Abstract
High-temperature structural behavior of a stuffed derivative of α-quartz, Mg0.5AlSiO4, has been investigated using in situ synchrotron-based angle-dispersive powder X-ray diffraction (XRD) from 299 to 1273 K. Rietveld analysis of the XRD data indicates that the framework of Mg0.5AlSiO4 remains isostructural with α-quartz throughout the temperature range tested. As in α-quartz, unit-cell parameters a and c and cell volume V of Mg0.5AlSiO4 increase with increasing temperature, primarily due to progressive tilting of [(Al,Si)O4] tetrahedra along the a axes. However, the rates of increase in the cell parameters and the rate of decrease in the tetrahedral tilt angle (δ) are much smaller for Mg0.5AlSiO4 than for α-quartz. This behavior can be attributed to the occupancy of Mg2+ over the octahedral channel sites in the α-quartz-type framework, effectively hindering the [(Al,Si)O4] tetrahedral tilting. As a result, the α- to β-quartz phase transformation, which exists in silica at 846 K, does not occur in Mg0.5AlSiO4 up to 1273 K, and probably beyond, to its melting point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although natural quartz is intolerant of ionic substitutions (mostly at the levels of hundreds of ppm) (Keith and Tuttle 1952; Ghiorso et al. 1979; Smith and Steele 1984), replacement of Si4+ cations by the larger Al3+ can facilitate incorporation of several small cations, including Li+, Mg2+, Zn2+, H+ and Na+, into the quartz framework (Schulz 1971; Schulz et al. 1971a, b; Tscherry et al. 1972; Guth and Heger 1979; Müller et al. 1988, 1990; Paulus et al. 1990; Sternitzke and Müller 1991; Palmer 1994; Xu et al. 1999a, b, c, 2000a, 2001a; Heaney 2000; Phillips et al. 2000). The charge-coupled substitution, Si4+ → Al3+ + M+ or ½ M2+ (M+ = Li+, H+, Na+; M2+ = Mg2+, Zn2+), produces a series of phases that Buerger (1954) classified as “stuffed derivatives of quartz”.
Depending on the size and concentration of substituting cations, stuffed derivatives of quartz adopt either the α- or β-quartz structure. As the stable polymorph of silica at room temperature, α-quartz (space group P3121 or P3221) transforms to the more expanded α-quartz (space group P6422 or P6222) at 846 K via the rotation of rigid tetrahedra about the a axes (Wright and Lehmann 1981; Will et al. 1988; Heaney 1994). Although pure β-quartz is not quenchable, the incorporation of small cations into its structural channels can prop open the framework and stabilize the β-quartz structure at room temperature. For example, the so-called LAS (Li2O–Al2O3–SiO2) phases with compositions Li1–xAl1–xSi1+xO4, 0 ≤ x < ~ 0.65, possess the β-quartz structure. Like β-quartz, these phases exhibit negative coefficients of thermal expansion (CTE) (Pillars and Peacor 1973; Lichtenstein et al. 1998, 2000; Xu et al. 1999c, 2001a). As a result, they have served as major components of high-temperature glass–ceramic products used in domestic cookware and in many high-precision machines such as jet engines (Beall 1994; Müller 1995; Roy 1995; Ramalingam and Reimanis 2012). However, when the content of incorporated Li+ falls below a certain threshold, i.e., ~ 0.65 < x ≤ 1 in Li1–xAl1–xSi1+xO4, the LAS phases adopt the α-quartz structure and possess positive CTEs. Furthermore, these α-quartz-like phases transform to their β-quartz-like counterparts at higher temperatures, and the transition temperature scales inversely with the Li content (Xu et al. 2001a).
To tailor the thermal properties of LAS phases for specific applications, other small cations, mainly Mg2+ and Zn2+, have been used to partially replace Li+ (Petzoldt 1967; Beall 1994). Whereas Zn0.5–0.5xAl1-xSi1+xO4, such as β-quartz and Li1-xAl1-xSi1+xO4 (x < ~ 0.65), exhibits negative CTEs, Mg0.5-0.5xAl1-xSi1+xO4 exhibits positive CTEs (Schreyer and Schairer 1961; Müller et al. 1988; Sternitzke and Müller 1991). In our recent study (Xu et al. 2015), we grew a Mg0.5AlSiO4 phase from glass and determined its structure using synchrotron XRD, transmission electron microscopy (TEM) and 29Si nuclear magnetic resonance (NMR) spectroscopy. Our results indicate that this phase possesses an α-quartz-like structure with Mg occupying octahedral channel sites, in contrast to the tetrahedral coordination of Li in the β-quartz-type framework for β-eucryptite, LiAlSiO4.
