Abstract
Background
Esophageal cancer has a poor prognosis because of its rapid progression and early and extensive lymph node metastasis. Simple, objective indicators for predicting long-term outcomes are needed to select optimal perioperative treatment and appropriate follow-up for patients with esophageal cancer. The aim of this study is to investigate the relationship between the lymphocyte-to-C-reactive protein ratio (LCR) and the survival of patients with esophageal cancer, by performing time-dependent receiver operating characteristic (ROC) curve analysis. The results were compared to those of traditional inflammation-based markers.
Methods
This study enrolled 495 patients who underwent thoracic esophagectomy for esophageal cancer as the primary treatment between 2000 and 2019 in our department. We investigated the predictability of the LCR for oncological outcomes compared to that of other traditional inflammatory markers.
Results
The 3-year overall survival (OS) and recurrence-free survival (RFS) were 72.6% and 57.5%, respectively. Low LCR was significantly associated with higher cancer stage, included depth of invasion (p < 0.001), lymph node metastasis (p < 0.001) and cStage (p < 0.001). The LCR had the highest AUC value (0.675) for predicting OS compared to the other examined inflammatory markers. In multivariate analysis, the LCR (optimal cutoff threshold = 19,000) was identified as a significant predictor of death (hazard ratio, 2.24; 95% confidence interval [CI], 1.61–3.12; p < 0.001) and recurrence (hazard ratio, 1.97; 95%CI, 1.48–2.63; p < 0.001).
Conclusion
The LCR is novel indicator for oncological outcomes for patients with esophageal cancer and may assist to facilitate personalized multidisciplinary treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical esophagectomy is regarded as the most promising treatment to prolong survival of patients with esophageal cancer. However, despite improvements in surgical techniques and the development of multimodal treatments, including neoadjuvant therapy, esophageal cancer still has poor long-term prognoses, because of its rapid progression and early and extensive lymph node metastasis [1, 2]. The pathological TNM stage is the gold standard for predicting oncological outcomes after surgery; however, neoadjuvant treatment is performed as a standard treatment for resectable esophageal cancer. Therefore, especially in these patients, it is difficult to evaluate true disease progression using a surgical specimen alone. As a result, simple, objective markers for predicting survivals are needed to choose optimal perioperative treatment and appropriate follow-up for esophageal cancer patients.
Tumors can directly and indirectly interact with the inflammatory immune response to upregulate cytokines, inflammatory mediators, inhibit apoptosis, promote angiogenesis and induce DNA damage [3]. Several previous studies, including our own, have demonstrated several prognostic markers for long-term prognosis after esophagectomy using the preoperative inflammation-based score [4,5,6]. In particular, inflammation-based markers of the systemic inflammatory response, such as the neutrophil-to-lymphocyte ratio (NLR) [7, 8], lymphocyte-to-monocyte ratio (LMR) [9, 10], platelet-to-lymphocyte ratio (PLR) [11, 12] and the C-reactive protein (CRP)-to-albumin ratio (CAR) [13, 14], have been known as prognostic factors, not only in esophageal cancer but also in other type of cancers. Our institution also showed that preoperative FA score, which is a prognostic indicator based on preoperative plasma fibrinogen and serum albumin levels, was significantly associated with postoperative survival in patients with esophageal cancer [4, 5].
Recently, Okugawa et al. suggested the feasibility of the novel lymphocyte-to-CRP ratio (LCR) as a prognostic biomarker for predicting oncological outcomes in patients with colorectal cancer [15]. However, no study has shown the usefulness of the LCR for large groups of patients with esophageal cancer. Therefore, the aim of the current study was to investigate the relationship between the LCR and the survival of patients with esophageal cancer, compared to traditional inflammation-based markers.
Methods
Patients
This study enrolled 495 patients who underwent thoracic esophagectomy for esophageal cancer as the primary treatment at Keio University Hospital, Tokyo, Japan, between 2000 and 2019. Patients who underwent salvage esophagectomy after definitive chemoradiotherapy or macroscopic residual tumor (R2) resection were excluded. We retrospectively evaluated the patients’ clinical information, pathologic findings and prognosis obtained from hospital records. The clinical stage of the cancer was determined according to the Union Against Cancer, 8th edition [16]. This study was conducted with the approval of the Ethics Committee of Keio University School of Medicine.
