Abstract
Background
Liver resection represents the curative treatment of choice for patients with colorectal liver metastases (CRLM). Laparoscopic hepatectomy in CRLM is considered a safe approach. However, the information on their oncological results in the different series is deficient. This study aimed to compare the surgical margin, overall survival (OS), and disease-free survival (DFS) in patients with oncological resections of CRLM according to the type of surgical approach performed.
Methods
Between April 2007 and June 2017, 263 patients with CRLM underwent hepatic resection. Inclusion criteria were initial resectability, tumor size ≤ 50 mm, 3 or less metastases, no bilobar involvement, and absence of extrahepatic disease. A propensity score was performed to adjust the indication bias.
Results
Eighty-two patients were included (56 open and 26 laparoscopic). Twenty-eight (50%) patients had synchronous presentation in the open approach and 6 (23%) in the laparoscopic approach (p = 0.021), with more frequent simultaneous open resections (p = 0.037). The resection margin was positive (R1) in 5 patients with an open approach and 2 with a laparoscopic approach (8.9% and 7.6% respectively; p = 0.852). Nine patients (16%) with conventional approach and 2 (7.7%) with laparoscopic approach had local complications (p = 0.3). There was one death in the open group and none in the laparoscopic. There were no significant differences in OS and DFS rate between both groups (1–3 years, OS: 92–77% and 96–75% respectively; 1–3 years, DFS: 63–20% and 73–36% respectively).
Conclusions
There were no significant differences in terms of surgical margin, OS rate, and DFS rate between the laparoscopic and open approach in patients with CRLM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer remains one of the leading causes of cancer-related deaths worldwide [1]. Surgical resection represents the only chance of long-term survival. The goal of surgery should be to resect all metastases with negative histological margins while preserving sufficient functional hepatic parenchyma [2]. Currently, the possibility of improving survival in patients with CRLM is favored by advances in medical therapy, such as systemic chemotherapy, and the improvement in surgical techniques for metastatic disease [3, 4].
The laparoscopic approach is associated with a decrease in intraoperative bleeding, fewer postoperative complications, shorter hospital stay, and decrease in health care costs [5]. However, information on long-term oncological outcomes in laparoscopic hepatectomy (LH) for CRLM is limited [6,7,8,9].
A recently published randomized trial (OSLO-COMET) [10] analyzed short-term and long-term outcomes, demonstrating these advantages in oncologic liver surgery. A recent systematic review published by Haney et al. demonstrated that laparoscopic liver surgery for minor resections shows clear perioperative benefits over open liver surgery with moderate to high certainty of evidence. They also report certain limitations in the long-term results in relation to laparoscopic liver resection [11]. Moreover, the status of surgical resection margins is an additional factor that has been evaluated in many series due to its influence on long-term survival [12]. Currently, there is a little evidence about the results of laparoscopic surgical resections margins when compared to open resections.
The aim of this study is to analyze the oncologic outcomes (surgical margin, overall survival, and disease-free survival) in patients with laparoscopic or open liver resection due to CRLM.
Methods
This is a retrospective analysis of a prospectively maintained database with consecutive patients treated with hepatic resection for CRLM in our center between April 2007 and June 2017. Laparoscopic vs open resection was compared. This study complies with ethical standards and was approved by the Institutional Review Board of Hospital Italiano de Buenos Aires (Protocol number 3900). Patients were divided into two groups (laparoscopic vs open). The patients were selected for laparoscopic versus open resection approach (LR vs OR) based on surgeon preference and experience in minimally invasive liver surgery. Only patients who met our institutional inclusion criteria for minimally invasive approach (initial resectability, tumor size ≤ 5 cm, number of metastases ≤ 3, without bilobar disease, absence of extrahepatic disease) were included. Patients who received concomitant treatment with ablative methods were excluded and patients who died within the first 90 postoperative days were included in the study.