The motivation of this work is to examine the high-temperature structural behavior of Mg0.5AlSiO4. Since this phase is structurally analogous to α-quartz, will it undergo an α-to-β transformation on heating? How does the framework structure evolve as a function of temperature? What is the role of extra-framework Mg2+? To answer these questions, we collected high-energy synchrotron XRD data of powdered Mg0.5AlSiO4 from 299 K to 1273 K and high-resolution synchrotron XRD data at 523 K and 748 K. Rietveld analysis indicates that Mg0.5AlSiO4 maintains its α-quartz-like structure throughout this temperature range. As in α-quartz, unit-cell dimensions increase with increasing temperature. However, the rates of increase are significantly smaller than those for α-quartz. This behavior can be attributed to the occupancy of Mg2+ over the octahedral channel sites, effectively hindering the [(Al,Si)O4] tetrahedral tilting, which is the structural mechanism for thermal expansion of α-quartz-type framework and its transformation to the β-quartz-type configuration (Megaw 1973).
Experimental methods
Mg0.5AlSiO4 sample
The Mg0.5AlSiO4 sample was synthesized from heat-treatment of a glass of the same composition at 1173 K for ~ 7 h. Detailed synthesis procedures have been described in Xu et al. (2015). TEM observation and synchrotron XRD showed that the sample is homogeneous and phase pure. Energy-dispersive spectroscopy (EDS) analysis revealed that the chemical composition is essentially identical to the nominal Mg0.5AlSiO4 (Xu et al. 2015).
High-temperature synchrotron X-ray diffraction
We conducted two sets of powder synchrotron XRD experiments on Mg0.5AlSiO4 at high temperatures (Fig. 1). One set included high-resolution measurements at 523 K and 748 K with a linear position-sensitive detector at beam line X7A of the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory (BNL) (Fig. 1a). The goal was to accurately determine the structure of Mg0.5AlSiO4 at high temperatures. The wavelength used was 0.700789 Å (17.7 keV in energy), as calibrated with a CeO2 standard. Sample powders were sealed in a quartz-glass capillary of 0.02-mm diameter, which was mounted in a furnace consisting of a 1.25 inch-diameter wire-wound BN tube and outer water-cooled Al tube. Sample temperatures were registered with a Chromel–Alumel thermocouple positioned just below the center of the capillary, and was found to be stable within 1 K. To minimize preferred orientation, the capillary was fully rotated during data collection. Data were collected from 7° to 56° 2θ in the step-scan mode using step sizes of 0.25° and counting times of 10 s (7–15°), 20 s (15–30°), 40 s (30–45°), and 80 s (45–56°) per step.
Comparison of the strongest XRD peaks, (101), of Mg0.5AlSiO4 from room-temperature synchrotron data collected at a NSLS (Xu et al. 2015) and b APS. The NSLS data offer higher resolution (narrower peaks), whereas the APS data have superior counting statistics (compare the intensity scales). The slight difference in the (101) peak positions of the two patterns reflects systematic errors in the two diffractometers that were corrected during data processing
Another set of XRD experiments included high-energy synchrotron measurements performed in a transmission mode at beam line 11-ID-C of the advanced photon source (APS), Argonne National Laboratory (ANL), taking advantage of quick data collection using high-energy synchrotron X-rays (Fig. 1b). The goal was to collect more XRD data at closely spaced temperature points within a larger range to gauge the critical temperature of the possible α–β transition in Mg0.5AlSiO4. The wavelength used was 0.11798 Å (105.1 keV in energy), as calibrated with a CeO2 standard. Sample powders were packed into the chamber (8 mm in diameter and 3 mm deep) of a Linkam high-temperature furnace TS1500, and two-dimensional (2D) XRD patterns were collected continuously using a PerkinElmer large area detector when the sample was heated from room temperature to 1273 K with a heating rate of 10 K/min. A total of 747 data points were obtained. The 2D patterns were calibrated and converted to the conventional patterns of intensity versus 2θ using the Fit2d software. Selected temperature-dependent XRD patterns are shown in Fig. 2. Details of the experimental setup and data treatment can be found in Chen et al. (2011).
