Abstract
We report two cases of intraperitoneal bleeding from superior mesenteric artery (SMA) pseudoaneurysm after pancreaticoduodenectomy for pancreatic head carcinoma. In both cases, a stent-graft was deployed on the main SMA to exclude pseudoaneurysm and to preserve blood flow to the bowel. Bleeding stopped after the procedure. One patient was able to be discharged but died from carcinoma recurrence 4 months later. The other patient died of sepsis and stent-graft infection 5 months later. These patients remained free of intraperitoneal rebleeding during the follow-up period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreaticoduodenectomy is the standard treatment for pancreatic head carcinoma. The operative mortality rate was recently reported as being <5%; however, the rate of complications, such as bleeding, anastomotic leakage, and localized infection, is relatively high at 30% to 40%. Bleeding, which can be life-threatening, occurs with a frequency of 2% to 16% [1, 2]. Bleeding after pancreaticoduodenectomy is categorized into two groups: early and delayed. Early bleeding, which is mainly caused by intraoperative arterial injury and inadequate hemostasis, is the most common type. In contrast, delayed bleeding, which occurs ≥5 days after surgery, is caused by pseudoaneurysm of the main visceral arteries. In this article, we report two cases of bleeding superior mesenteric artery (SMA) pseudoaneurysm after pancreaticoduodenectomy. Stent-grafts were deployed in each case.
Case Reports
Case 1
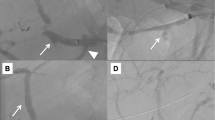
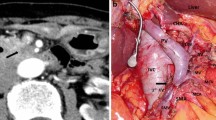
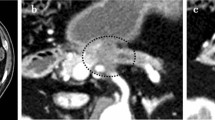
A 58-year-old woman underwent pancreaticoduodenectomy with resection of the portal vein and intraoperative radiation therapy for pancreatic head carcinoma. The patient developed pancreatic anastomotic leakage and biliary anastomotic leakage after surgery. Contrast-enhanced computed axial tomography (CAT) showed fluid collection just posterior and to the right of the SMA (Fig. 1). The fluid was discharged from an abdominal drain, which was placed during surgery posterior to the pancreaticojejunal anastomosis. The drain fluid was culture negative. The patient developed massive arterial bleeding from the drain 30 days after surgery. We performed emergency angiography to identify the bleeding vessel. An SMA pseudoaneurysm was visible at the stump of the replaced right hepatic artery (Fig. 2). We embolized the pseudoaneurysm with microcoils. The SMA blood flow was preserved to prevent bowel ischemia. However, rebleeding from the drain occurred 4 days after coil embolization. Emergency angiography was performed again. SMA angiography showed an enlarged pseudoaneurysm (Fig. 3A). This patient could not undergo additional surgery because of severe postoperative intra-abdominal adhesions. We therefore decided to use a stent-graft for the SMA pseudoaneurysm. In this urgent situation, only a 4 × 19–mm balloon-expandable stent-graft (Jostent GraftMaster Coronary Stent Graft System; Abbott, Redwood, CA), which was polytetrafluoroethylene–covered coronary stent-graft, was available. First, a 7F guiding catheter was progressed to the orifice of the SMA by way of the femoral artery. A 0.014-inch guidewire was advanced to the distal SMA, and the stent-graft was progressed over the wire just across the pseudoaneurysm. The stent-graft was then deployed, and postdilatation was performed using a 5-mm percutaneous transluminal angioplasty (PTA) balloon catheter. Then the drain was pulled out slightly to check hemostasis. However, superior mesenteric arteriography showed type 1 endoleak at the distal side of the stent-graft and contrast extravasation into the drain (Fig. 3B). The drain was immediately reinserted. Endoleak and bleeding disappeared with compression of the drain from outside. Superior mesenteric arteriography 13 days after stent-graft deployment showed no contrast extravasation or endoleak (Fig. 4). The patient was discharged 6 weeks later. Although the patient died from recurrence of carcinoma 4 months after discharge, no intraperitoneal bleeding occurred during the follow-up period.
