Abstract
Background
Routine contrast esophagram has been shown to be increasingly limited in diagnosing anastomotic leaks after esophagectomy.
Methods
Patients undergoing esophagectomy from 2013 to 2014 at Huai’an First Peoples’ Hospital were identified. We retrospectively analyzed patients who underwent routine contrast esophagram on postoperative day 7 (range 6–10) to preclude anastomotic leaks after esophagectomy.
Results
In 846 patients who underwent esophagectomy, a cervical anastomosis was performed in 286 patients and an intrathoracic anastomosis in 560 patients. There were 57 (6.73%) cases with anastomotic leaks, including cervical leaks in 36 and intrathoracic leaks in 21 patients. In the cervical anastomotic leak patients, 13 were diagnosed by early local clinical symptoms and 23 underwent routine contrast esophagram. There were 7 (30.4%) true-positive, 11 (47.8%) false-negative, and five (21.8%) equivocal cases. In the intrathoracic anastomotic leak patients, four (19%) were diagnosed by clinical symptoms, 16 (76.2%) were true positives, and one (4.8%) was a false negative. Aspiration occurred in five patients with cervical anastomoses and in eight patients with intrathoracic anastomoses; aspiration pneumonitis did not occur in these cases.
Conclusions
Gastrografin and barium are safe contrast agents to use in post-esophagectomy contrast esophagram. Because of the low sensitivity in detecting cervical anastomotic leaks, routine contrast esophagram is not advised. For patients with intrathoracic anastomoses, it is still an effective method for detecting anastomotic leaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastomotic leak is one of the most common complications after esophagectomy, with a probability of 5–35% [1, 2]. Routine contrast esophagram can detect anastomotic leaks after esophagectomy prior to the resumption of oral feeding, thus reducing oral intake complications such as mediastinal infection, pyothorax, wound infection, septicemia, and death [3, 4]. However, a growing body of evidence highlights the limitations of routine contrast esophagram in the detection of anastomotic leaks, due to its low sensitivity [5, 6], especially in the diagnosis of cervical anastomotic leaks [7, 8]. In China, routine contrast esophagram is generally used in thoracic surgery centers [9]. We retrospectively analyzed the clinical data of patients who underwent esophagectomy at our center and assessed the clinical usefulness and safety of a contrast swallow study to predict anastomotic leaks.
Methods
The clinical data of patients who underwent esophagectomy in our institution by three surgeons between January 1, 2013 and December 31, 2014 were collected. Patient demographics, location of esophageal lesion, operative approach, clinical signs of anastomotic leaks, complications, time of esophagram, esophagram results, complications of the swallow studies, and time to resumption of oral diet were recorded. For the purpose of the analysis, the anatomic location of the anastomosis was divided into cervical and intrathoracic levels.
Generally, esophagram was performed the first week (median postoperative day 7, range 6–10 days) after esophagectomy. After an iodine allergy test was confirmed to be negative, patients were instructed to swallow 60 ml of gastrografin for the preliminary assessment of anastomotic integrity. When there was no evidence of an obvious leak, 50–70 ml of oral barium was administered to further confirm the integrity of the anastomosis. The anastomosis was carefully observed in at least three different positions (anteroposterior, left posterior oblique, and right posterior oblique) during swallowing to assess for extravasation of contrast material. Radiographic spot films were obtained in each position, and in the presence of extravasation, additional radiographs were obtained to document this finding. Overhead radiographs were obtained at the conclusion of the examination, centered on the mid-thoracic or cervical area (depending on the location of anastomosis performed) in the anteroposterior and lateral positions. A single anteroposterior upper abdominal film was also obtained. Esophagram results were interpreted by both the attending surgeon and a radiologist specializing in upper gastrointestinal fluoroscopic assessment.
A clinically apparent anastomotic leak required the presence of at least two of the following criteria: (a) the appearance of saliva, stomach contents or pus through the wound or drains, (b) the presence of fever, leukocytosis, and local signs of inflammation, (c) anastomotic leakage assessed by other diagnostic examination (e.g., endoscopy or computed tomography (CT) scan), (d) anastomotic breakdown seen at re-exploration. A radiological leak was defined as any extravasation of the contrast medium at the site of the anastomosis.