Rietveld analysis of XRD data
The above NSLS and APS synchrotron XRD data were analyzed by the Rietveld method with the general structure analysis system (GSAS) program (Larson and Von Dreele 2000) and GSAS-II (Toby and Von Dreele 2013), respectively. The starting structural parameters were taken from our previous study of the same sample at room temperature (Xu et al. 2015). Our refinements proceeded as follows: after the scale factor and four Chebyshev polynomial background terms had converged, specimen displacement and lattice parameters were added and optimized. Twelve (for the NSLS data) or eight (for the APS data) additional background terms were then added, and the peak profiles were fitted by refining isotropic and anisotropic broadening parameters and a Gaussian particle size coefficient in a pseudo-Voigt function (Thompson et al. 1987; Cox et al. 1988; Finger et al. 1994). On convergence of the preceding parameters, atomic positions and isotropic temperature factors for Mg, Al/Si, and O were refined.
Results and discussion
Framework structure
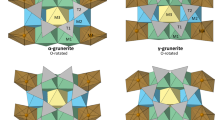
Xu et al. (2015) studied the structure of Mg0.5AlSiO4 using a combined approach of synchrotron XRD, electron diffraction and 29Si NMR spectroscopy. The results indicate that the aluminosilicate framework adopts the structure of α-quartz with Al and Si being disordered over the tetrahedral sites at long-range scales (i.e., no doubled periodicity along the c axis, as would be expected due to Al/Si ordering in accordance with the so-called “Al avoidance” principle (Loewenstein 1954); though they are partially ordered at short-range scales, revealed by NMR). Mg exhibits different site occupancies within the neighboring channels parallel to the c axis, resulting in doubled periodicities along a relative to that of α-quartz. Thus the final refined structure was based on an a-doubled α-quartz-like superstructure (Xu et al. 2015). We analyzed the high-resolution high-temperature XRD data collected at NSLS using this superstructure model, and the refined structural parameters at 523 K and 748 K are listed in Tables 1 and 2, respectively. The corresponding fitted XRD patterns are plotted in Fig. 3a (for 523 K, refinement agreement indices Rwp = 4.4%, χ2 = 2.37) and 3B (for 748 K, Rwp = 5.1%, χ2 = 2.06). The analyses indicate that at high temperature, the aluminosilicate framework of Mg0.5AlSiO4 remains isostructural with α-quartz. Moreover, difference electron Fourier maps reveal that Mg occupies octahedral channel sites along the c axis (Fig. 4), as was observed at room temperature (Xu et al. 2015), and thus the site occupancies of Mg were fixed for all the temperatures in our refinements.
Fitted synchrotron XRD patterns of Mg0.5AlSiO4 based on the α-quartz superstructure model with doubled a axes (space group P3221) at a 523 K and b 748 K. Data are shown as plus signs, and the solid curve is the best fit to the data. Tick marks below the pattern show the positions of allowed reflections, and the lower curve represents the difference between the observed and calculated profiles. The inset shows the details of the profile from 15° to 38° 2θ
Nevertheless, for ease of describing structural variations in the α-quartz-like framework with temperature, especially with respect to the β-quartz-like configuration, one may neglect the superstructure and use the basic quartz unit cell to represent the Mg0.5AlSiO4 framework. In fact, the superlattice peaks corresponding to the a-doubled periodicities are hardly visible in synchrotron XRD patterns, especially those collected at APS with moderate resolution (when the peaks are more diffuse) and at high temperatures (which may indicate possible disappearance of the superstructure at higher T). Hence, neglecting these weak, broad peaks in Rietveld analyses of XRD data does not induce significant errors in the refined Mg0.5AlSiO4 framework structure, and we used the average, basic α-quartz structure model to analyze all the high-temperature XRD data collected at APS, yielding refinement agreement indices of Rwp = 4.6% (1273 K)–6.8% (299 K) and χ2 = 0.60 (1273 K)–1.19 (299 K). The derived unit-cell parameters, atomic coordinates and atomic displacement parameters at a large number of closely spaced temperatures allow accurate characterization of framework variations in Mg0.5AlSiO4 as a function of temperature, as discussed below.
