20 min after application of Gd-EOB-DTPA was used for liver and tumor volumetry prior to RE and for evaluation of tumor response. The T2-weighted TSE sequence with fat saturation was used for detection of ascites prior to and 6 weeks after completed RE. Volumetry measurements (liver, tumor) were taken with the image processing software Osirix (©Antoine Rosset, 2003–2011).
Liver Function Impairment
Liver function impairment was defined as an elevation of liver-related laboratory parameters (ALT, AST, bilirubin, ALP) and/or decreased serum albumin level as graded by CTCAE ≥ 2 and/or an elevation of bilirubin of ≥30 µmol/l (i.e., ≥1.8 mg/dl; corresponds to an elevation of 1.5 time upper limit of normal) and/or development of ascites.
Statistics
Results of continuous data are displayed as means and standard deviations, results of frequency data as counts and percentages. For two-group comparisons of the means, Student’s t test was used. Correlations were calculated using a two-sided Pearson’s test. Differences between patients undergoing different RE protocols (whole-liver RE, sequential RE, one lobe RE) were assessed by two-sided Kruskall–Wallis tests, and differences by entity and tumor progression were assessed by Fisher’s exact test.
The Kaplan–Meier method was used for estimates of overall survival.
Cox regression was used to identify factors significantly associated with overall survival in the study population. For factors with p values of <0.10, Kaplan–Meier curves were calculated along with median survival and interquartile range. Wilcoxon tests were used to evaluate differences between the groups. No imputations for missing values were performed.
The evaluation of potential predictors for liver damage at 6 weeks after completed RE as well as for survival after RE was performed using a logistic regression model, including the factors: baseline cytokine levels and laboratory parameters before first RE, sex, tumor load, cytokine levels and laboratory parameters during follow-up, and the time point. The final model for each factor was chosen by backward variable selection using p < 0.05 as selection criterion. Receiver operator characteristic (ROC) analysis was performed on significant predictors to calculate the accuracy and other diagnostic parameters and to determine a cutoff point at the maximum sum of sensitivity and specificity.
For factors with p values of <0.05, Kaplan–Meier curves were calculated using the cutoffs as identified in the ROC analysis along with median survival and interquartile range (IQR). Wilcoxon tests were used to evaluate differences between the groups.
Two-sided p values <0.05 were regarded as statistically significant. This exploratory study was performed to obtain basic data and to generate hypotheses. Therefore, the sample size was based on clinical and practical considerations rather than power calculations.
The statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Cytokine Levels After RE
The means of the specified cytokines at baseline and follow-up are displayed in Table 2. Significant changes over time were seen in interleukin 1 (increase from 0.4 pg/ml (±0.7) at baseline to 1.1 pg/ml (±1.4) 3 days after RE (p = 0.02)) and in interleukin 6 (increase from 16.8 pg/ml (±21.8) at baseline to 54.6 pg/ml (±78.2) 3 days after RE (p = 0.003)).
Liver Function Prior/After RE
Prior to RE, 5/34 patients showed a grade 2 (3 cases) or grade 3 (2 cases) liver function impairment according to CTCAE-graded laboratory parameters (increase in ALP in all but one cases and AST increase in the remaining patient). Six weeks after completed RE, 10/34 patients showed a grade 2 (9) or grade 3 (1) liver function impairment (increase in bilirubin, 5 cases; increase in ALP, 4 cases; increase in AST, 1 case), with no significant change over time.
Serum bilirubin increased significantly from 11.8 µmol/l (±6.0) prior to RE to 18.0 µmol/l (±17.5) 6 weeks after completed RE (p = 0.04), whereas serum albumin and cholinesterase activity showed a slight but significant decrease (from 39.9 (±3.6) to 37.6 g/l (±5.6)) and from 99.0 (±34.1) to 87.3 µmol/ls (±37.1), respectively) (Table 3).