Treatment
Neoadjuvant treatment has been the standard treatments in patients with clinical node-positive or T2-4 cancer without distant metastases since 2007. Neoadjuvant therapy comprised 5-fluorouracil (5-FU) and cisplatin, 5-FU and cisplatin combined with docetaxel, or 5-FU and cisplatin combined with a radiation dose of 40–50 Gy. We performed thoracotomy with right thoracic incision, and video-assisted thoracic surgery (VATS) was performed in the hybrid position combining the left decubitus and prone positions [17]. Robot-assisted thoracic surgery (RATS) was performed in the prone position since 2018. The Clavien–Dindo classification was used to assess postoperative complications [18].
Laboratory Measurements
Preoperative routine laboratory tests of the samples obtained in the 7 days prior to the surgery were performed, with or without neoadjuvant treatment. To confirm the absence of infection and inflammation in patients at the time of measurement, the patients’ physical statuses were examined for general signs of infection on the same day. Imaging modalities, including computed tomography, were performed during the 1–2 weeks prior to surgery.
A total of five combinations were calculated as follows:
LCR: Lymphocyte count (number/μL)/CRP (mg/dL).
NLR: Neutrophil count (number/μL)/lymphocyte count (number/μL).
PLR: Platelet count (number/μL)/lymphocyte count (number/μL).
CAR: CRP (mg/dL)/ALB (g/dL).
LMR: Lymphocyte count (number/μL)/monocyte count (number/μL).
Statistical Analysis
Time-dependent receiver operating characteristic (ROC) curve analysis was used to validate the model performance for the abovementioned five inflammatory markers[19]. The optimal cutoff values of markers for the prediction of death were determined with the Youden index. Moreover, two area under the ROC curve values were compared using the DeLong test. In terms of the univariate analysis of background characteristics and risk factors for short-term outcomes, categorical variables were analyzed using the Chi-square test and continuous variables were analyzed using the Mann–Whitney U test to test for differences between two groups and one-way analysis of variance (one-way ANOVA) to test for differences among three groups. Prognoses were investigated using the Kaplan–Meier method and log-rank tests. Moreover, variables with p-values < 0.05 in the univariate analysis were entered into a Cox hazard regression model for multivariate analysis. Overall survival (OS) was calculated from the surgery date until death, and recurrence-free survival (RFS) was calculated from the surgery date until recurrence or death from any cause. Statistical analyses were performed using Stata/IC 16 for Mac (StataCorp, TX, USA) and R, version 3.1.2, using the packages “timeROC” and “survival” (R Foundation Statistical Computing, Vienna, Austria).
Results
Patient Characteristics, Relationship between Preoperative LCR and TNM Stage
The patient population comprised 421 males (85%) and 74 females (15%), with a median age of 65 years (range 34–82). The median follow-up duration for the surviving patients without recurrence was 51 months. Neoadjuvant treatment was carried out in 256 patients (52%). The thoracoscopic approach included VATS and RATS and was performed on 400 patients (81%), while 95 patients (19%) underwent thoracotomy. Other clinicopathologic characteristics of the study patients are shown in Table 1.
LCR Value Predicts Oncological Outcomes with Comparison of other Inflammatory Markers
In the study cohort, the 1-year, 2-year, 3-year and 5-year OS were 89.3%, 79.4%, 72.6% and 65.3%, respectively, and the 1-year, 2-year, 3-year and 5-year RFS were 73.0%, 62.1%, 57.5% and 55.8%, respectively. A total of 180 patients (36.4%) developed recurrence and 159 patients (32.1%) died. To compare the accuracy of inflammatory markers for predicting OS, we performed ROC curve analysis of LCR, CAR, LMR, NLR and PLR. Our results demonstrated that the LCR had the highest AUC value (0.675) compared to the others (Fig. 1). For predicting RFS, the AUC of the LCR was also superior to the other parameters tested (0.670). The time-dependent ROC curve of the LCR was continuously superior to that of the other markers 30 months postoperatively. After 30 months, the AUC value of the LCR was similar to that of the CAR (Fig. 2). Using the DeLong test, the AUC value of the LCR on OS was significantly higher than that of the NLR and PLR at 3, 4 and 5 years after surgery and was also significantly higher than that of the LMR at 4 years and 5 years after surgery (Supplemental Table 1).