Regarding the results of the initial contrast swallow study, patients were classified as follows: (1) true positive—a positive initial contrast swallow study in a patient who developed clinically apparent leak, irrespective of whether the leak appeared before or after the first esophagram; (2) true negative—a negative initial contrast swallow study in a patient who remained asymptomatic after oral intake throughout the entire hospitalization; (3) false positive—a positive initial contrast swallow study in a patient who remained asymptomatic throughout the entire hospitalization; (4) false negative—a normal initial contrast swallow study in a patient who had a clinical leak regardless of the time at which it appeared.
Until the contrast swallow examination was executed, oral intake was prohibited and patients were fed through a jejunal feeding tube. Furthermore, nasogastric suction was maintained to decompress the gastric conduit. When the esophagram did not show contrast leakage, oral intake was gradually resumed, starting with water. However, in equivocal cases, confirmation of an anastomotic leak was achieved by computed tomography (CT). When a radiographic leak was detected, oral intake was omitted. If these patients were without clinical symptoms (radiologically diagnosed, asymptomatic leaks) such as fever, leukocytosis, or local signs of inflammation after the initial contrast swallow study, a second study was performed to determine the integrity of the anastomosis after one week in intrathoracic patients; cervical patients would resume oral intake after 3–7 days.
Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for routine contrast esophagram. Data were analyzed using SPSS statistical software for Windows (version 19.0, SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
The study was approved by our institutional review board. Informed consent from patients whose records were included was not required for this study.
Results
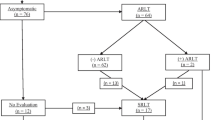
From January 1, 2013 to December 31, 2014, 883, patients who underwent esophagectomy were reviewed. Patients were excluded from the study for the following reasons: 23 cases who had postoperative complications other than anastomotic leaks and required prolonged stays in the intensive care unit; three deaths that occurred before scheduled contrast swallows because of stroke, cardiac arrest, or pulmonary infarction; and 11 with recurrent laryngeal nerve paralysis, leaving 846 (95.8%) eligible. Patient demographic characteristics are shown in Table 1. Of these, 846 patients underwent esophageal imaging. Circular stapling was applied for side-to-end anastomosis after the gastric conduit was pulled through the esophageal bed in all esophageal anastomoses. Two hundred and eighty-six patients had a cervical anastomosis. One hundred and sixty-one patients underwent thoracoscopic esophageal resection, and 125 patients had a McKeown esophagectomy. A thoracic anastomosis was performed in 560 patients, using the Ivor-Lewis, Sweet, or left thoracoabdominal approaches. Anastomotic leaks were found in 57 patients (6.73%), including cervical leaks in 36 and thoracic leaks in 21 cases.
In 23 cases of cervical anastomotic leaks, esophagram was performed prior to the development of local clinical symptoms. There were seven true positives, 11 false negatives, and five equivocal cases. Of the 250 patients without leak, there were six false-positive cases who had no local symptoms after esophagography in another 3–7 days (median 3 days), who resumed oral intake immediately did not develop a clinical leak. There were 241 true-negative cases, where no leak occurred after resumption of oral intake. Five of eight equivocal patients had clinical anastomotic leaks in 3 days. The other three cases who did not have local signs of inflammation in 3–7 days (median 3 days) were allowed oral intake but did not have leaks (Fig. 1).
In patients with intrathoracic anastomosis, a total of 21 patients developed anastomotic leaks. Four patients were diagnosed by clinical symptoms. The postoperative diagnosis times were 3, 5, 8, and 11 days. In the first three cases, the chest tube drained pus, the patient was febrile, and the swallowing methylene blue test was positive. One of the four patients with anastomotic bleeding had an anastomotic leak which appeared as a pyothorax on postoperative day 11. One patient whose esophagram result was a false negative developed a pyothorax after resuming oral intake, and the left posterolateral incision was infected. Sixteen cases were true positives. Eleven of 16 cases had accompanying fever, but five were without fever before esophagram. After contrast swallow study, all 16 patients had fever in 48 h and leukocytosis. Esophageal imaging did not demonstrate an anastomotic leak in 535 patients; there were no false-positive results. On CT, the four highly suspicious leaks showed visualization of the tip of the gastric stumps (Fig. 2).