Prior to RE, 4 patients showed detectable ascites (all grade 1 according to CTCAE). Six weeks after completed RE, ascites was detectable in 16 patients [grade 1 (n = 12) and grade 2 (n = 4)] (p = 0.04).
Liver function impairment according to the definition described above was seen in 8 patients at baseline and in 19 patients 6 weeks after completed RE (p = 0.005).
Cancer type, history of chemotherapy, and progression of disease (in 5 cases, all other patient showed a disease control) at follow-up showed no significant correlation with development of liver function impairment.
Overall Survival
Median overall survival after RE was 7.4 months (IQR 5–7.9 months; 95% CI 5.4–10.7 months). Type of RE showed no significant influence on survival (whole-liver RE: 4.8 months, sequential whole-liver RE: 9 months, unilobar RE: 5.8 months, p = 0.08).
Cancer type, history of chemotherapy and progression of disease during follow-up showed no significant influence on survival.
Type of RE
No differences between the type of RE treatments performed (whole liver, sequential whole liver, unilobar treatment) were detected for sex (p = 1.0), age (p = 0.703), cancer type (p = 0.38), tumor load (p = 0.6128), tumor volume (p = 0.7674), liver volume (p = 0.3237), overall survival (p = 0.47), and disease progression during follow-up (p = 0.68). The applied activity was found to differ significantly between patients receiving whole-liver versus unilobar treatment (whole liver: 1.7 GBq, unilobar: 0.9 GBq; p = 0.0302), but not for sequential and whole liver or sequential and unilobar. Global differences between the groups with regard to cytokine levels and liver function were not seen; however, specific differences were as follows: At baseline, total bilirubin and IL-2 were significantly elevated in the group of patients eventually receiving unilobar (bilirubin) and whole-liver (IL-2) treatment compared to the other groups (p = 0.0385 and p = 0.0083, respectively). Two hours after RE, IL-2 and IL-4 levels showed significant differences between groups, with IL-2 being highest and IL-4 being lowest in the whole-liver treatment group (p = 0.0354 and p = 0.0083, respectively). At later follow-up, only IL-1 (3 days after RE) and GLDH (6 weeks after RE) showed significant differences between the treatment groups, with both parameters being highest in the sequential treatment group (p = 0.0138 and p = 0.0486, respectively). All other laboratory parameters were not significantly different between treatment groups at any time point.
Threshold Analysis for Prediction of Liver Function Impairment at 6 weeks After RE and Survival
The final model showed a significant influence only for baseline IL-6 and IL-8.
Interleukin 6 at Baseline
The optimal cut point as extracted from the ROC by the Youden Index was 6.53 pg/ml with a sensitivity of 83.3% and specificity of 86.7% (area under the curve (AUC) 87%), corresponding to a positive predictive value of 88% and a negative predictive value of 81% for liver function impairment according to the above-mentioned definition.
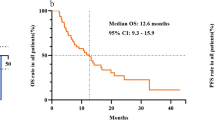
The 6.53 pg/ml cutoff for baseline IL-6 was also significantly associated with overall survival in the Cox regression analysis (p < 0.0001). Median survival was 13.2 months (Q25: 6 months; Q75: 20.4 months; 95% CI 6–20.4) if baseline IL-6 was ≤6.53 pg/ml, whereas it was 5 months (Q25: 4.1 months; Q75: 7 months; 95% CI 3.2–6.6) in patients with a baseline value of >6.53 pg/ml. (Figure 1a).
Interleukin 8 at Baseline
The optimal cut point as extracted from the ROC by the Youden Index was 60.8 pg/ml with a sensitivity of 66.7% and specificity of 86.7% (AUC 81%), corresponding to a positive predictive value of 86% and a negative predictive value of 68% for liver function impairment according to the above-mentioned definition.