Comparison of the AUC between the LCR and other systemic inflammatory markers for overall survival (OS). LCR: lymphocyte-to-C-reactive protein ratio, c: clinical, LMR: lymphocyte-to-monocyte ratio, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, CAR: C-reactive protein-to-albumin ratio
Optimal LCR Cutoff Value for Predicting Survival
We set the optimal cutoff value of LCR for OS using the Youden index. The best cutoff value was 19,000, and under this cutoff value, the sensitivity and specificity were 67.3% and 58.8%, respectively.
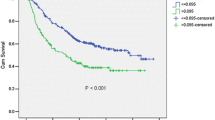
Low LCR was Associated with Poor Oncological Outcomes
According to cutoff value of LCR, we divided all patients into two groups: low LCR and high LCR group. The group of 272 patients with a LCR ≧ 19,000 (high LCR group) had a significantly higher OS and RFS than the group of 223 patients with a LCR < 19,000 (low LCR group) (3-year OS, former 80.4% vs. latter 62.4%; p < 0.001 and 3-year RFS, former 67.4% vs. latter 45.0%; p < 0.001) (Fig. 3). Of all 180 patients with recurrence, the low LCR group had a higher recurrence rate (46.2%) than the high LCR group (28.3%, p < 0.001). In terms of pathological findings, the high LCR group had a significantly higher OS than the low LCR group in patients with pStage II or higher (p = 0.002) as well as in patients with pStage I or lower (p < 0.001). Similar results were observed in patients with and without neoadjuvant treatment. The high LCR group had a significantly higher OS than the low LCR group without neoadjuvant (p < 0.001) or with neoadjuvant (p = 0.002) treatment.
In the univariate analyses, elderly patients (age ≧ 65 years), cStage III/IV (vs. cStage I/II), thoracotomy (vs. thoracoscopy), amount of bleeding (≧ 180 ml), residual cancer and low LCR (< 19,000) were identified as significant risk factors for death. In the multivariate analysis, low LCR was identified as a significant independent predictor of death (hazard ratio [HR], 2.24; 95%CI, 1.61–3.12; p < 0.001) (Table 2). The LCR was also identified as a significant predictor of recurrence (HR, 1.97; 95%CI, 1.48–2.63; p < 0.001) (Table 3).
Preoperative LCR was not Associated with Postoperative Complications
Postoperative complications were observed in 338 patients (68.3%); of whom, 140 patients (28.3%) had complications greater than Clavien–Dindo grade 2. Recurrent laryngeal nerve palsy [107/495 patients (21.6%)] was the most commonly observed complication after esophagectomy, followed by pneumonia [83/495 patients (16.8%)], anastomotic leakage [74/495 patients (15.0%)] and atrial fibrillation [36/495 patients (7.3%)]. Preoperative LCR was not associated with postoperative complications and complications greater than Clavien–Dindo grade 2 (Supplemental Table 2).
Discussion
The present study demonstrated that the LCR is novel indicator of oncological outcomes compared to other inflammatory markers in patients who underwent esophagectomy for esophageal cancer. We also determined the optimal cutoff value for the prediction of survival, which will make the clinical use of the LCR clinical easier and also facilitate improved decision making.
Although several institutions have reported the usefulness of inflammation-based markers of the systemic inflammatory response as predictors of survival for solid cancers, including esophageal cancer, few studies have shown the feasibility of this new prognostic marker. In particular, with regard to colorectal cancer, Okugawa et al. showed the reliability of the LCR as a prognostic biomarker for predicting long-term survival [15]. Suzuki et al. indicated that the LCR is a useful predictive factor for oncological outcomes in patients with Stage II or III colon cancer [20], while the LCR was also reported as novel prognostic marker in gastric cancer [21, 22]. Furthermore, Yamamoto et al. recently reported the usefulness of LCR in patients with esophageal cancer [23]; however, the study was analyzed with relatively small number of cases, i.e., 153 patients with esophageal cancer. Our present study included approximately 500 patients with esophageal cancer and analyzed the relationship among multiple inflammation-based markers, such as LMR, PLR and CAR, which were not included in the study reported by Yamamoto et al.