Preoperative evaluations consisted of abdominal and chest computed tomography scans (CT) and/or magnetic resonance imaging (MRI) and clinical biochemistry including carcinoembryonal antigen (CEA). The patients were categorized as resectable after evaluation by a multidisciplinary team comprising surgeons, pathologist, oncologist and radiologist.
Synchronous metastases were defined as cases in which patients received a diagnosis of CRLM at the same time or within 6 months of the diagnosis of the primary tumor. Major hepatectomies were defined as resections of three or more liver segments [13].
Demographic and clinicopathological data were collected from each patient, including sex, age, tumor characteristics, preoperative, perioperative, and postoperative variables. The Clavien-Dindo classification system was used to describe postoperative complications (major morbidity DC ≥ 3) [14]. We also analyzed the oncological outcomes: overall survival (OS) (was calculated from the time of resection to death or last follow-up) and disease-free survival (DFS) (from the time of resection to the presence of evidence compatible with recurrence). R0 resection margin was defined as a disease-free margin ≥ 1 mm and R1 as a disease-free margin < 1 mm [15]. R1 resections were classified as follows: R1 vascular when the tumor was detected at the level of the first or second-order Glissonean pedicle or hepatic veins within their last 4 cm before confluence at the inferior vena cava. The tumor was exposed exclusively along the vessel; in R1 parenchymal, the tumor was exposed along the transection plane. OS and DFS were adjusted for the presence of synchronous disease.
Patient follow-up included serum CEA levels and CT of the chest, abdomen, and pelvis every 3 months, the first 2 years, and every 6 months from the third year after liver resection, according to most recent NCCN guidelines [16].
Surgical technique.
The laparoscopic surgical technique used has already been previously described by our group [17].
Statistical analysis
Statistical analyses were carried out using Stata Software for MAC version 13. The distribution of quantitative variables was analyzed graphically using histogram and probability graphs and analytically using the Shapiro-Wilks test. Variables with normal distribution were expressed as mean and standard deviation (SD) and variables with non-normal distribution as the median and interquartile range (IQR). Categorical data was expressed as absolute frequency and percentage. Comparisons between groups for categorical variables were performed by means of the chi-square test or Fisher’s exact test when appropriate.
The Kaplan–Meier method was applied to calculate mean survival values and to construct survival curves. The log-rank test was used to compare survival among the groups. A Cox regression analysis was performed to adjust to overall survival and disease-free survival in relation to other potential confounding factors (synchronicity disease, size, and the number of metastases, bilaterality, preoperative CEA, location of the primary tumor). Statistical significance was reached at p values lower than 0.05.
The Kaplan–Meier method was applied to calculate mean survival values and to construct survival curves. The log-rank test was used to compare survival among the groups. A Cox regression analysis was performed to adjust to overall survival and disease-free survival in relation to other potential confounding factors (synchronicity disease, size, and the number of metastases, bilaterality, preoperative CEA, location of the primary tumor). Statistical significance was reached at p values lower than 0.05.
A propensity score was performed to adjust the indication bias. The predictive model with the largest area under the curve (AUC of 0.76 (CI: 0.64) and the lowest Akaike information criterion (94.5) was selected. The model was used to predict the probability of laparoscopy and the overlap of the Propensity Scores was tested (PS) between the patients who were approached laparoscopically and those who were not in a graphical way with histograms.
The PS was used to adjust in a logistic regression model the positive margin and the global and disease-free survival, reporting the raw OR/HR and adjusted with their respective 95 CI.