Thermal expansion
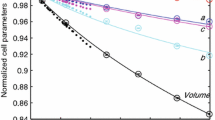
Figure 5 reveals the dependence of unit-cell parameters a, c, and unit-cell volume V of Mg0.5AlSiO4 on temperature. On heating, both a and c increase, and thus V also increases. To obtain the coefficients of thermal expansion (CTEs), we calculated a linear fit of the cell-parameter data as a function of temperature (T):
The mean CTEs of Mg0.5AlSiO4 in the temperature range 299–1273 K are: αa = 6.09(1) × 10−6 K−1; αc = 4.62(2) × 10−6 K−1; and αV = 1.690(4) × 10−5 K−1. These parameters are much lower than those of pure α-quartz, which first exhibits steady increases in a, c and V and then steep increases prior to its transformation to β-quartz at 846 K (Kihara 1990, 2001; Carpenter et al. 1998). For comparison, linear fittings of the a, c and V values of α-quartz in the temperature range 293–699 K (based on the XRD data of Carpenter et al. 1998) yield: αa = 20(1) × 10−6 K−1; αc = 13(1) × 10−6 K−1; and αV = 5.2(4) × 10−5 K−1, which are about three times larger than the corresponding CTEs of Mg0.5AlSiO4. The CTEs of α-quartz in the temperature range prior to its transition to β-quartz (e.g., 700–846 K) are even larger, and they increase drastically as the temperature approaches the critical point, 846 K.
Mechanism of thermal expansion
As in α-quartz, the dominant mechanism responsible for the positive thermal expansion of Mg0.5AlSiO4 is a rotation of nearly rigid Si- or Al-tetrahedra around the a axes (in addition to the correlated changes in tetrahedral dimensions (Grimm and Dorner (1975)), which is associated with the low-frequency, high-amplitude phonon modes, most of which have negative Grüneisen coefficients (Xu and Heaney 1997; Smirnov 1999; Tucker et al. 2001). Figure 6 shows the temperature dependence of the tetrahedral rotation angle δ (Grimm and Dorner 1975)
With increasing temperature, δ decreases gradually from ~ 9.4° at 299 K to ~ 7.1° at 1273 K. In other words, the Al/Si-tetrahedral framework progresses towards the β-quartz configuration, where δ = 0. However, even at the highest temperature tested (1273 K), δ does not reach zero, indicating that the framework structure remains in the α-quartz configuration. Extrapolation of the fitted δ–T linear relation:
to δ = 0 yields a hypothetical α–β transition temperature of ~ 4383 K, which is well above the melting temperature (< 1573 K, Xu et al. 2015; Strnad 1986) of Mg0.5AlSiO4. By contrast, the rate of decrease in δ in α-quartz with increasing temperature is much larger; δ decreases from 15.8° at 298 K to 7.4° at 844 K, followed by a steep drop of 7.4° to reach δ = 0° in β-quartz from 844 to 847 K, a 3 K interval (Antao 2016). Temperature-dependent δ values have been fitted to an order parameter to describe the α–β quartz transition (Grimm and Dorner 1975; Carpenter et al. 1998).
The smaller rate of decrease in δ of Mg0.5AlSiO4 with increasing temperature and the absence of its β-quartz counterpart at high temperature can be attributed to the occupancy of the octahedral channel sites by Mg in Mg0.5AlSiO4. As shown in Fig. 7a, Mg2+ cations are situated at approximately the same z value as two O atoms in [Si/AlO4] tetrahedra from the two helical chains that spiral about c. On heating, the tetrahedra rotate about the a axes (Fig. 7b), as reflected by the decreases in δ. However, the presence of Mg in the octahedral channel sites hinders the tetrahedral tilting, resulting in a smaller rate of decrease in δ than occurs in α-quartz. The inhibitory effect of the channel Mg cations on tetrahedral tilting also explains the absence of a transition to a β-quartz-like Mg0.5AlSiO4 polymorph at high temperature.
