Abstract
Background
Anatomical resection (AR) for colorectal liver metastasis (CLM) is disputable. We investigated the impact of AR on short-term outcomes and survival in CLM patients.
Methods
Patients having hepatectomy with AR or nonanatomical resection (NAR) for CLM were reviewed. Comparison was made between AR and NAR groups. Group comparison was performed again after propensity score matching with ratio 1:1.
Results
AR group (n = 234 vs n = 89 in NAR group) had higher carcinoembryonic antigen level (20 vs 7.8 ng/mL, p ≤ 0.001), more blood loss (0.65 vs 0.2 L, p < 0.001), more transfusions (19.2% vs 3.4%, p = 0.001), longer operation (339.5 vs 180 min, p < 0.001), longer hospital stay (9 vs 6 days, p < 0.001), more tumors (p < 0.001), larger tumors (4 vs 2 cm, p < 0.001), more bilobar involvement (20.9% vs 7.9%, p = 0.006), and comparable survival (overall, p = 0.721; disease-free, p = 0.695). After propensity score matching, each group had 70 patients, with matched tumor number, tumor size, liver function, and tumor marker. AR group had more open resections (85.7% vs 68.6%, p = 0.016), more blood loss (0.556 vs 0.3 L, p = 0.001), more transfusions (17.1% vs 4.3%, p = 0.015), longer operation (310 vs 180 min, p < 0.001), longer hospital stay (8.5 vs 6 days, p = 0.002), comparable overall survival (p = 0.819), and comparable disease-free survival (p = 0.855).
Conclusion
Similar disease-free survival and overall survival of CLM patients were seen with the use of AR and NAR. However, AR may entail a more eventful postoperative course. NAR with margin should be considered whenever feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatectomy is potentially curative for patients with resectable colorectal liver metastasis (CLM), with a 5-year survival rate up to 58% [1,2,3]. There are many factors that affect long-term survival, including node-positive primary disease, carcinoembryonic antigen level, extrahepatic disease, poor tumor grade, the presence of more than one liver metastasis, tumor diameter>3 cm, and positive resection margin [4]. Factors that can be altered by surgical technique should be surgeons’ top concern, and therefore, anatomical resection (AR) and nonanatomical resection (NAR) for the removal of CLM have been studied. NAR has been advocated to reduce postoperative morbidity and mortality. And it could reduce the chance of liver failure [5]. This is particularly important in the presence of liver damage (such as hepatic sinusoidal obstruction, periportal inflammation, and steatohepatitis [6]) after the use of neoadjuvant chemotherapy. On the other hand, the intraoperative results [7,8,9,10] and postoperative outcomes of AR might be more detrimental than those of NAR [10] as the surgical stress induced by major resection is usually high. Furthermore, preserving more liver for future re-intervention [11,12,13] has been proven to have a survival benefit due to the high intrahepatic recurrence rate.
The primary objective of this study was to investigate the impact of AR on the short-term outcome and survival (overall and disease-free) in patients with CLM.
Methods
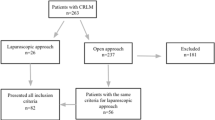
This retrospective study needed no institutional review committee approval since treatments given to patients were not influenced by the study. We reviewed the data of patients who underwent hepatectomy for CLM at our hospital from January 1990 to January 2017. All these patients had no extrahepatic disease. Patients who received treatment other than resection (e.g., radiofrequency ablation in addition to resection) were excluded. The decisions on treatment modality and operative procedure were based on tumor size, location, and number. All decisions were concluded by a multidisciplinary team of surgeons and oncologists.
Diagnosis of CLM
The patients were diagnosed with colorectal carcinoma by colonoscopy with biopsy proven. Computed tomography or magnetic resonance imaging was performed to check for liver metastasis. Liver biopsy was not done routinely.