As reported in our previous studies, proinflammatory cytokines, such as interleukin (IL)- 6 or IL-8, might correlate with cancer progression [24, 25]. CRP is widely known to be induced by IL-6 during the synthesis of acute-phase proteins. Lymphocytes are responsible for the host immune response, and a decrease in lymphocytes in host tumors is associated with a poor prognosis[26,27,28]. Therefore, the LCR may represent malignant status by reflecting both the anti-tumor immune status and the tumor-promoting environment. Moreover, like other conventional markers, such as the Glasgow Prognostic Score reported by McMillan, LCR may be related to cachexia, which is defined as a multifactorial syndrome involving loss of bodyweight or skeletal muscle mass [29,30,31]. This may also be one of the reasons for decreased survival. Early multimodal intervention including nutritional support should be especially considered for patients with low LCR.
Standard treatment for Stage II/III esophageal cancer is neoadjuvant followed by surgery, while upfront surgery is standard for Stage I esophageal cancer [32]. However, in some cases, the disease may be highly malignant and may recur early postoperatively [33]. Based on the LCR value, performing intensive adjuvant after surgery for patients with pathological Stage II or higher or performing neoadjuvant for Stage I may be an option. LCR can help to facilitate personalized multidisciplinary treatments for the patients with esophageal cancer.
This study has limitations. First, it was a retrospective, single-center study limited to a Japanese population, which could have introduced an element of selection bias. Moreover, since the time span (20 years) was long, there were changes in the management of postoperative complications and different surgical approaches during this time period. However, we recruited a large group, including approximately 500 patients, to overcome this bias. Second, we calculated the LCR in the 7 days before surgery, even in patients with neoadjuvant treatment. Wu et al. indicated change the amount of NLR and PLR predicted chemotherapy response in colorectal cancer [34]. Therefore, if the LCR indicates tumor characteristics, the amount of change in the LCR from pre- to post-neoadjuvant may reflect the response to chemotherapy. Further study of the ability of the LCR to predict response to chemotherapy is necessary.
In conclusion, the present study demonstrated that the LCR is a novel indicator for oncological outcomes for patients who undergo esophagectomy for esophageal cancer. LCR can help to facilitate personalized multidisciplinary treatments such as neoadjuvant for early esophageal cancer, or intensive adjuvant after surgery for advanced esophageal cancer.
References
Akutsu Y, Kato K, Igaki H et al (2016) The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: From the results of JCOG0502, a prospective multicenter study. Ann Surg 264:1009–1015
Aoyama J, Kawakubo H, Mayanagi S et al (2019) Discrepancy between the clinical and final pathological findings of lymph node metastasis in superficial esophageal cancer. Ann Surg Oncol 26:2874–2881
DeNardo DG, Johansson M, Coussens LM (2008) Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev 27:11–18
Matsuda S, Takeuchi H, Kawakubo H et al (2015) Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: Comparison with the Glasgow prognostic score. Ann Surg Oncol 22:302–310
Matsuda S, Takeuchi H, Kawakubo H et al (2021) Validation study of fibrinogen and albumin score in esophageal cancer patients who underwent esophagectomy: Multicenter prospective cohort study. Ann Surg Oncol 28:774–784
Takeuchi M, Takeuchi H, Kawakubo H et al (2018) Perioperative risk calculator predicts long-term oncologic outcome for patients with esophageal carcinoma. Ann Surg Oncol 25:837–843
Sato H, Tsubosa Y, Kawano T (2012) Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 36:617–622
Walsh SR, Cook EJ, Goulder F et al (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91:181–184
Hirahara N, Matsubara T, Mizota Y et al (2016) Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg 16:1–12
Chan JCY, Chan DL, Diakos CI et al (2017) The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg 265:539–546
Feng JF, Huang Y, Chen QX (2014) Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 12:1–6
