Abstract
Objectives
To prospectively evaluate a novel implant, Y-STRUT® (Hyprevention, Pessac, France), designed to provide prophylactic reinforcement of the proximal femur in metastatic patients.
Methods
Ten patients presenting lytic lesions of the proximal femur were to be treated. The device consisted of two components implanted in the proximal femur, combined with bone cement. Patients were followed at 2, 6 and 12 months to record technical feasibility, safety and efficacy parameters of the procedure.
Results
All patients (62 years, 67% male) presented a pertrochanteric lesion shown on imaging with an average Mirels’ score of 9.42 (range 8–11). Procedures were performed by interventional radiologists, under general anesthesia in 97 ± 28 min, with 9.2 ± 3.1 ml of cement injected. Hospitalization duration was 2.3 ± 1.4 days. A median follow-up of 237 days (range 24–411) was reported. Wound healing was achieved in all patients, with no case of wound infection, bleeding, leakage or inflammation. Among the patients evaluated, 86% could resume walking at hospital discharge. Visual Analogue Scale decreased from 3.6 ± 2.9 before treatment to 1.3 ± 0.8 at 1 year. OHS-12 score increased from 30 ± 10 at baseline to 37 ± 6 at 1 year.
Conclusions
Results from this first-in-man study conducted in patients with metastatic bone disease show the feasibility and the safety of the intervention, with a short hospitalization, when performed following the operating instructions. Initial data showing pain-level diminution and increase in OHS-12 score indicate that both symptomatic and functional conditions of the patients were improved 1 year after the implantation of this novel implant.
Level of Evidence
Level 4, Case Series.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proximal femur is the most common site of metastatic involvement in the appendicular skeleton [1]. Osteolytic metastases, most frequently localized at the trochanteric region and femoral neck [2], may cause pathological fractures of particularly serious consequences for these highly vulnerable patients. Life expectancy of patients who have suffered from a pathological fracture of the proximal femur is estimated to be <1 year on average [3].
Early detection and prophylactic fixation are therefore critical to maintain function and prevent complications associated with these fractures [1]. Standard surgical osteosynthesis treatment can be performed [4–9] but remains at risk for this patient profile. Furthermore, osteosynthesis may require temporary chemotherapy cessation and impede further radiation therapy, thus interfering with the patients’ therapeutic management.
Based on positive preclinical results, a first-in-man clinical study was performed to assess the feasibility, safety and clinical efficacy of a novel implant designed to prevent fracture of the proximal femur in patients with metastatic lesions.
Materials and Methods
Device Description
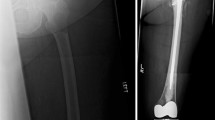
The CE-marked device Y-STRUT® (Hyprevention, Pessac, France) was specifically developed in order to provide biomechanical reinforcement of the proximal femur. The device consists of two components made of radiotransparent PEEK (polyetheretherketone) polymer, which connect in situ (Fig. 1) and reinforce the structure of the bone.
Numerical simulations were performed in order to determine optimal sizing and positioning of the implants. Then, device implantation in cadaveric femurs resulted in an increase of fracture load and energy at break without modifying the failure mode, showing the potential of this innovative device to improve the biomechanical performance of the proximal femur [10, 11].
Operative Technique
The device is implanted through a minimally invasive approach (two small incisions) in the femoral head under imaging guidance—there is no tumor ablation prior to device implantation, but implant positioning is planned (entry points, axis, distal points, 3D view positioning) to ensure that the implant will go through the tumor and that its three extremities will be well anchored in healthy bone (Fig. 2). A dedicated set of instruments (Fig. 3) is provided to ensure proper insertion and connection of the implants. The technique consists in introducing a guidewire in the axis of the femoral neck and drilling of implant #1 bed. A pilot enables the guidance of the drilling for implant #2 and guides the introduction and connection of the two components. Both implants are inserted through metal tubes, thus preventing soft tissues from being contaminated by cancerous cells.