The 60.8 pg/ml cutoff for baseline IL-8 was also significantly associated with overall survival in the Cox regression analysis (p < 0.0001). Median survival was 13.2 (Q25: 6.9 months; Q75: 20.4 months; 95% CI 6–20.4) if baseline IL-8 was ≤60.8 pg/ml, whereas it was 5.1 months (Q25: 4.1 months; Q75: 6.7 months; 95% CI 3.2–6.7) in patients with a baseline value of >60.8 pg/ml. (Figure 1b).
mCRC Subgroup Analysis
The results for the largest subgroup, patients with mCRC, were very similar to the overall data with only baseline IL-6 and IL-8 being significantly associated with a liver function impairment in follow-up in the logistic regression with an optimal cutoff of baseline IL-6 and IL-8 at 6.4 and 54.95 pg/ml, respectively. The overall survival, as stratified by these values, was again significantly different with 13.4 versus 4.7 months (p = 0.004) for IL-6 (≤ vs.>6.4 pg/ml) and 13.4 versus 6.7 months (p = 0.049) for IL-8 (≤ vs. >54.95 pg/ml).
In the overall population, IL-6 and IL-8 baseline values correlated significantly but to a weak extent (p = 0.049, R2 = 0.345).
Prior exposure to chemotherapy was not associated with elevated IL-6 or IL-8 values (according to calculated threshold values).
A low but significant positive correlation was found between tumor load and the baseline value of IL-6 (p = 0.013, R 2 = 0.43) and IL-8 (p = 0.001, R 2 = 0.58). Analysis with regard to tumor entity (HCC and metastatic disease) did not change results except for IL-8 in HCC (IL-6 and IL-8 in metastatic disease: p = 0.013, R 2 = 0.52 and p = 0.06, R 2 = 0.40; IL-6 and IL-8 HCC: p = 0.05, R 2 = 0.60 p = 0.28, R 2 = 0.35). However, IL-6 and IL-8 were associated with later liver function impairment and survival in the multivariate analysis, not tumor load.
Discussion
In the presented observational study, we sought to determine relevant cytokine levels and changes in RE patients before and during early follow-up after treatment. In line with reported results, we were able to reproduce the increase in pro-inflammatory cytokines (which was most pronounced for IL-6) after RE [32, 33]. In a second step, we were able to identify baseline levels of IL-6 and IL-8 as good predictors of liver function impairment and survival after RE, with a cutoff value of 6.53 pg/ml for IL-6 and 60.8 pg/ml for IL-8, indicating the strong relation between tumor infiltration and induced inflammation. A significant correlation of tumor burden and IL-6 and IL-8 baseline values augments this interpretation. The implementation of these results might help to develop an individualized, and, hence, safer, approach for RE treatment.
RE is gaining increasing importance in the treatment of liver-dominant malignancies (both primary and secondary). To date, most available data have been derived from heavily pretreated patients in a salvage situation; in this scenario, RE can afford a longer progression-free survival and overall survival as compared to best supportive care or systemic chemotherapy. However, clinically relevant liver function impairment (REILD) following RE is a concern [13, 14]. Among others, preexisting damage to the liver parenchyma (e.g., after administration of chemotherapy or in case of underlying liver cirrhosis) is a paramount risk factor for the development of REILD. Up until now, it is still unclear whether this is related to a higher susceptibility of the parenchyma to radiation-induced injury or just to a reduced functional reserve. Several prophylactic regimens are recommended to reduce the risk of a REILD in these patients (sequential lobar treatment, no whole-liver administration, reduced applied yttrium 90 activity, medication with low-dose steroids, ursodeoxycholic acid, enoxaparin, and pentoxifylline) [13, 15, 19]. However, evidence for prophylactic regimens remains low. Furthermore, treatment of REILD is merely symptomatic, whereas a causal treatment is not available. Hence, patient selection is of major interest to identify patients with high probability of liver-related adverse events or short survival prior to RE.