Results
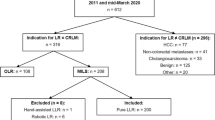
From an initial cohort of 263 patients operated for CRLM, open hepatic resection was performed in 237 patients and laparoscopic resection in 26 patients. Of the 237 conventional resection, only 56 presented the established selection criteria similar to laparoscopic resection (Fig. 1). The demographic characteristic and preoperative variables of the two groups are described in Table 1. The analysis shows a synchronous presentation in 28 patients (50%) with an open resection and 6 patients (23%) with a laparoscopic resection (p = 0.021). The extrahepatic disease was present in 3 patients (11.5%) with LR, with a significant difference (p = 0.029). These three cases were intraoperative findings. Simultaneous resection was performed in 24 patients (42%) with an OR and in 5 patients (19%) with LR (p = 0.037). The major hepatectomy (more than 3 segments) was performed in 11 (19.6%) patients with an open approach and 2 (7.6%) patients with a laparoscopic approach (p = 0.209) (Table 2). Postoperative complications were observed in 9 patients (16%) of the open resection group and 2 patients (7.7%) of the laparoscopic resection group (p = 0.3). There was a statistically significant difference in major morbidity; it occurred in 8 patients (14.2%) with OR and the patients with LR did not present complications (p = 0.042). One patient (1.7%) died in the open resection group and there were no deaths in the laparoscopic resection group (p = 0.493) (Table 3). Postoperative hospital stay was significantly shorter after laparoscopic resection (4.5 days; range, 3–6 vs 7 days; range, 5–12; p = 0.001), demonstrating similar results to studies such as Casting et al. (open resection: 28%—laparoscopic resection: 8%) and Nachmany et al. (open resection: 11.4%—laparoscopic resection: 9.6%) among others [18] [19]. The conversion rate of laparoscopic liver resections was 19.2% (5 patients).
At final pathologic analysis, the resection margin was found positive (R1) in 5 patients with OR and 2 patients with LR (Table 3). In the crude analysis and adjusted by PS, laparoscopic surgery does not increase the positive margin odds (OR: 0.85, 95% CI: 0.15–4.7, p 0.852, adjusted OR: 1.21, 95% CI: 0.18–8.2, p = 0.842). Of these 7 patients, 4 had positive vascular margins and 3 positive parenchymal margins.
The overall survival rate at 1 and 3 years for the open resection group was 92% and 77% respectively and for the laparoscopic resection group was 96% and 75% respectively (p = 0.739). The risk of death was similar in both groups, even after adjusting for PS (HR 0.72, 95% CI: 0.28, p = 0.506; adjusted HR: 0.42, p = 0.169).
Disease-free survival (DFS) at 1 and 3 years was similar in the two groups (Figs. 2 and 3). The risk of recurrence was similar in both groups even after adjusting for PS (HR: 0.74, 95 CI: 0.40, p = 0.344, adjusted HR: 0.67, CI: 5.33, p = 0.280). When adjusting the OS and DFS rates according to the presence or absence of synchronous disease, the risk of recurrence did not increase (p = 0.94; p < 0.001 respectively) (Figs. 4 and 5). The median follow-up was 30 months (range CI: 12–46).
Discussion
Colorectal cancer is one of the leading causes of cancer-related death worldwide. Liver metastases occur in 60–70% of cases; of these, 20–30% are synchronic and 30–50% metachronic [20]. Currently, surgical resection is considered the only curative treatment for CRLM, with 5-year survival rates ranging between 30 and 58% [21,22,23,24]. Although the oncological outcomes in the different published series are similar, regardless of the type of approach, with the advent of technology in recent years, liver surgeons acquired more and better management of the laparoscopic approach [25]. Furthermore, several studies demonstrated lower intraoperative blood loss, hospital stay, and morbidity rates with the LR [26, 27][28][26, 27]. Our results are consistent with the literature in terms of less intraoperative blood loss, less hospital stay, and morbidity rates with LR (Tables 2 and 3).
Oncological outcomes are analyzed in different retrospective studies, meta-analysis, and propensity score matching where no significant differences are observed in terms of the type of approach. Slim et al. demonstrated equivalent morbidity rates with the literature and improvements in oncological resection but without demonstrating results in terms of OS and DFS [29]. In our series, there was an OS at 1 and 3 years of 92–77% for OR and 96–75% for LR, respectively. DFS at 1 and 3 years was 63–20% for OR and 73–36% for LR respectively.