Consolidation of an impending pathological fracture of the proximal femur secondary to osteolytic lesions with the implantation of the studied device combined with bone cement. Radiographs showing the consecutive steps of the operative technique: positioning of the guide wire 1 (A), drilling implant 1 location site (B), positioning the template and the guide wire 2 (C), drilling implant 2 location site (D), positioning of implant 1 (visualizing marker on the head side over the guide wire) (E), positioning of implant 2 and control of implants positioning (the 2 distal markers form a V shape) (F), cement injection on both sides (G, H)
The device is finally fixed into the bone by injecting low-viscosity, radio-opaque PMMA (poly(methyl methacrylate)) bone cement with a high temperature of polymerization through the perforated implants. Cement injection allows to fill the lytic lesion. Radiographic control is performed during the procedure to follow in situ steps, as well as CT scan acquisitions to control 3D implantation, according to the physician’s need.
Study Design
The study was designed as a multicentre, single-arm, prospective, pilot study. The targeted population consisted of ten patients for whom pre-fractural metastatic bone lesions were diagnosed in the proximal femur and for whom device treatment indication was validated during a multidisciplinary meeting. Main inclusion criteria were documented lytic lesions located in the proximal femur, lesion size <2/3 of the cortex and a Mirels’ score ≥8 [12, 13], Eastern Cooperative Oncology Group (ECOG) score <4 [14] and American Society of Anesthesiologists (ASA) physical status classification <5 [15] (Fig. 2).
The exclusion criteria were lytic lesions located in the diaphysis below or in the acetabulum and previous cementoplasty in the targeted area.
Evaluation of the procedure feasibility and tolerance was based on a composite endpoint consisting of two feasibility and safety criteria (procedural parameters and intraoperative adverse events) and six device tolerance criteria (walking recovery, pain self-assessment by Visual Analogue Scale (VAS), Oxford Hip Score (OHS-12) [16], radiographic control, adverse events and device explantation). Clinical efficacy was assessed by fractures reported during follow-up. Wound status was qualitatively recorded at specific time points following the intervention. Implanted patients were to be followed for a period of 1 year with visits planned at 2, 6 and 12 months.
Data Collection, Management and Analysis
Data were collected using electronic case report forms, and monitoring visits were performed by clinical research associates. Data management and statistical analysis were performed by ITEC Services (Cenon, France) using SAS version 9.3 (SAS Institute, North Carolina, USA).
Results
A total of twelve patients were enrolled between July 2013 and February 2015 in two investigational sites (Table 1). Most patients were male (67%), with a mean age of 62 ± 6 years (range 53–71) and an average BMI of 25 ± 3 kg/m2. All patients were somewhat ambulatory with an ECOG score equal to or lower than 3, and none of them was bedbound [14]. The primary cancers were lung in majority (n = 5), then kidney (n = 2) and one rectum, mesothelioma, multiple myeloma and a mucinous adenocarcinoma of unknown origin. All patients had a Mirels’ score equal to or greater than 8 [12]. Eight patients (67%) presented with an ASA category of 2 (mild), while the remaining four patients (33%) presented with an ASA category of 3 (severe) [15].
Two patients enrolled in the study, with a Mirels’ score of 10 and 11 and both lung cancer, experienced femoral fracture due to the metastatic lesion prior to the intervention and were neither treated nor followed in accordance with the protocol. The remaining ten patients were implanted.
All treated patients (n = 10) were implanted with the investigational device following the presented operative technique. No ablation of the metastatic lesions was performed prior to the implantation. All ten implantations were performed by interventional radiologists in the operating theater under general anesthesia and lasted on average 97 ± 28 min.
The total quantity of cement injected was 9.2 ± 3.1 ml (range 3–15). Four cement leakages occurred (in soft tissue only, at the level of the entry point) without any symptoms.
Four of the ten patients from the study were discharged the day following the intervention. The duration of the hospitalization was 2.3 ± 1.4 days (range 1–5), when excluding a patient who stayed hospitalized after the intervention due to severe cancer progression until his death (non-procedure-related event).
An average follow-up of 237 days (range 24–411) was reported, totalizing 2374 days (6.50 years) for the cohort (Table 1). Night patients could be followed for 2 months and six for 6 months, and four patients completed the 1-year follow-up.
The qualitative status recorded during the study showed that wound healing was achieved in 89% of cases at 2 months and all cases at 6 months. There was no case of wound infection, bleeding, leakage or inflammation reported.
The average pain level on the implanted site as assessed using a VAS was 3.6 ± 2.9 (n = 10) at baseline and 1.3 ± 0.8 (n = 4) at 12 months (Fig. 4). The pain decreased of 28% for the patients who were followed during 1 year. Four patients experienced some pain following the intervention which resolved spontaneously with no sequelae.