Preoperative assessment
Biochemical and radiological assessments were done before hepatectomy. Complete blood count, clotting profile, and liver and renal function were routinely checked. Hepatitis serology, carcinoembryonic antigen, and Child–Pugh score were documented. Most patients received indocyanine green clearance test. Indocyanine green retention rate of 18% at 15 min after injection was the cutoff level for major hepatectomy, and 22% was the cutoff level for minor hepatectomy [14]. The test might not be given to patients who did not have cirrhosis or abnormal liver function. To define the liver anatomy and to check for systemic involvement, computed tomography or magnetic resonance imaging with or without 18-F 2-fluoro-2-deoxy-d-glucose positron emitted tomography was performed. A computed tomographic volumetric study was required for major hepatectomy (resection of ≥3 Couinaud segments). The minimum ratio of future liver remnant volume to estimated standard liver volume was 30% for normal livers [15]. In the case of marginal liver volume, portal vein embolization or ALPPS was considered on an individual basis.
Hospital mortality was defined as death happening after the operation and before discharge from hospital. Operative mortality was defined as death happening within 90 days of the operation. Postoperative complications were graded according to the Clavien–Dindo classification [16]. The UICC TNM classification, 7th edition, was used for cancer staging [17].
Surgical technique
All open and laparoscopic hepatectomies were performed by the same team of liver surgeons led by at least one consultant specialist. The laparoscopic method was the choice whenever possible.
In open hepatectomy, a right subcostal incision with a sternal extension was usually used. Ultrasonic examination was performed to confirm the tumor location, size, and number. AR was preferred to NAR most of the time, and a 1-cm resection margin, if possible, was the goal. Transection of the liver parenchyma was performed along the demarcation line that appeared after occlusion of the Glissonean pedicle confined to the segment to be resected. In NAR, we marked the line for transection with the aid of ultrasound. A Cavitron ultrasonic surgical aspirator was used for parenchymal transection. During transection, the central venous pressure was kept low (<5 mmHg). Abdominal drain was not routinely used.
In laparoscopic hepatectomy, the French position was adopted. Usually, 2–3 working ports were used for minor hepatectomy and 4–5 ports were used for major hepatectomy. Ultrasonography was performed. An ultrasonic dissector was used for superficial parenchymal transection, whereas a Cavitron ultrasonic surgical aspirator was used for deep transection. For major hepatectomy, the dissection was similar to the open approach. The intra-Glissonian approach was performed for individual isolation of the inflow. The hepatic artery was controlled by metal clips, and the main branch of the portal vein was controlled by Hemolock clips. An endovascular stapler or Hemolock was used to control the main bile duct. A stapler was used to control the major hepatic vein. The liver specimen was delivered through a subumbilical or Pfannenstiel incision. Drainage tube was not routinely placed [18, 19].
For AR, the Brisbane 2000 Terminology of Liver Anatomy and Resections was applied [20]. NAR was performed for small tumors located peripherally. Major resection was performed for centrally located tumors. For tumors that were close to the major vessel (i.e., main pedicles), AR was preferred. With AR, the liver segment supplied by the vasculature would be removed. The aim was to remove the tumorous segment while minimizing the area of ischemia in the liver remnant. For tumors that were close to the major hepatic veins, AR would be more feasible as these lesions would more likely be deeply seated lesions. A 1-cm gross margin was aimed for. A positive resection margin was defined as the presence of tumor cells at the line of transection due to microscopic involvement by the tumor, venous permeation, or microsatellite nodules.
Histological measurement
We used standardized histological assessment method and reporting format, which featured the number of tumor, size of the largest tumor, and numerical report of surgical margin width in tenths of a millimeter. The final margin width was judged as the distance of the lesion closest to the transected surface. In the case of multiple tumors, the closet margin was recorded as final margin.
Chemotherapy
Patients who had high-risk factors for colorectal cancer alone were subjected to adjuvant chemotherapy. Fluorouracil was used before 2004. Since 2004, FOLFOX (folinic acid, fluorouracil and oxaliplatin) was adopted. Furthermore, XELOX (capecitabine and oxaliplatin) was started since 2010. In the presence of KRAS mutation, bevacizumab was added to FOLFOX or XELOX. For patients who had wild-type KRAS, the choice of chemotherapy was FOLFOX or XELOX and cetuximab. All patients were considered for postoperative adjuvant chemotherapy unless they had undergone chemotherapy adjuvant to bowel resection within the previous 12 months, according to the unit protocol. Chemotherapy was offered to patients whose tumors were considered technically unresectable as an attempt to downstage the disease.