Patients with cervical anastomotic leaks were treated by open neck incision and drainage. Patients with thoracic anastomotic leaks, after adequate drainage and enteral nutrition, were cured as confirmed by repeat imaging or endoscopy in 15–92 days.
In the patients with cervical anastomosis, there were five cases of aspiration (5/273, 1.83%). In the patients with thoracic anastomosis, there were eight cases of aspiration (8/556, 1.4%). The incidence of aspiration in cervical anastomotic patients was higher than in patients with intrathoracic anastomosis (p < 0.05). None of the patients developed contrast-induced aspiration pneumonia.
If equivocal studies are excluded from analysis, esophagram in the neck is 38.9% sensitive, 97.6% specific, and have positive and negative predictive values of 53.8 and 95.6%, respectively. In the intrathoracic group, the sensitivity is 93.3, specificity is 100%, and the positive and negative predictive values are 100 and 99.8%, respectively (Tables 2, 3).
Discussion
Anastomotic leak is one of the most serious complications after esophagectomy [3, 6–8]. It is reported that the mortality rate was as high as 35% in anastomotic leak patients, accounting for approximately 25–50% of deaths post-esophagectomy [10, 11]. Prior to resuming an oral diet, diagnosing an anastomotic leak is very important [5, 6, 12]. However, many studies have shown that routine contrast esophagram is a redundant method of detection.
At the Mayo Clinic, 505 patients who underwent screening for anastomotic integrity using a water-soluble contrast agent 7 days after esophagectomy were retrospectively analyzed. The sensitivity of this test was 40.4%, the specificity was 94.7%, and the false-negative rate was 59.5%. This article concludes that the radiologic assessment of esophageal anastomoses is insufficient for it to be worthwhile as a screening procedure [5]. A retrospective study of 221 patients who underwent barium esophagram 7 days post-esophagectomy showed a sensitivity of 45.5% and specificity of 97.8%, with a change in treatment course in 3.6% of patients based on imaging results; 2.3% underwent intervention, compared to 1.4% of patients with false positives who had a delay of resumption of oral intake. It should also be mentioned that the barium contrast agent used for screening anastomotic leaks is likely to cause aspiration and mediastinitis, and compromise the subsequent radiographic studies. It also shows the limitations of routine contrast esophagram after esophagectomy to detect anastomotic leaks [13].
In cervical anastomosis, the sensitivity of routine contrast esophagram was 38.9%, the specificity was 97.6%, the positive predictive value was 53.8%, and the negative predictive value was 95.6%. Of 286 patients who underwent cervical anastomosis after esophageal resection, 13 patents had anastomotic leaks diagnosed by early clinical symptoms, seven by esophagram, and 16 by local clinical symptoms after oral intake or swallowing contrast agents in 3 days in false negative and five equivocal patients. Only 2.6% of patients (7/273) with cervical anastomosis were identified to have a leak by routine contrast esophagram. Although seven patients were diagnosed as anastomotic leaks, it does not change the result that the neck required reopening in 1–3 days. Most of them had local signs of inflammation. Thus, we consider esophageal imaging in cervical anastomosis inappropriate. In a meta-analysis by Sheraz et al., contrast esophagram was deemed unnecessary for patients with cervical anastomoses [7].
In 560 patients with intrathoracic anastomosis, the sensitivity was 93.3%, the specificity was 100%, and the positive and negative predictive values were 100 and 99.8%, respectively. The only one false-negative case, after resumption of an oral diet, developed fever, posterolateral incision infection, and pyothorax. This was the only patient who required a thoracic drainage tube again after esophagram.