Among the data available at hospital discharge, 86% of the patients could resume walking. One patient did not recover walking, but he presented the most severe ECOG score at baseline (ECOG 3, i.e., symptomatic and more than 50% of time in bed during the day).
The OHS-12 was 30 ± 10 (n = 10) at baseline and 37 ± 6 (n = 4) at 12 months (Fig. 4). For the patients who reached the 1-year follow-up, their condition progressively improved (+23% at 1 year).
No case of osteolysis or implant loosening was observed, but one case of asymptomatic femoral neck fracture associated with a fracture of the implant was diagnosed at 6 months. This event was attributed to a non-optimal placement of the implant associated with tumor progression. This fracture was treated surgically (implant explantation and orthopedic treatment of the fracture) without sequelae.
Discussion
This prospective study was the first trial evaluating this new device specifically designed for prophylactic fixation of the proximal femur. Thus, this implant provides a reproducible, percutaneous approach for patients meeting well-defined indications and offering a standardized treatment option to clinicians to facilitate the management of patients with osteolytic metastases.
Most of the primary tumors were located in the lung (42%) or the kidney (17%), two cancers cited among the tumors that most commonly metastasize to bone [1].
According to the Mirels’ scoring system and the associated clinical guidance [12, 13], prophylactic fixation is highly recommended for a lesion with an overall score of 9 or greater that is associated with a fracture risk ≥33%. Baseline parameters—and specifically Mirels’ score with a median of 9—illustrate the severity of the bone lesions treated in this study. Furthermore, the occurrence of two fractures prior to implantation among the twelve patients initially enrolled (17% of cases), as well as one case of asymptomatic femoral neck fracture post-implantation (1/10) reported on this study, confirms the validity of the eligibility criteria and the severity of the patients’ condition.
The procedures were performed by interventional radiologists in 97 ± 28 min on average, including per-operative CT scan acquisitions. This result is on the same ranges as others existing percutaneous techniques developed for the same indication. Indeed, Deschamps reported 110 ± 43 min in cohorts of 12 and 35 oncologic patients using triple screwing combined with cementoplasty [4, 5]; 80 ± 7.5 min has been reported by Tian in a cohort of 19 patients with impeding pathological fractures treated with needles implantation and cementoplasty [7] and 60 min for Kelekis in a cohort of 12 oncologic patients with symptomatic lesions of long bones treated with multiple micro-needles implantation combined with cement [9]. The procedure duration of this new technique shows that it is technically feasible and promising.
The total quantity of cement injected was 9.2 ± 3.1 ml (range 3–15) in this study. As expected, the quantity of cement injected increased significantly with the Mirels’ score [12], which takes the size of the lesion into account, but never exceeding 10–15 ml. In comparison, Tian who studied femoroplasty with or without needle prior insertion [7] injected almost three times more cement, with 24.67 ± 5.59 (range 15–40) for femoroplasty alone and 31.21 ± 6.30 ml (range 21–45) when combined with needles. The drawbacks of using a large amount of cement are the occurrence of subtrochanteric atypical fractures and the associated difficulty of the revision procedure with such an amount of cement to be removed and even more when combined with needles. Therefore, these risks are likely reduced by using an implant made of PEEK material and combined with a limited amount of cement [10]. In this study, the single case of fracture was an anatomical neck fracture, showing that the presence of the device did not cause an atypical fracture mode and thus did not compromise its revision.
The minimally invasive surgery approach allows to achieve wound healing in all cases with no access site complication and a short duration of the hospitalization (2.3 ± 1.4 days). It should also be noted that 40% of the patients were discharged the day following the intervention. These results appear very short compared to the 19.3 days (range 1–105) reported by Ristevski in a cohort of 201 patients with femoral metastatic lesions treated by standard surgical fracture fixation device [6]. In addition, favorable results observed with regard to walking recovery (86% at hospital discharge) indicate that the intervention does not impede fast walking recovery. Overall, these favorable results show the interest of minimally invasive surgery and suggest that the intervention could be performed as a day surgery case.
Despite a context of rapid cancer progression in final phase, illustrated by the high percentage of deceased patients, and the use of a subjective pain scale where focal discomfort cannot easily be distinguished from diffuse pain, a reduction in pain level (–28%) could be observed at 1 year. Similarly, the OHS-12 showed a progressive and sustained improvement in the patients’ functional condition throughout the follow-up period (increasing by +23% at 1 year).