Follow-up and recurrence
All patients were followed up at our outpatient clinic within 2 weeks after discharge home and then every 3 months in the first year. Afterwards, they would be seen every 6 months. Blood tests (for liver function and carcinoembryonic antigen level checks, etc.) were taken before every follow-up visit, and cross-sectional imaging was arranged half-yearly or more frequently. Disease recurrence was declared if contrast computed tomography showed features of local recurrence or distant metastasis.
Statistics
Continuous variables were described as median with range included in brackets. The Chi squared test or Fisher’s exact test, where appropriate, was used to analyze categorical variables. The Mann–Whitney U test or the t test, where appropriate, was used to analyze parametric variables. In the subgroup analysis, liver function, tumor marker, tumor number, and tumor size were matched in a 1:1 ratio so that they were the same in both groups. Disease-free survival and overall survival were calculated from the day of discharge to the day of disease recurrence or census, that is, death or last follow-up visit, using the Kaplan–Meier method. The log-rank test was used for survival comparison between groups. p value <0.05 was considered statistically significant. The software SPSS, version 20, was used for all statistical analyses.
Results
In the study period, 323 patients received hepatectomy for CLM; 234 patients underwent AR and 89 patients underwent NAR. These two groups of patients were comparable in terms of age (AR 60.5 years; NAR 62.0 years old; p = 0.275) and sex (AR 143 men and 91 women; NAR 58 men and 31 women; p = 0.502). More patients suffered from respiratory disease in the NAR group (14.6% vs 6.8%, p = 0.029). Patients in the AR group had higher creatinine level (79 vs 76 μmol/L, p = 0.032), aspartate transaminase level (27 vs 24 U/L, p = 0.009), and carcinoembryonic antigen level (20 vs 7.8 ng/mL, p ≤ 0.001). All patients in the NAR group received minor resection. Intraoperatively, the NAR group had less blood loss (0.2 vs 0.65 L, p < 0.001), less blood transfusion (3.4% vs 19.2%, p = 0.001), shorter operation duration (180 vs 339.5 min, p < 0.001), and shorter hospital stay (6 vs 9 days, p < 0.001). Pathological examination revealed that the AR group had more tumor nodules (p < 0.001), larger tumor size (4 vs 2 cm, p < 0.001), and more bilobar involvement (20.9% vs 7.9%, p = 0.006). The two groups were comparable in terms of resection margin involvement (AR 6.7%; NAR 8.2%; p = 0.788), microvascular invasion (AR 21.7%; NAR 18.3%; p = <0.001), time to disease recurrence (AR 14.2 months; NAR 12.7 months; p = 0.507), and pattern of recurrence. Although patients in the AR group had more and larger tumors, they did not have inferior survival. This group had a median of 47.0 months of overall survival and a 41.6% 5-year overall survival rate. Correspondingly, the NAR group had a median of 47.4 months and a 5-year rate of 41.2% (p = 0.721). When it comes to disease-free survival, the AR group had a median of 14.5 months and a 5-year rate of 25.3%. Correspondingly, the NAR group had a median of 15.6 months and a 5-year rate of 26.5% (p = 0.695).