Our results are comparable with the literature; Koffron et al. published a series of 300 patients in 2007, demonstrating that laparoscopic liver resection is feasible and safe, and offers better outcomes in terms of blood loss, blood transfusion, hospital stay, and morbidity rates. Due to the presence of patients with different types of tumors and poor follow-up, oncological outcomes were not obtained [30].
Published studies regarding oncological outcomes associated with laparoscopy are scarce and poorly defined. More recent series (multicenter studies and meta-analyses) are limited by their heterogeneity [18, 31, 32]; Castaing et al. published a series of two centers that include 120 patients with CRLM in 2009 where half underwent a laparoscopic approach and showed that the surgical approach had no impact on survival or disease recurrence.
In 2015, Shiffman et al. published a meta-analysis that included 610 patients, where they found no differences in oncological outcomes in relation to the type of approach performed.
Furthermore, Zhang et al. in 2017 performed a systematic review that showed that there are no oncological differences regardless of the type of approach.
Robles et al. published in 2019 a prospective randomized study with 204 patients, where they demonstrated similar oncological results in both types of approaches [33]. In another multicenter study, Nguyen et al. demonstrated that the laparoscopic liver resection for colorectal metastasis is safe, feasible, and oncologically compared to open liver resection when performed by experienced surgeons [34].
In our study, the disease presented synchronously in 28 patients (50%) with OR and in 6 patients (23%) with LR (p = 0.021), in accordance with different studies in the literature [35].
The OS and DFS outcomes were adjusted according to the presence or absence of synchronous disease, without significant differences. Multiple clinicopathologic have been established as important determinants of treatment failure for colorectal liver metastases, including the width of the resection margin. Most authors report that this margin is a significant factor that influences OS and DFS. In the past decades, the risk of resection margin < 10 mm was considered a contraindication for liver resection. Ekberg et al. reported margins ≥ 10 mm necessary to improve long-term survival [36]. Currently, different studies show that disease-free resection margins of 1 to 10 mm present the same oncological results as those margins > 10 mm [15]. In a comparative study with propensity score matching Untereiner et al. demonstrated that the negative resection margin rates were similar in both groups [37]. In our study, the resection limit was 1 mm, resulting in resection margin positive (R1) in 5 patients with OR and 2 patients with LR (8.9% and 7.6% respectively; p = 0.852). Results are comparable with diverse studies published to date; the latest systematic review published in the literature did not show significant differences regarding the resection margins according to the type of approach [38].
Vigano et al. published in 2016 the differences between vascular R1 and parenchymal R1. They demonstrated that R1 parenchymal resection is not adequate for CRLM because it is associated with a higher local recurrence risk and worse survival. R1 vascular resection allows local control of the disease equivalent to R0 resection and adequate survival [39]. In our study, the R1 vascular occurred in 4 patients and R1 parenchymal in 3 patients.
Our study has some limitations; it is a retrospective review of data collected prospectively in a single institution. The selections of patients for the type of approach were not strictly random, and the surgical skill set of each surgeon may have influenced their decisions. Furthermore, overall survival and disease-free survival at 5-year outcomes could not be obtained due to difficulties in patients follow-up.
In our single-center study, the oncological outcomes do not show significant differences in terms of surgical margin, overall survival, and disease-free survival according to the surgical approach used in patients with CRLM. We recommend the use of the laparoscopic approach in selected patients, by specialist surgeons and in centers with sufficient experience.