In the literature, cytokines were evaluated in cancer patients with different primaries and their potential was studied to predict outcome and overall survival for different types of therapies (chemotherapy and resection) [21, 22, 24, 26, 27, 30]. In line with our study results, from all tested cytokines mostly IL-6 emerged as the best predictor for outcome and overall survival. Interestingly, our reported cutoff for baseline IL-6 of 6.53 pg/ml is in the range of reported cutoff values for baseline IL-6 prior to oncological treatments other than RE in various types of advanced cancers (with or without liver metastases). Soubrane and colleagues determined a cutoff value for baseline IL-6 of 5 pg/ml to predict survival in patients with metastatic malignant melanoma treated with biochemotherapy (9.7 vs. 24.6 months) [26]. In patients with operable gastric cancer, survival correlated well with preoperative IL-6 with an optimal cutoff at 6.77 pg/ml (overall survival at 24 months 96.2 vs. 80.7%) [22]. Nikiteas et al. were able to show that baseline IL-6 of more than 8 pg/ml was associated with a poorer survival in patients with metastatic colorectal cancer [24]. Several studies were able to show that IL-6 is an independent prognostic parameter in patients with metastatic breast cancer associated with extent of disease and survival. For example, Zhang et al. [27] were able to show a sixfold increased risk of death in patients with IL-6 higher than 5 pg/ml. Interestingly, also patients with HCC and cirrhosis show a comparable pattern. Shao et al. [34] analyzed the outcomes of patients who received sorafenib with metronomic chemotherapy as first-line therapy for advanced HCC and demonstrated that high pretreatment plasma IL-6 or IL-8 levels were associated with poor PFS and OS.
Summary of data shows that in contrast to IL-6, evidence for usability of baseline IL-8 as prognosticator is low.
Tumor load and IL-6/IL-8 showed a low but significant positive correlation, indicating a true association between stage of disease and IL-6 and IL-8 levels. This finding is consistent with results from other studies showing a correlation of IL-6 with tumor burden in metastatic malignant melanoma and breast cancer [23, 27]. If confirmed, IL-6 and IL-8 may have an advantage over tumor volumetry due to their easy ascertainability. Further on, IL-6 and IL-8 were significantly associated with liver toxicity and overall survival in the multivariate model, not tumor load.
To our knowledge, no other study has reported on the predictive value of baseline cytokines in RE patients. Fernandez-Ros et al. and Wickremesekera et al. were also able to show an increase in IL-6 after RE; however, an analysis regarding the predictive value of cytokines for toxicities during follow-up or survival was not performed [33]. Interestingly, and in line with Fernandez-Ros et al., we were not able to identify a significant change in TNF-α after RE. In contrast to these findings, in vitro experiments have shown a TNF-α release of irradiated Kupffer cells and sinusoidal endothelial cells [31]. However, IL-6 and IL-1 are also known to be important for the initiation of the pro-inflammatory effects in hepatocytes after irradiation, by inducing an acute phase response with subsequent activation of either pro-apoptotic or pro-survival pathways [31].
Several limitations apply to this study, including the small number of patients, which may affect the statistical validity of our results. However, the data acquired in this observational study were used to design a confirmatory study, the aim being to verify the predictive value of IL-6 for side effects and survival following RE.