Thus, the question remains, in patients with an intrathoracic anastomosis who have a low probability of leak (3.75%, 21/560), whether there is any value of using routine contrast esophagram after all esophagectomies, or whether there are other ways to screen these patients where a leak is suspected through symptoms such as fever. We found that patients with leaks (64.7%, 15/21) have symptoms of fever, but hydrothorax, pneumonia, wound infection, and upper respiratory tract infection (11.13%, 60/539) also present with fever. The incidence of fever in patients with leaks was higher than in those without fever (p < 0.05; Table 1). If contrast esophagram was only used to screen febrile patients, we would have been unable to identify the six afebrile patients in whom anastomotic leaks occurred.
When patient’s condition allowed, routine contrast esophagram was used beginning on postoperative day 7 (median postoperative day 7, range 6–10 days) in our center. First, 60 ml of gastrografin was used as contrast medium; if no obvious leak was found, then barium was used. Because a water-soluble iodine preparation such as gastrografin is absorbable, if an anastomotic leak is present, this can reduce the occurrence of mediastinitis [14, 15]. As barium has a higher emission density, it is more conducive to detecting anastomotic integrity [4]. Gastrografin is less radiopaque than barium and when used alone, may miss some esophageal perforations or leaks [16]. Several studies have shown that water-soluble contrast agents are unable to detect 15–25% of thoracic and 50% of cervical esophageal perforations [14–18]. In the true-positive intrathoracic anastomosis leak patients, there were six cases that needed barium to detect them (6/16, 37.5%), while 10 cases were detected by gastrografin alone. Because of the anatomy of the neck, five (5/7, 71.4%) cases were confirmed by barium in the seven true-positive cervical anastomosis patients.
In our study, there were no deaths in anastomotic leak patients. Cervical anastomotic leaks were always accompanied by cervical incision clinical symptoms such as erythema, edema, warmth, and pain of skin, as well as the outflow of saliva or pus. These were cured by fasting and local incision drainage. In intrathoracic anastomotic leak patients whose esophagram was positive, we withheld oral intake. Those intrathoracic anastomotic leaks were confined around the local mediastinum without the further development of pyothorax. They did not require replacement of thoracic drainage tube. The false-negative case developed a pyothorax after resumption of oral intake.
There were several limitations to this retrospective study. Bias can occur in the review of patients who did not suffer leaks but where the esophagram may be false positive, patients with an equivocal leak who may have a positive esophagram, patients who did not have an esophagram, and patients with delayed oral intake after a positive esophagram. Most true leak patients had clinical signs in 0–3 days (median 1 day) after swallowing contrast agents. Our results are in line with what has previously been reported regarding the sensitivity and specificity of contrast esophagram in cervical anastomosis patients, but the sensitivity and specificity were all higher in intrathoracic patients than in previous studies. The reasons for this may be that we performed duplicate esophagrams, in which almost all patients swallowed gastrografin and barium in sequence; a surgeon was in attendance during the imaging diagnosis; and patients classified as false positive on esophagram were counted into the equivocal leak group. In our single institution, routine contrast esophagram to detect cervical anastomotic leaks is not necessary. The test does not avoid cervical incision drainage. Further, the integrity of the cervical anastomosis can be tested by drinking small amounts of water with simultaneous observation of the cervical wound and routine contrast esophagram is abandoned [19]. In patients with intrathoracic anastomosis, although the leak probability is low, the sensitivity and specificity of esophagram are high. We can withhold oral intake in intrathoracic anastomotic leak patients whose esophagram is positive. Conversely, resuming an oral diet can result in pyothorax, wound infection, enlargement of an existing anastomotic leak, slow healing, an extended length of hospital stay, increasing costs, and even death [3, 6–8, 20]. Our center still uses routine contrast esophagram to preclude intrathoracic anastomotic leak. At the same time, radiography can identify patients with pneumonia and pleural effusion and can determine whether there is a downstream obstruction [12]. Computed tomography, gastroscopy, or reoperation is other screening methods. Some articles discussed the role of endoscopy to predict a leak after esophagectomy in patients in whom a leak is suspected [21, 22]. However, this requires an experienced operator, gastric emptying of the conduit, and patient sedation. Iatrogenic injury of the anastomotic region should be avoided, as this method is not routine [22].