Further analysis was performed to match the patients’ tumor characteristics for better comparison, including aspartate transaminase level, tumor size, and tumor number. Propensity score matching was done with a ratio of 1:1, resulting in 70 patients in each group. Table 1 is a group comparison of perioperative and histopathological characteristics after propensity score matching. After matching, the two groups were comparable in demographic characteristics, comorbidity, preoperative liver function, and carcinoembryonic antigen level (AR 8.8 ng/mL; NAR 8.9 ng/mL; p = 0.729). All patients in the NAR group and 45.7% of the patients in the AR group underwent minor resection (p < 0.001). More patients in the AR group received open resection (85.7% vs 68.6% in the NAR group, p = 0.016). The NAR group had less intraoperative blood loss (0.3 vs 0.556 L, p = 0.001), less blood transfusion (4.3% vs 17.1%, p = 0.015), shorter operation time (180 vs 309.5 min, p < 0.001), and shorter hospital stay (6 vs 8.5 days, p = 0.002). The overall complication rate was similar (AR 15.7%; NAR 11.4%; p = 0.622). The groups had matched tumor number (p = 0.226) and tumor size (p = 0.927) and were comparable in terms of resection margin involvement (AR 5.8%; NAR 8.6%; p = 0.404) and follow-up period (AR 35.1 months; NAR 39.8 months; p = 0.902). However, more patients in the AR group had microvascular invasion (AR 28.3%; NAR 21.9%; p = 0.002). This group had a median overall survival of 41.0 months and a 45.0% 5-year overall survival rate. Correspondingly, the NAR group had a median overall survival of 47.4 months and a 5-year rate of 44.1% (p = 0.819) (Fig. 1). When it comes to disease-free survival, the AR group had a median of 17.9 months and a 5-year rate of 22.6%. Correspondingly, the NAR group had a median of 16.7 months and a 5-year rate of 31.0% (p = 0.855) (Fig. 2).
Discussion
AR refers to en bloc removal of a liver portion supplied by a major branch of the portal vein and hepatic artery. Each segment of the liver is an independent functional unit, isolated from other segments. AR has been proposed to improve survival in hepatocellular carcinoma resection [21,22,23,24,25]. In hepatocellular carcinoma resection, AR aims to decrease chances of intrahepatic metastasis and local regional recurrence by clearance of microvascular invasion arising from the tumor-bearing portal branches and the corresponding liver parenchyma. However, in contrast to hepatocellular carcinoma, CLM develops from blood-borne tumor cells circulating throughout the systemic circulation. The idea of AR for CLM might not be applicable, as previous studies showed contradictory results [5, 7, 9, 10, 26,27,28]. A meta-analysis reported that NAR for CLM did not compromise oncological outcomes and AR and NAR showed no difference in terms of postoperative morbidity and mortality [29]. Another study recommended complete macroscopic removal of all lesions with negative resection margins, irrespective of width [30].
Given that our overall results showed no differences in overall survival and disease-free survival between groups, it seems that NAR would be favorable in view of the better operative outcomes and short-term recovery. However, the AR group in fact had more and larger liver metastases. It has been reported that tumor size and tumor number in CLM are adverse prognostic factors for long-term survivors [31,32,33,34,35]. Despite the poorer tumor biology, the AR group had overall survival and disease-free survival similar to the NAR group (p = 0.784 and 0.612, respectively), which could be explained by the more radical hepatectomy in the AR group. After factors biased by liver function (aspartate transaminase level, tumor size, and tumor number) were eliminated by propensity score matching, comparable survival outcomes were seen in the two groups.
After all, the decision to offer AR or NAR to the patients depended very much on tumor location. NAR was usually used to treat peripherally located tumors. For tumors close to the major vessel or pedicle, AR would be an easier and feasible option, as seen in our results. Blood loss and blood transfusion requirement were both lower in the NAR group. Hepatic lobectomy, which is one of the common forms of AR, was a risk factor for perioperative transfusion, while transfusion itself was associated with coagulopathy, allergic reactions, tumor promoting action [36], greater in-hospital mortality, complication, and longer hospital stay. This was further confirmed by a recent meta-analysis by Lyu et al. [37]. The study showed the association between perioperative blood transfusion in CLM resection and various adverse outcomes, including overall morbidity, major complications, mortality, and length of hospital stay. Specifically, more cases of postoperative infection and liver failure were seen [38, 39]. In addition to the more extensive resection in the AR group, blood loss and blood transfusion in this group could be factors contributing to the relatively inferior postoperative outcomes.