A further limitation is that patients with different types of cancer were included. Different types and lines of chemotherapy in different cancer types may be associated with different levels of preexisting liver parenchyma damage, resulting in different susceptibility for RE-related side effects. Additionally, livers affected by underlying cirrhosis (as is frequently the case in patients with hepatocellular carcinoma) might react in a different way to RE as non-cirrhotic livers do. To avoid bias induced by underlying liver cirrhosis, patients with a liver function status beyond Child-Pugh grade A were not included in this analysis. Importantly, tumor type was without significant influence on the development of liver function impairment and overall survival, and a subgroup analysis on mCRC population revealed similar results regarding the independent predictive value of baseline IL-6 and IL-8 values on liver function impairment and survival. Furthermore, published data support that increased IL-6 levels are a general finding in malignant liver disease of various origins including HCC in cirrhosis, respectively [21, 22, 24, 26, 27, 30, 34]. A further limitation is that patients with unilobar, whole liver sequential, and whole-liver RE treatments were included. The different amount of applied activity (unilobar vs. whole liver) or the treatment regimen (sequential whole liver vs. whole liver) might have led to a bias. However, both the types of RE and the amount of applied activity were without influence on following liver function impairment and overall survival. This finding is in contrast to published data on adverse events after RE, where the sequential treatment approach was associated with significant less liver function impairment compared to the whole-liver approach [15]. We assume that this difference may be associated with varying definitions of liver function impairment in our and other studies. Further on, influence of the prophylactic coil embolization on cytokine values, especially baseline values, cannot be excluded. However, since other observational studies on patients receiving no RE report about similar magnitudes of cytokines at baseline (as outlined above), we rate this influence as neglectable. Finally, the overall survival after RE is comparatively low in our study cohort, indicating the salvage situation of the treated patients. Thus, transferability of the results to other cohorts treated at an earlier stage of disease is limited.
Conclusions
In this study, we were able to reproduce changes in pro- and anti-inflammatory cytokines after RE. Furthermore, we were able to identify baseline biomarkers (IL-6 and IL-8 baseline values) to be associated with later liver dysfunction and survival. We hypothesize that these biomarkers are potential prognosticators and might help in patient selection for RE.
References
Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60(5):1552–63.
Bester L, Meteling B, Pocock N, Pavlakis N, Chua TC, Saxena A, Morris DL. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23(1):96–105.
Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(3):324–31.
Hilgard P, Hamami M, Fouly AE, Scherag A, Muller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, Gerken G, Antoch G. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52(5):1741–9.
Kennedy AS, McNeillie P, Dezarn WA, Nutting C, Sangro B, Wertman D, Garafalo M, Liu D, Coldwell D, Savin M, Jakobs T, Rose S, Warner R, Carter D, Sapareto S, Nag S, Gulec S, Calkins A, Gates VL, Salem R. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1494–500.
Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Inarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–78.
Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, Kettner E, Hildebrandt B, Dudeck O, Pech M, Amthauer H, Ricke J. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc IntervRadiol. 2012;35(5):1066–73.
Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E, Delatte P, Delaunoit T, Personeni N, Paesmans M, Van Laethem JL, Flamen P. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28(23):3687–94.
Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64.
Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, Gupta R, Vouche M, Gates VL, Miller FH, Omary RA, Salem R. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58(1):73–80.
Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155e2–1163e2.
van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland AH, Ferguson T, Rodriguez J, Kroning H, Wolf I, Ganju V, Walpole E, Boucher E, Tichler T, Shacham-Shmueli E, Powell A, Eliadis P, Isaacs R, Price D, Moeslein F, Taieb J, Bower G, Gebski V, Van Buskirk M, Cade DN, Thurston K, Gibbs P. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(15):1723–31.
Gil-Alzugaray B, Chopitea A, Inarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, Benito A, Dominguez I, D’Avola D, Herrero JI, Quiroga J, Prieto J, Sangro B. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57(3):1078–87.
Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, Chopitea A, Inarrairaegui M, Arbizu J, Bilbao JI. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112(7):1538–46.
Seidensticker R, Seidensticker M, Damm R, Mohnike K, Schuette K, Malfertheiner P, Van Buskirk M, Pech M, Amthauer H, Ricke J. Hepatic toxicity after radioembolization of the liver using Y-90-microspheres: sequential lobar versus whole liver approach. Cardiovasc Interv Radiol. 2012;35(5):1109–18.
Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, Snyder M, Lewis SK, Allerton JP, Thompson JM. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128(12 Pt 1):975–81.