At that stage of the knowledge, this new technique shows promising clinical benefits that need to be developed through larger data collection. Indeed, this first study presents limitations, such as a small number of patients implanted with the studied device (n = 10) and a short follow-up due to cancer progression leading to death (only four patients completed the 1-year follow-up, and the remaining six patients could be followed for 142 days on average). But the available data are sufficient to evaluate the feasibility and safety of the studied device. An operator-dependent bias can also be noted as five different physicians performed the ten interventions (two physicians performed only one single procedure, two physicians performed two procedures, and one physician performed four procedures). But, compared to the existing percutaneous techniques developed for the same indication, this novel implant is associated with instruments specifically designed to facilitate the intervention and thus enhance its reproducibility.
Conclusion
Results from this first-in-man study conducted in patients with metastatic bone disease show the feasibility and the safety of this novel implant intervention. This minimal invasive procedure appears as a promising consolidation technique for oncologic patients with poor performance status, with a short hospitalization stay and no postoperative fracture, when performed following the operating instructions. Additional studies should now be conducted on a greater number of subjects to confirm these clinical benefits.
References
Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31(4):515–28.
Schneiderbauer MM, von Knoch M, Schleck CD, Harmsen WS, Sim FH, Scully SP. Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am Vol. 2004;86(A(8)):1684–9.
Parker MJ, Khan AZ, Rowlands TK. Survival after pathological fractures of the proximal femur. Hip Int: J Clin Exp Res Hip Pathol Ther. 2011;21(5):526–30.
Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T. Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2012;35(6):1428–32.
Deschamps F, de Baere T, Hakime A, Pearson E, Farouil G, Teriitehau C et al. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol. 2016;26(6):1631–9.
Ristevski B, Jenkinson RJ, Stephen DJG, Finkelstein J, Schemitsch EH, McKee MD, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52(4):302–8.
Tian Q-H, He C-J, Wu C-G, Li Y-D, Gu Y-F, Wang T et al. Comparison of percutaneous cementoplasty with and without interventional internal fixation for impending malignant pathological fracture of the proximal femur. Cardiovasc Intervent Radiol. 2016;39(1):81–9.
Arvinius C, Parra JLC, Mateo LS, Maroto RG, Borrego AF, Stern LL-D. Benefits of early intramedullary nailing in femoral metastases. Int Orthop. 2014;38(1):129–32.
Kelekis A, Filippiadis D, Anselmetti G, Brountzos E, Mavrogenis A, Papagelopoulos P, et al. Percutaneous augmented peripheral osteoplasty in long bones of oncologic patients for pain reduction and prevention of impeding pathologic fracture: the rebar concept. Cardiovasc Intervent Radiol. 2016;39(1):90–6.
Szpalski M, Gunzburg R, Aebi M, Delimoge C, Graf N, Eberle S, et al. A new approach to prevent contralateral hip fracture: evaluation of the effectiveness of a fracture preventing implant. Clin Biomech. 2015;30(7):713–9.
Ferrari S, Reginster J-Y, Brandi ML, Kanis JA, Devogelaer J-P, Kaufman J-M, et al. Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos. 2016;11(1):37.
Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256–64.
Jawad MU, Scully SP. In brief: classifications in brief: mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res®. 2010;468(10):2825-2827.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281–4.
Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br Vol. 1996;78(2):185–90.
Acknowledgements
None.
Funding
This study was funded by Hyprevention, Pessac, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Laëtitia Rodrigues and Charlène Maas are employees of Hyprevention, Pessac, France. Vincent Cabane (Tree House Consulting, Paris, France) is a consultant for Hyprevention, Pessac, France. François Cornelis and Frédéric Deschamps are members of the scientific board of Hyprevention, Pessac, France. Thibault Carteret, Thierry De Baere, Bruno Lapuyade and Lambros Tselikas declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cornelis, F.H., Tselikas, L., Carteret, T. et al. A Novel Implant for the Prophylactic Treatment of Impending Pathological Fractures of the Proximal Femur: Results from a Prospective, First-in-Man Study. Cardiovasc Intervent Radiol 40, 1070–1076 (2017). https://doi.org/10.1007/s00270-017-1613-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1613-5