References
RL Siegel KD Miller A Jemal 2020 Cancer statistics, 2020 CA Cancer J Clin 70 7 30
B Nordlinger H Sorbye B Glimelius GJ Poston PM Schlag P Rougier 2013 Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial Lancet Oncol 14 1208 1215
AA Schnitzbauer SA Lang H Goessmann S Nadalin J Baumgart SA Farkas 2012 Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings Ann Surg 255 405 414
A Falcone S Ricci I Brunetti E Pfanner G Allegrini C Barbara 2007 Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest J Clin Oncol 25 1670 1676
Cheung TT, Poon RTP, Yuen WK, Chok KSH, Tsang SHY, Yau T, et al. Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ Journal of Surgery. 2013. pp. 847–852. :https://doi.org/10.1111/j.1445-2197.2012.06270.x
AD Guerron S Aliyev O Agcaoglu E Aksoy HE Taskin F Aucejo 2013 Laparoscopic versus open resection of colorectal liver metastasis Surg Endosc 27 1138 1143
R Montalti G Berardi S Laurent S Sebastiani L Ferdinande LJ Libbrecht 2014 Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: oncological outcomes of a case-control matched-pairs analysis Eur J Surg Oncol 40 536 544
Hasegawa Y, Nitta H, Sasaki A, Takahara T, Itabashi H, Katagiri H, et al. Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: a comparative analysis of 168 consecutive cases at a single center. Surgery. 2015. pp. 1065–1072. :https://doi.org/10.1016/j.surg.2015.01.017
Robert M. Cannon, MD , Charles R. Scoggins, MD, MBA, Glenda G. Callender, MD, Kelly M. McMasters, MD, PhD, Robert C.G. Martin II. Laparoscopic versus open resection of hepatic colorectal metastases. Surgery. 2012 [cited 22 Aug 2020]. :https://doi.org/10.1016/j.surg.2012.07.013
ÅA Fretland VJ Dagenborg GMW Bjørnelv AM Kazaryan R Kristiansen MW Fagerland 2018 Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial Ann Surg 267 199 207
Haney CM, Studier-Fischer A, Probst P, Fan C, Müller PC, Golriz M, et al. A systematic review and meta-analysis of randomized controlled trials comparing laparoscopic and open liver resection. HPB . 2021. :https://doi.org/10.1016/j.hpb.2021.03.006
B Cady RL Jenkins GD Steele Jr WD Lewis MD Stone WV McDermott 1998 Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome Ann Surg 227 566 571
Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. Journal of Hepato-Biliary-Pancreatic Surgery. 2005. pp. 351–355. :https://doi.org/10.1007/s00534-005-0999-7
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Annals of Surgery. 2004. pp. 205–213. :https://doi.org/10.1097/01.sla.0000133083.54934.ae
Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241: 715–22, discussion 722–4.
Kawaguchi Y, Kopetz S, Lillemoe HA, Hwang H, Wang X, Tzeng C-WD, et al. A new surveillance algorithm after resection of colorectal liver metastases based on changes in recurrence risk and RAS mutation status. J Natl Compr Canc Netw. 2020;18: 1500–1508.