Case 2
A 70-year-old woman underwent pancreaticoduodenectomy with resection of the portal vein and intraoperative radiation therapy for pancreatic head carcinoma. The patient developed refractory pancreatic anastomotic leakage and intraperitoneal abscess after surgery. Escherichia coli were cultivated from an abdominal drain that was placed during surgery posterior to the pancreaticojejunal anastomosis. Antibiotic therapy was continued. The patient developed massive arterial bleeding from the abdominal drain 70 days after surgery, and we therefore performed emergency angiography. There was no contrast extravasation on celiac arteriography or superior mesenteric arteriography, even when the abdominal drain was pulled out slightly to induce intraperitoneal bleeding. Celiac arteriography showed protrusion and dilatation at the stump of the gastroduodenal artery (GDA) (Fig. 5A). Although it turned out to be our misjudgment later, we diagnosed the stump of the GDA as the cause of bleeding. Therefore, we embolized the common hepatic artery with microcoils. Then the drain was pulled out slightly again to check hemostasis. However, arterial bleeding occurred suddenly from the drain. And, superior mesenteric arteriography showed contrast extravasation from the main SMA to the drain (Fig. 5B). The drain was reinserted immediately, and the bleeding stopped with compression of the drain. This patient could not undergo additional surgery because of the postoperative severe intra-abdominal adhesion and infection. We therefore decided to use a stent-graft for the SMA bleeding. Stent-graft treatment was performed the next day. A lateral superior mesenteric arteriography view showed a small pseudoaneurysm where the drain crossed the SMA (Fig. 6A). The acute angle of the SMA origin to the aorta was not suitable for the deployment of a self-expandable stent-graft from the femoral arterial approach. Therefore, the left brachial artery was punctured, and a 4F catheter was advanced to the SMA. The catheter was then exchanged over a guidewire for an 8F guiding sheath. Subsequently, a 6 × 40 mm Dacron-covered self-expandable stent-graft (PASSAGER; Boston Scientific, Natick, MA) was loaded into the guiding sheath, and the stent-graft was deployed by a pusher rod. Postdilatation was performed using a 5-mm PTA balloon catheter. Subsequent arteriography showed complete exclusion of the pseudoaneurysm and good blood flow to the bowel through the stent-graft (Fig. 6B). Intraperitoneal bleeding stopped, and antibiotic therapy was continued after stent-graft deployment. The localized infection improved temporarily. However, the refractory pancreatic anastomotic leakage remained unhealed. Three weeks later, abscess formation recurred around the SMA, and the localized infection continued. E. coli and Staphylococcus aureus were cultivated from the drain. The patency of the stent-graft was confirmed several times on follow-up contrast-enhanced CAT. Four months later, the general condition of the patient worsened, and blood cultures grew E. coli and Pseudomonas aeruginosa. Therefore, contrast-enhanced CAT was performed to check the abscess and the patency of the stent-graft. CAT showed stent-graft occlusion, air within the occluded stent-graft, and fluid collection around the SMA (Fig. 7). The presence of air and fluid collection indicated graft infection. The patient subsequently died of multiorgan failure caused by sepsis 5 months after stent-graft deployment. During the follow-up period, the patient was free of further bleeding after the stent-graft deployment.
In the present two cases, we obtained written informed consent from the patients, and treatment and presentation was approved by our hospital’s Institutional Review Board.
Discussion
Although the exact mechanism of delayed bleeding after pancreaticoduodenectomy remains elusive, localized infection and pancreatic leakage after pancreaticoduodenectomy are thought to be the main causes of pseudoaneurysm formation. It is probable that the localized infection and pancreatic juice weaken and erode the arterial wall [2]. In the present cases, both patients had pancreatic leakage around the SMA, and one had localized infection. In addition, the abdominal drain, which was placed during surgery posterior to the pancreaticojejunal anastomosis, crossed the SMA. Therefore, this mechanical stimulus on the arterial wall also promoted pseudoaneurysm formation.