Tumor cells of CLM are cells derived from the primary lesion that have traveled into the liver [40], and hence, these cancer cells are likely delivered evenly. As such, there is the opinion that AR is better for treating CLM. However, the results from our patients suggested that, given similar tumor size and similar tumor number, NAR could achieve survival similar to AR, while AR might result in a more eventful postoperative course. For peripherally located lesions, NAR would also allow preservation of a larger amount of liver parenchyma for possible hepatectomy in case of local recurrence of disease [7, 10]. It is believed that minimal scarification of the liver parenchyma decreases surgical stress and operative risk, thereby reducing postoperative morbidity and mortality [29]. Unfortunately, both AR and NAR did not confer any survival benefit. Since some of our patients (30.8% in the AR group and 22.9% in the NAR group) would receive further resection for intrahepatic recurrence, NAR, if feasible, would have been more suitable.
Only a minority (< 10%) of the recurrences were solely intrahepatic, suggesting that more effective means of systemic chemotherapy or target therapy are needed. Chemotherapy has been shown to improve the disease-free and progression-free survival of CLM patients [41]. In this study, the proportion of patients who underwent chemotherapy (before or after surgery) was similar between groups, which points to the importance of more radical resection in improving disease-free survival. We opted for upfront hepatectomy if possible and subsequent adjuvant chemotherapy. For patients who had undergone neoadjuvant chemotherapy, we allowed a 4-week chemotherapy-free period to let the liver recover before hepatectomy in order to reduce surgical morbidity.
Synchronous disease and metachronous disease have different tumor behaviors. Patient survival after resection of metachronous lesions has been shown to be better than that of synchronous lesions [42]. The difference is likely due to the fact that metachronous lesions occur later in the course of disease progression [43, 44] and that synchronous lesions may represent a more disseminated disease [45]. Synchronous liver metastasis might have a poorer tumor biology [46]. However, it would be controversial to compare the postoperative survival outcomes of synchronous metastasis and metachronous metastasis as it is a general practice to offer neoadjuvant chemotherapy to patients with liver metastasis before they receive resection [46, 47]. Our study had similar proportions of synchronous disease and metachronous disease, and thus, the difference in their tumor biology was minimum, rendering the effect of surgery more prominent.
Laparoscopic surgery has been gaining popularity, and laparoscopic hepatectomy (both major and minor resections) has been proven to improve short-term outcomes, including shortened hospital stay and reduced morbidity [48,49,50,51,52]. The fact that our patients in the NAR group had more laparoscopic wedge resections, apart from that the AR group had more extensive resection, could also be a contributing factor to the better operative and postoperative outcomes. Laparoscopic monosegmentectomy using the Glissonean pedicle approach is now a valid option [53]. However, tumors in difficult locations may make some laparoscopic resections difficult—for example, anatomical segmentectomy for posteriosuperior lesions would be difficult due to poor access and visualization [54, 55]. These cases would call for specific techniques or patient positions [54, 56] or the placement of additional trocars [55]. According to a meta-analysis of AR versus NAR for CLM, NAR can reduce blood loss, postoperative morbidity, and hospital stay [29], and it is hoped that laparoscopy can further reduce surgical stress and improve short-term outcomes of CLM resection [57].
The retrospective nature of this study has given rise to its major limitation. The possibility of selection bias, missing data, and treatment heterogeneity throughout the years could not be completely avoided. In addition, due to the dilution of number of patients in subgroup analysis using propensity score matching, it would be difficult to further subdivide the patients into synchronous and metachronous metastasis groups, although such disease nature might have an impact on the results of the study. Furthermore, the effect of neoadjuvant and adjuvant chemotherapies, which could potentially influence survival, was not investigated in the current study. A single-center series also limits interobserver variability and treatment heterogeneity in terms of perioperative management and operative technique. While it would be most ideal to carry out a randomized controlled trial on this topic, this retrospective study with a reasonable sample size can still shed some light on the surgical approach in CLM management.
Conclusion
Similar disease-free survival and overall survival of CLM patients were seen with the use of AR and NAR. However, AR may entail a more eventful postoperative course. NAR with margin should be considered whenever feasible.