Clariá RS, Ardiles V, Palavecino ME, Mazza OM, Salceda JA, Bregante ML, et al. Laparoscopic resection for liver tumors. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 2009. pp. 388–391. :https://doi.org/10.1097/sle.0b013e3181bb9333
Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Annals of Surgery. 2009. pp. 849–855. :https://doi.org/10.1097/sla.0b013e3181bcaf63
I Nachmany N Pencovich N Zohar A Ben-Yehuda C Binyamin Y Goykhman 2015 Laparoscopic versus open liver resection for metastatic colorectal cancer Eur J Surg Oncol 41 1615 1620
Y-W Chen M-T Huang T-C Chang 2019 Long term outcomes of simultaneous laparoscopic versus open resection for colorectal cancer with synchronous liver metastases Asian J Surg 42 217 223
Curley S. Outcomes after surgical treatment of colorectal cancer liver metastases. Seminars in Oncology. 2005. pp. 109–111. :https://doi.org/10.1053/j.seminoncol.2005.06.011
Rees M, Tekkis PP, Welsh FKS, OʼRourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer. Annals of Surgery. 2008. pp. 125–135. :https://doi.org/10.1097/sla.0b013e31815aa2c2
Y Cheng L Zhang H Li L Wang Y Huang L Wu 2017 Laparoscopic versus open liver resection for colorectal liver metastases: a systematic review J Surg Res 220 234 246
MA Choti JV Sitzmann MF Tiburi W Sumetchotimetha R Rangsin RD Schulick 2002 Trends in long-term survival following liver resection for hepatic colorectal metastases Ann Surg 235 759 766
JF Buell D Cherqui DA Geller N O’Rourke D Iannitti I Dagher 2009 The international position on laparoscopic liver surgery: The Louisville Statement, 2008 Ann Surg 250 825 830
KT Nguyen JW Marsh A Tsung JJL Steel TC Gamblin DA Geller 2011 Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal Arch Surg 146 348 356
A Rao G Rao I Ahmed 2012 Laparoscopic or open liver resection? Let systematic review decide it Am J Surg 204 222 231
Pekolj J, Clariá Sánchez R, Salceda J, Maurette RJ, Schelotto PB, Pierini L, et al. Laparoscopic liver resection: a South American experience with 2887 Cases. World J Surg. 2020. :https://doi.org/10.1007/s00268-020-05646-4
A Slim M Garancini S Sandro Di I Mangoni A Lauterio A Giacomoni 2012 Laparoscopic versus open liver surgery: a single center analysis of post-operative in-hospital and post-discharge results Langenbecks Arch Surg 397 1305 1311
Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246: 385–92; discussion 392–4.
Schiffman SC, Kim KH, Tsung A, Wallis Marsh J, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015. pp. 211–222. :https://doi.org/10.1016/j.surg.2014.08.036
T Beppu G Wakabayashi K Hasegawa N Gotohda T Mizuguchi Y Takahashi 2015 Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study J Hepatobiliary Pancreat Sci 22 711 720
R Robles-Campos V Lopez-Lopez R Brusadin A Lopez-Conesa PJ Gil-Vazquez Á Navarro-Barrios 2019 Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial Surg Endosc 33 3926 3936
KT Nguyen A Laurent I Dagher DA Geller J Steel MT Thomas 2009 Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes Ann Surg 250 842 848
Okuno M, Goumard C, Mizuno T, Omichi K, Tzeng C-WD, Chun YS, et al. Operative and short-term oncologic outcomes of laparoscopic versus open liver resection for colorectal liver metastases located in the posterosuperior liver: a propensity score matching analysis. Surg Endosc. 2018;32: 1776–1786.
H Ekberg KG Tranberg R Andersson C Lundstedt I Hägerstrand J Ranstam 1986 Determinants of survival in liver resection for colorectal secondaries Br J Surg 73 727 731
X Untereiner A Cagniet R Memeo S Tzedakis T Piardi F Severac 2016 Laparoscopic hepatectomy versus open hepatectomy for colorectal cancer liver metastases: comparative study with propensity score matching Hepatobiliary Surg Nutr 5 290 299
Ciria R, Ocaña S, Gomez-Luque I, Cipriani F, Halls M, Fretland ÅA, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surgical Endoscopy. 2020. pp. 349–360. :https://doi.org/10.1007/s00464-019-06774-2
L Viganò F Procopio MM Cimino M Donadon A Gatti G Costa 2016 Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? An Observational Cohort Ann Surg Oncol 23 1352 1360
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was performed according to the Declaration of Helsinki and approved by the local ethics committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nicolás, M., Czerwonko, M., Ardiles, V. et al. Laparoscopic vs open liver resection for metastatic colorectal cancer: analysis of surgical margin status and survival. Langenbecks Arch Surg 407, 1113–1119 (2022). https://doi.org/10.1007/s00423-021-02396-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02396-2