Pseudoaneurysm after pancreaticoduodenectomy is more often seen at the stump of the GDA than at the SMA. The hepatic artery and the splenic artery can also be sites of bleeding [3]. Transcatheter arterial embolization is usually performed for such pseudoaneurysms [4]. Certainly, embolization is effective for bleeding from the GDA stump and the hepatic artery; however, it is not suitable for pseudoaneurysms and bleeding of the main SMA because blood flow of this vessel must be preserved to prevent bowel ischemia. In such cases, stent-graft deployment is considered a useful treatment. However, to our knowledge, only one report of stent-graft for SMA pseudoaneurysm after pancreaticoduodenectomy has been published [5]. In that case, thrombin was also injected into the pseudoaneurysm.
Although stent-graft deployment is effective for controlling acute bleeding and pseudoaneurysm, there is always the risk of stent-graft infection, particularly in patients with infection in the region. The indications for stent-graft deployment in infected pseudoaneurysm remain controversial. Some reports have mentioned good clinical courses after the procedure, without stent-graft infection [6, 7]. In contrast, there have been reports of stent-graft infection during follow-up [8, 9]. However, data from infected pseudoaneurysms from other anatomic sites may not be exactly applicable to the postpancreaticoduodenectomy setting, such as in our cases, because of the enzyme leak issue. Allen et al. advocated that stent-graft treatment for infected pseudoaneurysm should be performed in specific circumstances, such as infection with a low-virulence organism, absence of gross purulence, and high operative risk. Moreover, it may be valid to use stent-grafting as an interim measure while awaiting an improvement in general condition that would enable surgery [10]. In the present two patients, neither additional surgery (such as ligation of the pseudoaneurysm and reconstruction of the SMA) nor SMA embolization were considered appropriate. Only stent-graft deployment was able to exclude the pseudoaneurysm and preserve blood flow to the bowel. Although stent-graft infection occurred in one patient, stent-grafting was highly effective in delivering life-saving hemostasis.
In conclusion, stent-graft treatment is effective in palliating acute bleeding from the SMA. However, more data are needed to evaluate long term prognosis.
References
Miedema BW, Sarr MG, van Heerden JA et al (1992) Complications following pancreatoduodenectomy. Arch Surg 127:945–950
Rumstadt B, Schwab M, Korth P et al (1998) Hemorrhage after pancreatoduodenectomy. Ann Surg 227:236–241
Santoro R, Carlini M, Carboni F et al (2003) Delayed massive arterial hemorrhage after pancreaticoduodenectomy for cancer. Management of a life-threatening complication. Hepatogastroenterology 50:2199–2204
Yoon YS, Kim SW, Her KH et al (2003) Management of postoperative hemorrhage after pancreatoduodenectomy. Hepatogastroenterology 50:2208–2212
Wallace MJ, Choi E, Mcrae S et al (2007) Superior mesenteric artery pseudoaneurysm following pancreaticoduodenectomy: management by endovascular stent-graft placement and transluminal thrombin injection. Cardiovasc Intervent Radiol 30:518–522
Kwon K, Choi D, Choi SH et al (2002) Percutaneous stent-graft repair of mycotic common femoral artery aneurysm. J Endovasc Ther 9:690–693
Sanada J, Matsui O, Arakawa F et al (2005) Endovascular stent-grafting for infected iliac artery pseudoaneurysms. Cardiovasc Intervent Radiol 28:83–86
Burks JA Jr, Faries PL, Gravereaux EC et al (2001) Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg 34:1055–1059
Gonzalez-Fajardo JA, Gutierrez V, Martin-Pedrosa M et al (2005) Endovascular repair in the presence of aortic infection. Ann Vasc Surg 19:94–98
Allen RC, Sebastian MG (2001) The role of endovascular techniques in aortoesophageal fistula repair. J Endovasc Ther 8:602–663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, K., Mori, Y., Komada, T. et al. Stent-Graft Treatment for Bleeding Superior Mesenteric Artery Pseudoaneurysm After Pancreaticoduodenectomy. Cardiovasc Intervent Radiol 32, 762–766 (2009). https://doi.org/10.1007/s00270-009-9502-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9502-1