References
Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T et al (2000) Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg 231(4):487–499
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239(6):818–825 (discussion 25–27)
Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C et al (2005) Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241(5):715–722 (discussion 22–24)
Spelt L, Andersson B, Nilsson J, Andersson R (2012) Prognostic models for outcome following liver resection for colorectal cancer metastases: a systematic review. Eur J Surg Oncol 38(1):16–24
Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK (2007) Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Brit J Surg 94(3):274–286
Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24(13):2065–2072
Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, Joanne IJ, Verhoef C (2011) Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 35(3):656–661. https://doi.org/10.1007/s00268-010-0890-9
Stewart GD, O’Suilleabhain CB, Madhavan KK, Wigmore SJ, Parks RW, Garden OJ (2004) The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur J Surg Oncol 30(4):370–376
Sarpel U, Bonavia AS, Grucela A, Roayaie S, Schwartz ME, Labow DM (2009) Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann Surg Oncol 16(2):379–384
Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M et al (2001) Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg 181(2):153–159
Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Moriya Y, Sugihara K (1999) Repeat liver resection for recurrent colorectal liver metastases. Am J Surg 178(4):275–281
Shaw IM, Rees M, Welsh FK, Bygrave S, John TG (2006) Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable long-term survival. Brit J Surg 93(4):457–464
Petrowsky H, Gonen M, Jarnagin W, Lorenz M, DeMatteo R, Heinrich S et al (2002) Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg 235(6):863–871
Fan ST (2010) Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Surg 17(4):380–384
Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y et al (2006) Estimating liver weight of adults by body weight and gender. World J Gastroenterol 12(14):2217–2222
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A (eds) (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Cheung TT, Poon RT, Dai WC, Chok KS, Chan SC, Lo CM (2016) Pure laparoscopic versus open left lateral sectionectomy for hepatocellular carcinoma: a single-center experience. World J Surg 40(1):198–205. https://doi.org/10.1007/s00268-015-3237-8
Cheung TT, Poon RT, Yuen WK, Chok KS, Tsang SH, Yau T et al (2013) Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ J Surg 83(11):847–852
Terminology Committee of the IHPBA (2000) Terminology of liver anatomy and resections. HPB Surg 2:333–339
Ueno S, Kubo F, Sakoda M, Hiwatashi K, Tateno T, Mataki Y et al (2008) Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg 15(5):493–500
Wakai T, Shirai Y, Sakata J, Kaneko K, Cruz PV, Akazawa K et al (2007) Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma. Ann Surg Oncol 14(4):1356–1365
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M et al (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242(2):252–259
Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y et al (2011) Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci 56(6):1626–1633
Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD (2012) A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol 19(12):3697–3705
DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y (2000) Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg 4(2):178–184
Finch RJ, Malik HZ, Hamady ZZ, Al-Mukhtar A, Adair R, Prasad KR et al (2007) Effect of type of resection on outcome of hepatic resection for colorectal metastases. Brit J Surg 94(10):1242–1248
Guzzetti E, Pulitano C, Catena M, Arru M, Ratti F, Finazzi R et al (2008) Impact of type of liver resection on the outcome of colorectal liver metastases: a case-matched analysis. J Surg Oncol 97(6):503–507
Tang H, Li B, Zhang H, Dong J, Lu W (2016) Comparison of anatomical and nonanatomical hepatectomy for colorectal liver metastasis: a meta-analysis of 5207 patients. Sci Rep 6:32304
de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R (2008) R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 248(4):626–637
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–318 (discussion 18–21)
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P et al (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 77(7):1254–1262
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247(1):125–135
Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S (2007) Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol 14(4):1336–1346