It should be noted that the absence of an α–β transition at high temperature also occurs in other α-quartz-like phases with formula MXO4 (e.g. GaPO4), which have a M–O–X bridging angle of ≤ 136° or a rotational angle δ of ≥ 22° at room temperature. An inverse linear relation exists between M–O–X angle and δ, and the larger the distortion of the tetrahedral chain, the smaller the M–O–X angle and the larger the value of δ (Philippot et al. 1994, 1996). However, the α-quartz-like Mg0.5AlSiO4 has an Al/Si–O–Al/Si angle of 148.4° and a rotational angle δ of ~ 9.4°. Therefore, the absence of the α–β transition in Mg0.5AlSiO4 is not due to its framework distortion (which is small). Rather, it results from the partial occupancy of Mg within the octahedral channel sites, hindering [Si/AlO4] tetrahedral rotation about the a axes.
Variations of atomic displacement parameters with increasing temperature
Figure 8 shows variations in the isotropic displacement factors (Uiso) of Mg, Al/Si and O as a function of temperature. At a given temperature, Uiso(O) > Uiso(Mg) > Uiso(Al/Si), which is consistent with the general trend that the lighter the element the larger the Uiso. For a given element, its Uiso increases with increasing temperature, as U (= kT/f, where k is the Boltzman constant, T is absolute temperature, and f is the bond force constant) is proportional to the temperature. However, the rate of increase of Uiso(Mg) on heating is much larger than those of Uiso(Al/Si) and Uiso(O) (The somewhat scattered Uiso(Mg) data points may be associated with the partial occupancy of Mg over the octahedral channel sites). This behavior may be explained in terms of the bonding configuration of Mg2+ with its neighboring atoms. In the α-quartz-like Mg0.5AlSiO4 structure, Mg is situated in the framework tunnels and thus has relatively weaker electrostatic interactions with its adjacent framework O, rendering greater vibrations with increasing temperature.
Effects of channel cation on structural variations of the quartz framework with temperature
The occupancy of the octahedral channel sites by Mg in Mg0.5AlSiO4 (Fig. 7a) is in striking contrast with that of the tetrahedral sites by Li in LiAlSiO4. This disparity is responsible for not only the difference in the type of quartz-like framework at room temperature (α-quartz for Mg0.5AlSiO4 and β-quartz for LiAlSiO4) but also its variations with temperature. Xu et al. (1999c) demonstrated that the β-quartz structure of LiAlSiO4 persists down to low temperatures (20 K was the lowest temperature tested) without a β- to α-quartz-like transformation. Although Li positionally disorders within the structural channels along c at high temperature (Xu et al. 1999c; Zhang et al. 2003), the framework configuration does not experience a symmetry-breaking distortion in response to the order–disorder behavior of the Li+ cations. In other words, the incorporation of Li into the quartz framework props open the β-quartz chain configuration throughout the temperature range from 20 K (and likely from 0 K) to the melting point of LiAlSiO4. As a result, LiAlSiO4 exhibits negative thermal expansion in unit-cell volume, as in β-quartz, with its cell parameter c decreasing as a increases with increasing temperature. Likewise, the absence of the α- to β-quartz-like transition in Mg0.5AlSiO4 at high temperature implies that the α-quartz structure of Mg0.5AlSiO4 likely persists from 0 K to its melting point as well. Since the α-quartz configuration is the low-temperature polymorph, no transition is expected in Mg0.5AlSiO4 upon cooling. Moreover, as Mg is localized within the available octahedral channel sites (Figs. 4 and 7a), there is no order–disorder transition within a given channel parallel to c upon heating, as occurred with Li in LiAlSiO4. Correspondingly, the thermal expansion coefficients of Mg0.5AlSiO4 are positive along both the a and c axes, as in α-quartz, though the magnitudes are smaller. Therefore, the occupancy of Li+ and Mg2+ over different channel sites in the quartz framework results in different polymorphs not only at room temperature but also above and below it.