Or R, Nagler A, Shpilberg O, Elad S, Naparstek E, Kapelushnik J, Cass Y, Gillis S, Chetrit A, Slavin S, Eldor A. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61(7):1067–71.
Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, Jung CW, Im YH, Yoon SS, Kang WK, Park K, Park CH, Kim SW. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transp. 2002;29(2):137–43.
Seidensticker M, Seidensticker R, Damm R, Mohnike K, Pech M, Sangro B, Hass P, Wust P, Kropf S, Gademann G, Ricke J. Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity PLoS ONE. 2014;9(11):e112731.
Damm R, Seidensticker R, Ulrich G, Breier L, Steffen IG, Seidensticker M, Garlipp B, Mohnike K, Pech M, Amthauer H, Ricke J. Y90 Radioembolization in chemo-refractory metastatic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer. 2016;16(1):509.
Chung YC, Chang YF. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003;50(54):1910–3.
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, Kim KH, Han JY, Kim HJ. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155.
Mouawad R, Benhammouda A, Rixe O, Antoine EC, Borel C, Weil M, Khayat D, Soubrane C. Endogenous interleukin 6 levels in patients with metastatic malignant melanoma: correlation with tumor burden. Clin Cancer Res. 1996;2(8):1405–9.
Nikiteas NI, Tzanakis N, Gazouli M, Rallis G, Daniilidis K, Theodoropoulos G, Kostakis A, Peros G. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World J Gastroenterol. 2005;11(11):1639–43.
Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–6.
Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res. 2005;15(3):199–204.
Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19(2B):1427–32.
Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat. 2007;102(2):129–35.
Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. 2010;25(2):135–40.
De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A, Catalano G. Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep. 1998;5(3):649–52.
Richter C, Seco J, Hong TS, Duda DG, Bortfeld T. Radiation-induced changes in hepatocyte-specific Gd-EOB-DTPA enhanced MRI: potential mechanism. Med Hypotheses. 2014;83(4):477–81.
Wickremesekera JK, Chen W, Cannan RJ, Stubbs RS. Serum proinflammatory cytokine response in patients with advanced liver tumors following selective internal radiation therapy (SIRT) with (90)Yttrium microspheres. Int J Radiat Oncol Biol Phys. 2001;49(4):1015–21.
Fernandez-Ros N, Inarrairaegui M, Paramo JA, Berasain C, Avila MA, Chopitea A, Varo N, Sarobe P, Bilbao JI, Dominguez I, D’Avola D, Herrero JI, Quiroga J, Sangro B. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015;35(5):1590–6.
Shao YY, Hsu CH, Huang CC, Cheng AL. Use of plasma angiogenesis-related factors to investigate the association of interleukin 8 and interleukin 6 levels with efficacy of sorafenib-based antiangiogenic therapy in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011;29(suppl 4):199.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Max Seidensticker reports grants and personal fees from SIRTEX Medical, grants and personal fees from Bayer Healthcare, outside the submitted work. Maciej Powerski, Robert Damm, Maurice Klopffleisch have no conflict of interest. Ricarda Seidensticker reports grants from SIRTEX Medical, personal fees from Bayer Healthcare, outside the submitted work. Benjamin Garlipp reports grants and personal fees from Sirtex medical, outside the submitted work. Holger Amthauer reports grants and other from Sirtex, outside the submitted work. Jens Ricke reports grants and personal fees from Sirtex Medical, personal fees from Siemens, grants from Bayer Healthcare, outside the submitted work. Maciej Pech reports grants and personal fees from Sirtex medical, outside the submitted work.
Ethical Approval
The authors state that the study was conducted in compliance with ethical standards.
Rights and permissions
About this article
Cite this article
Seidensticker, M., Powerski, M., Seidensticker, R. et al. Cytokines and 90Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc Intervent Radiol 40, 1185–1195 (2017). https://doi.org/10.1007/s00270-017-1622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1622-4