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S (2006) Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 13(5):668–676
Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F et al (2013) Perioperative blood transfusions and survival in patients with non-small cell lung cancer: a retrospective study. BMC Anesthesiol 13(1):42
Lyu X, Qiao W, Li D, Leng Y (2017) Impact of perioperative blood transfusion on clinical outcomes in patients with colorectal liver metastasis after hepatectomy: a meta-analysis. Oncotarget 8(25):41740–41748
Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP et al (2010) Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg 251(1):91–100
Laurent C, Sa Cunha A, Couderc P, Rullier E, Saric J (2003) Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Brit J Surg 90(9):1131–1136
Shirai Y, Wakai T, Ohtani T, Sakai Y, Tsukada K, Hatakeyama K (1996) Colorectal carcinoma metastases to the liver. Does primary tumor location affect its lobar distribution? Cancer 77(11):2213–2216
Ciliberto D, Prati U, Roveda L, Barbieri V, Staropoli N, Abbruzzese A et al (2012) Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep 27(6):1849–1856
Cossu ML, Ginesu GC, Feo CF, Fancellu A, Pinna A, Vargiu I et al (2017) Surgical outcomes in patients with hepatic synchronous and metachronous colorectal metastases. Ann Ital Chir 88:497–504
Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM (1984) Resection of hepatic metastases from colorectal cancer. Arch Surg 119(6):647–651
Scheele J, Altendorf-Hofmann A (1999) Resection of colorectal liver metastases. Langenbeck’s Arch Surg 384(4):313–327
Tsai MS, Su YH, Ho MC, Liang JT, Chen TP, Lai HS et al (2007) Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol 14(2):786–794
van der Pool AE, Lalmahomed ZS, Ozbay Y, de Wilt JH, Eggermont AM, Jzermans JN et al (2010) ‘Staged’ liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis 12(10 Online):e229–e235
Bockhorn M, Frilling A, Fruhauf NR, Neuhaus J, Molmenti E, Trarbach T et al (2008) Survival of patients with synchronous and metachronous colorectal liver metastases—is there a difference? J Gastrointest Surg 12(8):1399–1405
Allard MA, Cunha AS, Gayet B, Adam R, Goere D, Bachellier P et al (2015) Early and long-term oncological outcomes after laparoscopic resection for colorectal liver metastases: a propensity score-based analysis. Ann Surg 262(5):794–802
Ratti F, Catena M, Di Palo S, Staudacher C, Aldrighetti L (2015) Laparoscopic approach for primary colorectal cancer improves outcome of patients undergoing combined open hepatic resection for liver metastases. World J Surg 39(10):2573–2582. https://doi.org/10.1007/s00268-015-3127-0
Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y et al (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Surg 22(10):711–720
Fretland AA, Kazaryan AM, Bjornbeth BA, Flatmark K, Andersen MH, Tonnessen TI et al (2015) Open versus laparoscopic liver resection for colorectal liver metastases (the Oslo-CoMet Study): study protocol for a randomized controlled trial. Trial. 16:73
Hasegawa Y, Nitta H, Sasaki A, Takahara T, Itabashi H, Katagiri H et al (2015) Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: a comparative analysis of 168 consecutive cases at a single center. Surgery 157(6):1065–1072
Choi Y, Han HS, Sultan AM, Yoon YS, Cho JY (2014) Glissonean pedicle approach in laparoscopic anatomical liver resection. Hepatogastroenterology 61(136):2317–2320
Ikeda T, Toshima T, Harimoto N, Yamashita Y, Ikegami T, Yoshizumi T et al (2014) Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc 28(8):2484–2492
Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK (2014) Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Surg 21(8):E65–E68
Ishizawa T, Gumbs AA, Kokudo N, Gayet B (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256(6):959–964
Vibert E, Kouider A, Gayet B (2004) Laparoscopic anatomic liver resection. HPB 6(4):222–229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest.
Ethical approval
Institution research ethics committee approval was not needed for retrospective study of anonymous clinical data.
Informed consent
Informed consent to the study from patients was not needed as it is a review and analysis of anonymous clinical data and no individuals can be identified by the reported data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
She, W.H., Cheung, T.T., Ma, K.W. et al. Anatomical Versus Nonanatomical Resection for Colorectal Liver Metastasis. World J Surg 44, 2743–2751 (2020). https://doi.org/10.1007/s00268-020-05506-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05506-1