Conclusions/implications
Silicate phases with framework structures commonly occur in natural rocks (e.g., feldspars) and in synthetic ceramic/composite materials (e.g., glass–ceramic cookware). Extra-framework cations situated in channels and/or cavities may exert significant effects on framework topologies (Xu et al. 2000b; Balmer et al. 2001), to the point of inducing symmetry changes at ambient conditions (Heaney 2000; Xu et al. 2001b, 2002). This phenomenon in turn affects the structural behaviors of doped phases upon changing temperature or pressure (Zhang et al. 2002, 2005; Chen et al. 2016). This study demonstrates the different responses of the quartz framework to the occupancy of Mg2+ and Li+ within the channel sites. The presence of Mg2+ within the octahedral channel site stabilizes the α-quartz-like Mg0.5AlSiO4 structure up to its melting point, whereas the occupancy of Li+ over the tetrahedral channel site maintains the β-quartz-like LiAlSiO4 structure throughout the temperature range. Since the α- and β-quartz structures possess dramatically different thermal expansion properties (positive and negative thermal expansion for α- and β-quartz, respectively), one may design mixed Li/Mg-stuffed-quartz derivatives with tailored expansion properties (particularly zero thermal expansion). This information also has implications for the thermal behaviors of “impure” quartz and other framework aluminosilicate minerals during the formation of their host rocks.
References
Antao SM (2016) Quartz: structural and thermodynamic analyses across the α ↔ β transition with origin of negative thermal expansion (NTE) in β quartz and calcite. Acta Cryst B72:249–262
Balmer ML, Su Y, Xu H, Bitten E, McCready D, Navrotsky A (2001) Synthesis, structure determination, and aqueous durability of Cs2ZrSi3O9. J Am Ceram Soc 84:153–160
Beall GH (1994) Industrial applications of silica. Mineral Soc Am Rev Mineral 29:468–505
Buerger MJ (1954) The stuffed derivatives of the silica structures. Am Miner 39:600–614
Carpenter MA, Salje EKH, Graeme-Barber A, Wruck B, Dove MT, Knight KS (1998) Calibration of excess thermodynamic properties and elastic constant variations due to the α-β phase transition in quartz. Am Miner 83:2–22
Chen Z, Qin Y, Ren Y, Lu W, Orendorff C, Roth EP, Amine K (2011) Multi-scale study of thermal stability of lithiated graphite. Energy Environ Sci 4:4023–4030
Chen Y, Manna S, Narayanan B, Wang Z, Reimanis IE, Ciobanu CV (2016) Pressure-induced phase transformation in β-eucryptite: an X-ray diffraction and density functional theory study. Scripta Mater 122:64–67
Cox DE, Toby BH, Eddy MM (1988) Acquisition of powder diffraction data with synchrotron radiation. Aust J Phys 41:117–131
Finger LW, Cox DE, Jephcoat AP (1994) A correction for powder diffraction peak asymmetry due to axial divergence. J Appl Crystallogr 27:892–900
Ghiorso MS, Carmichael ISE, Moret LK (1979) Inverted high-temperature quartz. Contrib Miner Petrol 68:307–323
Grimm H, Dorner B (1975) On the mechanism of the α-β phase transformation of quartz. J Phys Chem Solids 36:407–413
Guth H, Heger G (1979) Temperature dependence of the crystal structure of the one-dimensional Li+-conductor β-eucryptite (LiAlSiO4). In: Vashista P, Mundy JN, Shenoy GK (eds) Fast ion transport in solids. Elsevier North Holland, New York, pp 499–502
Heaney PJ (1994) Structure and chemistry of the low-pressure silica polymorphs. Mineral Soc Am Rev Mineral Geochem 29:1–40
Heaney PJ (2000) Phase transformations induced by solid solution. Mineral Soc Am Rev Mineral Geochem 39:135–174
Keith ML, Tuttle OF (1952) Significance of variance in high-low inversion of quartz. Am J Sci 253a:203–280
Kihara K (1990) An X-ray study of the temperature dependence of the quartz structure. Eur J Mineral 2:63–77
Kihara K (2001) Molecular dynamics interpretation of structural changes in quartz. Phys Chem Miner 28:365–376