Conclusions
Our data do not support the use of contrast esophagram as a routine screening modality to detect anastomotic leaks in cervical anastomosis after esophagectomy. Routine contrast esophagram with gastrografin and barium in sequence remains a safe and effective way to determine the presence of an intrathoracic anastomotic leak.
References
Boone J, Rinkes IB, van Leeuwen M, van Hillegersberg R (2008) Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg 78(9):784–790
Honing J, Pultrum BB, van der Jagt EJ, Groen H, Plukker JT (2009) Routine or on demand radiological contrast examination in the diagnosis of anastomotic leakage after esophagectomy. J Surg Oncol 100(8):699–702
Agha FP, Orringer MB, Amendola MA (1985) Gastric interposition following transhiatal esophagectomy: radiographic evaluation. Gastrointest Radiol 10:17–24
Bains MS (1995) Ivor-Lewis esophagectomy. Chest Surg Clin North Am 5:515–526
Tirnaksiz MB, Tirnaksiz MB (2005) Effectiveness of screening aqueous contrast swallow in detecting clinically significant anastomotic leaks after esophagectomy. Eur Surg Res 37:123–128
Solomon DG, Sasaki CT, Salem RR (2012) An evaluation of the routine use of contrast radiography as a screening test for cervical anastomotic integrity after esophagectomy. Am J Surg 203(4):467–471
Markar SR (2013) Technical factors that affect anastomotic integrity following sophagectomy: systematic review and meta-analysis. Ann Surg Oncol 20:4274–4281
Jones CM, Heah R (2015) Should routine radiological assessment of anastomotic integrity be performed after oesophagectomy with cervical anastomosis? Best evidence topic (BET). Int J Surg 15:90–94
Shen Y, Wang H (2014) The effect of narrowed gastric conduits on anastomotic leakage following minimally invasive esophagectomy. Interact CardioVasc Thorac Surg 19:263–268
Alanezi K, Urschel JD (2004) Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 10:71–75
Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N (2001) Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg 88(10):1346–1351
Zwischenberger JB, Sankar AB (1995) Transhiatal esophagectomy. Chest Surg Clin North Am 5:527–542
Cools-Lartigue J, Andalib A (2014) Routine contrast esophagram has minimal impact on the postoperative management of patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol 21:2573–2579
Dodds WJ, Stewart ET, Vlymen WJ (1982) Appropriate contrast media for evaluation of esophageal disruption. Radiology 144:439–441
Phillips LG, Cunningham J (1984) Esophageal perforation. Radiol Clin North Am 22:607–613
Wong J, Cheung H, Lui R, Fan YV, Smith A, Siu KF (1987) Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery 101:408–415
Lowe L, Berkow AE (1978) Trauma to the esophagus. Gastrointest Radiol 2:305–321
Meyers MA, Ghahremani GG (1975) Complications of fiberoptic endoscopy. Esophagoscopy and gastroscopy. Radiology 115:293–300
Boone J, Rinkles IB (2008) Diagnostic value of routine aqueous cantrast swallow examination after oesophagectomy for detecting leakage of the cervical oesphagogastric anastomosis. ANZ J Surg 78:784–790
Siewert J, Stein H, Bartels H (2004) Anastomotic insufficiencies in the upper gastrointestinal tract. Chirurg 75:1063–1070
Oezcelik A, Banki F, Ayazi S et al (2010) Detection of gastric conduit ischemia or anastomotic breakdown after cervical esophagogastrostomy: the use of computed tomography scan versus early endoscopy. Surg Endosc 24:1948–1951
Schaible A, Ulrich A (2016) Role of endoscopy to predict a leak after esophagectomy. Langenbecks Arch Surg 401:805–812
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Z., Wang, X., An, X. et al. The Diagnostic Value of Routine Contrast Esophagram in Anastomotic Leaks After Esophagectomy. World J Surg 41, 2062–2067 (2017). https://doi.org/10.1007/s00268-017-3923-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3923-9