Yuk HD, Hwang EC, Park JY et al (2020) The number of metabolic features as a significant prognostic factor in patients with metastatic renal cell carcinoma. Sci Rep 10:475–481
Wei XL, Wang FH, Zhang DS et al (2015) A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer 15:1–11
Liu Z, Jin K, Guo M et al (2017) Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 24:561–568
Okugawa Y, Toiyama Y, Yamamoto A et al (2020) Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg 272:342–351
Rice TW, Patil DT, Blackstone EH (2017) 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann Cardiothorac Surg 6:119–130
Kaburagi T, Takeuchi H, Kawakubo H et al (2014) Clinical utility of a novel hybrid position combining the left lateral decubitus and prone positions during thoracoscopic esophagectomy. World J Surg 38:410–418
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Takeuchi M, Kawakubo H, Mayanagi S et al (2019) Perioperative risk calculator for distal gastrectomy predicts overall survival in patients with gastric cancer. Gastric Cancer 22:624–631
Suzuki S, Akiyoshi T, Oba K et al (2020) Comprehensive comparative analysis of prognostic value of systemic inflammatory biomarkers for patients with stage II/III colon cancer. Ann Surg Oncol 27:844–852
Okugawa Y, Toiyama Y, Yamamoto A et al (2020) Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr 39:1209–1217
Cheng C, Zhang Q, Zhuang LP, Sun J (2020) Prognostic value of lymphocyte-to-C-reactive protein ratio in patients with gastric cancer after surgery: A multicentre study. Jpn J Clin Oncol 50:1141–1149
Yamamoto A, Toiyama Y, Okugawa Y et al (2021) Clinical implications of the preoperative lymphocyte C-reactive protein ratio in esophageal cancer patients. Surg Today 51:745–755. https://doi.org/10.1007/s00595-020-02166-5
Okamura A, Takeuchi H, Matsuda S et al (2015) Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 22:3130–3135
Ogura M, Takeuchi H, Kawakubo H et al (2013) Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery 154:512–520
Lin EY, Pollard JW (2004) Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer 90:2053–2058
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation and cancer. Cell 140:883–899
Huber C, Bobek N, Kuball J et al (2005) Inhibitors of apoptosis confer resistance to tumour suppression by adoptively transplanted cytotoxic T-lymphocytes in vitro and in vivo. Cell Death Differ 12:317–325
Forrest LM, McMillan DC, McArdle CS et al (2003) Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable nonsmall- cell lung cancer. Br J Cancer 89:1028–1030
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev 39:534–540
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 12:489–495
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 16:1–24
Kato H, Fukuchi M, Miyazaki T et al (2005) Classification of recurrent esophageal cancer after radical esophagectomy with two- or three-field lymphadenectomy. Anticancer Res 25:3461–3467
Wu Y, Li C, Zhao J et al (2016) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol 14:1–8
Acknowledgements
We wish to thank Kumiko Motooka, a staff member at the Department of Surgery in Keio University School of Medicine, for her help with the preparation of this manuscript.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y.K. received lecture fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd, Asahi Kasei Pharma Corporation, Otsuka Pharmaceutical Factory Inc., Shionogi & Co., Ltd., Nippon Covidien Inc., Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb K.K. Y.K. was supported by grants from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd, Yakult Honsha Co. Ltd., Asahi KASEI Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Tsumura & CO., Kyouwa Hakkou Kirin Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., EA Pharma Co., Ltd., Astellas Pharma Inc., Toyama Chemical Co., Ltd., Medicon Inc., Kaken Pharmaceutical Co. Ltd., Eisai Co., Ltd., Otsuka Pharmaceutical Factory Inc., Teijin Pharma Limited., Nihon Pharmaceutical Co., Ltd. and Nippon Covidien Inc. Y.K. also holds an endowed chair provided by Chugai Pharmaceutical Co., Ltd. and Taiho Pharmaceutical Co., Ltd., outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Kawakubo, H., Hoshino, S. et al. Lymphocyte-to-C-Reactive Protein Ratio as a Novel Marker for Predicting Oncological Outcomes in Patients with Esophageal Cancer. World J Surg 45, 3370–3377 (2021). https://doi.org/10.1007/s00268-021-06269-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06269-z