Larson AC, Von Dreele RB (2000) GSAS–general structure analysis system. Los Alamos National Laboratory Report No. LAUR 86-748, p 179
Lichtenstein AI, Jones RO, Xu H, Heaney PJ (1998) Anisotropic thermal expansion in the silicate β-eucryptite: a neutron diffraction and density functional study. Phys Rev B 58:6219–6223
Lichtenstein AI, Jones RO, de Gironcoli S, Baroni S (2000) Anisotropic thermal expansion in silicates: a density functional study of β-eucryptite and related materials. Phys Rev B 62:11487–11493
Loewenstein W (1954) The distribution of aluminum in the tetrahedra of silicates and aluminates. Am Miner 39:92–96
Megaw H (1973) Crystal structures: a working approach. WB Saunders, Philadelphia
Müller G (1995) The scientific basis. In: Bach H (ed) Low thermal expansion glass ceramics. Springer-Verlag, Berlin, pp 13–49
Müller G, Hoffmann M, Neeff R (1988) Hydrogen substitution in lithium-aluminosilicates. J Mater Sci 23:1779–1785
Müller G, Paulus H, Darmstadt JS (1990) Synthesis and structure of β-quartz type Na0.5H0.5AlSi2O6 as compared to LiAlSi2O6. N Jb Miner Mh H11:493–503
Palmer DC (1994) Stuffed derivatives of the silica polymorphs. Mineral Soc Am Rev Mineral 29:83–122
Paulus H, Fuess H, Müller G, Darmstadt JS, Vogt T (1990) The crystal structure of β-quartz type HAlSi2O6. N Jb Miner Mh H5:232–240
Petzoldt J (1967) Metastabile mischkristalle mit quarzstruktur mit oxidsystem Li2O-MgO-ZnO-Al2O3-SiO2. Glastechnische Berichte 40:385–396
Philippot E, Goiffon A, Ibanez A, Pintard M (1994) Structure deformations and existence of the α-β transition in MXO4 quartz-like materials. J Solid State Chem 110:356–362
Philippot E, Palmier D, Pintard M, Goiffon A (1996) A general survey of quartz and quartz-like materials: packing distortions, temperature, and pressure effects. J Solid State Chem 123:1–13
Phillips BL, Xu H, Heaney PJ, Navrotsky A (2000) 29Si and 27Al MAS-NMR spectroscopy of β-eucryptite (LiAlSiO4): the enthalpy of Si, Al ordering. Am Miner 85:181–188
Pillars WW, Peacor DR (1973) The crystal structure of beta eucryptite as a function of temperature. Am Miner 58:681–690
Ramalingam S, Reimanis IE (2012) Effect of doping on the thermal expansion of β-eucryptite prepared by sol-gel methods. J Am Ceram Soc 95:2939–2943
Roy R (1995) Low thermal expansion ceramics: A retrospective. In: Stinton DP, Limaye SY (eds) Low-expansion materials, ceramic transactions, vol 52. The American Ceramic Society. Westerville, Ohio, USA, pp 1–4
Schreyer W, Schairer JF (1961) Metastable solid solution with quartz-type structure on the join SiO2-MgAl2O4. Z Kristallogr 116:60–82
Schulz H (1971) Influence of heat-treatment on the average structure of Mg[Al2Si3O10], a stuffed derivative of the high-quartz structure. Zeitschrift für Kristallographie Bd 134:253–261
Schulz H, Muchow GM, Hoffmann W, Bayer G (1971a) X-ray study of Mg-Al silicate high-quartz phases. Zeitschrift für Kristallographie Bd 133:91–109
Schulz H, Hoffmann W, Muchow GM (1971b) The average structure of Mg[Al2Si3O10], a stuffed derivative of the high-quartz structure. Zeitschrift für Kristallographie Bd 134:1–27
Smirnov MB (1999) Lattice dynamics and thermal expansion of quartz. Phys Rev B 59:4036–4043
Smith JV, Steele IM (1984) Chemical substitution in silica polymorphs. Neues Jahrbuch für Mineralogie Monatschefte 3:137–144
Sternitzke M, Müller G (1991) Crystal structure and thermal expansion of quartz-type aluminosilicates. J Mater Sci 26:3051–3056
Strnad Z (1986) Glass-ceramic materials. Glass science and technology, vol 8. Elsevier, Amsterdam, p 268
Thompson P, Cox DE, Hastings J (1987) Rietveld refinement of Debye-Scherrer synchrotron X-ray data for Al2O3. J Appl Crystallogr 20:79–83
Toby BH, Von Dreele RB (2013) GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J Appl Crystallogr 46:544–549
Tscherry V, Schulz H, Laves F (1972) Average and super structure of β-eucryptite (LiAlSiO4), part II, superstructure. Z Kristallogr 135:175–198
Tucker MG, Keen DA, Dove MT (2001) A detailed structural characterization of quartz on heating through the α–β phase transition. Mineral Mag 65:489–507
Will G, Bellotto M, Parrish W, Hart M (1988) Crystal structures of quartz and magnesium germanate by profile analysis of powder diffractometer data. J Appl Crystallogr 21:182–191
Wright AF, Lehmann MS (1981) The structure of quartz at 25 and 500°C determined by neutron diffraction. J Solid State Chem 36:371–380
Xu H, Heaney PJ (1997) Memory effects of domain structures during displacive phase transitions: a high-temperature TEM study of quartz and anorthite. Am Miner 82:99–108
Xu H, Heaney PJ, Navrotsky A, Topor L, Liu J (1999a) Thermochemistry of stuffed quartz-derivative phases along the join LiAlSiO4-SiO2. Am Miner 84:1360–1369
Xu H, Heaney PJ, Böhm H (1999b) Structural modulations and phase transitions in β-eucryptite: an in situ TEM study. Phys Chem Miner 26:633–643
Xu H, Heaney PJ, Yates DM, Von Dreele RB, Bourke MA (1999c) Structural mechanisms underlying near-zero thermal expansion in β-eucryptite: a combined synchrotron X-ray and neutron Rietveld analysis. J Mater Res 14:3138–3151
Xu H, Heaney PJ, Beall GH (2000a) Phase transitions induced by solid solution in stuffed derivatives of quartz: a powder synchrotron XRD study of the LiAlSiO4-SiO2 join. Am Miner 85:971–979
Xu H, Navrotsky A, Nyman MD, Nenoff TM (2000b) Thermochemistry of microporous silicotitanate phases in the Na2O–Cs2O–SiO2–TiO2–H2O system. J Mater Res 15:815–823
Xu H, Heaney PJ, Navrotsky A (2001a) Thermal expansion and structural transformations of stuffed derivatives of quartz along the LiAlSiO4-SiO2 join: a variable-temperature powder synchrotron XRD study. Phys Chem Miner 28:302–312
Xu H, Navrotsky A, Balmer ML, Su Y, Bitten ER (2001b) Energetics of substituted pollucites along the CsAlSi2O6–CsTiSi2O6.5 join: a high-temperature calorimetric study. J Am Ceram Soc 84:555–560
Xu H, Navrotsky A, Balmer ML, Su Y (2002) Crystal chemistry and phase transitions in substituted pollucites along the CsAlSi2O6-CsTiSi2O6.5 join: a powder synchrotron X-ray diffractometry study. J Am Ceram Soc 85:1235–1242
Xu H, Heaney PJ, Yu P, Xu H (2015) Synthesis and structure of a stuffed derivative of α-quartz, Mg0.5AlSiO4. Am Miner 100:2191–2198
Zhang J, Celestian A, Parise JB, Xu H, Heaney PJ (2002) A new polymorph of eucryptite (LiAlSiO4), ε-eucryptite, and thermal expansion of α- and ε-eucryptite at high pressure. Am Miner 87:566–571
Zhang M, Xu H, Salje EKH, Heaney PJ (2003) Vibrational spectroscopy of beta-eucryptite (LiAlSiO4): optical phonons and phase transition(s). Phys Chem Miner 30:457–462
Zhang J, Zhao Y, Xu H, Zelinskas MV, Wang L, Wang Y, Uchida T (2005) Pressure-induced amorphization and phase transformations in β-LiAlSiO4. Chem Mater 17:2817–2824
Acknowledgements
We thank J. L. Baker for plotting Fig. 2 and the two anonymous reviewers for helpful comments. This work was supported by the Laboratory Directed Research and Development (LDRD) program of Los Alamos National Laboratory (LANL). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract No. DE-AC02-06CH11357. Some experiments were carried out at the National Synchrotron Light Source, Brookhaven National Laboratory, which was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract No. DE-AC02-98CH10886. LANL, an affirmative action/equal opportunity employer, is managed by Triad National Security, LLC, for the National Nuclear Security Administration of the U.S. Department of Energy under contract 89233218CNA000001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H., Lü, X., Heaney, P.J. et al. Structural behavior of a stuffed derivative of α-quartz, Mg0.5AlSiO4, at high temperature: an in situ synchrotron XRD study. Phys Chem Minerals 46, 717–725 (2019). https://doi.org/10.1007/s00269-019-01033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-019-01033-1