Abstract
Background
For radiologists, the venous drainage of adrenal glands is a key to the technique of selective adrenal venous sampling. For endocrine surgeons, it is key to adrenalectomy for carcinoma and pheochromocytoma. This study aims to demonstrate direct anastomosis between the left adrenal vein, the diaphragmatic circulation and the azygos system. Anatomical textbooks only offer very little information concerning the left adrenal vein (LAV) and its potential anastomosis with the reno-lumbo-azygo trunk (RLAT) and the diaphragmatic circulation.
Methods
Between November 2014 and October 2015 in the LADAF (French Alps Anatomy Laboratory), we dissected 44 formalin-fixed adult cadavers.
Results
We found no direct anastomosis between the left adrenal vein and the reno-azygo-lumbar trunk and two anastomoses (4.5%) between the adrenal capsular vein and azygos system. A lumbo-azygo trunk has been found 38 times (86.3%), drained 35 times (79.5%) into the left renal vein and 3 times (6.8%) into the left genital vein. An inferior phrenic vein ending into an adrenal vein was highlighted in all cases, 6 times (13.7%) in a double adrenal vein and 38 times (86.3%) in a single one.
Conclusions
No connections have been found between left adrenal vein and the RLAT, and frequency of the IPV is discordant with the literature. However, our findings concerning the capsular vessels’ anastomosis with the azygos system, inferior diaphragmatic flow and double adrenal vein could have two clinical applications: Firstly, the ligation of the adrenal vein solely is not enough to entirely interrupt the adrenal vein drainage, and secondly, sampling of hormones in the LAV could be underestimated because of the risk of dilution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adrenal veins may present with a multitude of anatomical variants, which surgeons must be aware of when performing adrenalectomies. On a radiologic point of view, adrenal vein sampling is the gold standard for etiologic diagnosis in renin-independent mineralocorticoidism and is necessary to distinguish unilateral from bilateral disease [1–6]. Adrenalectomy for phaeochromocytoma is well known to be a dangerous surgery because of the risk of catecholamine release during the gland’s dissection. Few studies have precisely studied this anatomical area with well-defined methods and a substantial number of dissections. A demonstration of direct connections between the adrenal vein and azygos system could have clinical impact. It could explain hemodynamical disorders in phaeochromocytoma surgery after ligation of the main adrenal vein and misleading results in adrenal vein sampling. A specific anatomical study is necessary to explore the anatomical variations around the left adrenal vein; such knowledge could help radiologists and surgeons perform safer and more reproducible techniques.
Adrenal glands are retroperitoneal endocrine organs, located above and slightly medial to the kidney. The venous pathway is a remnant of the caudal portion of the subcardinal veins in the embryo. There are many communications between the subcardinal, posterior cardinal and supracardinal veins which leads to many possibilities in venous drainage in adults [1, 7–9]. The venous plexus inside of the left adrenal gland is drained into the left adrenal vein (LAV) which is longer than the right adrenal vein. The main endocrine products of the adrenal gland are secreted into the central medullary vein [1]. The LAV passes downward inferior, medial and posterior to the body of the pancreas. Most commonly, one central vein drains each adrenal gland; the LAV joins the inferior phrenic vein (IPV) before entering the superior border of the left renal vein 2–5 cm from the IVC. The left adrenal vein is approximately 4–5 mm in diameter and extends 1–4 cm in length to the inferior phrenic vein confluence. The confluence lies 1–3 cm from the left renal vein. The left adrenal vein drains into the inferior border of the left renal, opposite to the left gonadal vein ending [3, 10].
The reno-lumbar-azygo trunk (RLAT) is an inconstant structure; it is also subject to major anatomical variations and is sometimes called “arch,” “trunk” or “canal.” Lejars [11] in 1888 was the first to highlight a connection between the left renal vein and the azygos system on 62 of 80 cadavers (77.5%). The initial description of RLAT (“the Lejars arch”) was a posterior branch of the left renal vein dividing into a lumbal branch and a cranial branch reaching the root of the hemiazygos [12], but many variations exist, as double renal tributaries, or as a single parietal branch going to the lumbar veins or the hemiazygos [7]. One century later, Auvert in 1967 [11] found the RLAT in 42 of 50 specimens (84%). Monkhouse (1985) in 42 of 57 specimens (73.6%) [10]. Until now, the connections described between adrenal gland and azygos system were attributed to the capsular adrenal vein [10]. An anastomosis between the LAV and the azygos system could have a major impact and has not yet been described.
The study of the connections between the LAV and the IPV is particularly interesting because the literature is discordant regarding its description. In fact the morphology described above [7, 10, 13–16] was questioned by Loukas [17], who reported that the left inferior phrenic vein contributes to the left adrenal vein in only 34% of specimens studied.
Adrenalectomy requires a perfect knowledge of the anatomical variation of the venous pathway because of the risks of hemorrhage and hypertensive shock due of the catecholamine releases [18]. During the intervention, the surgeon must first ligate the left adrenal vein at the junction with the left renal vein before gland’s dissection. The presence of an anastomosis between the left adrenal vein (LAV) and the RLAT could compromise the hemodynamic stability by releasing catecholamine into the systemic circulation during the manipulation of the gland.
Anatomical variations of left adrenal venous drainage have been documented in large clinical studies of laparoscopic surgery [19]. Surgical studies have inherent constraints that limit the degree with which venous drainage can be explored. Furthermore, hemodynamic disorders after ligation of adrenal vein have been described during phaeochromocytoma surgery [20]. and necessity of primary ligation of the adrenal vein during this surgery is debated [21].
Adrenal venous sampling (AVS) is a difficult and invasive exam that needs a well-trained operator. In a previous publication, we reported on our experience in AVS and we insisted on those [6]. Preoperative AVS is mandatory for surgical decision making in the case of primary aldosteronism (PA) without any radiological target on abdominal CT scan, as it gives a definitive evaluation for the lateralization of PA [6, 22].
Its specificity is excellent, so much so that current guidelines recommend the use of cross-sectional imaging in conjunction with AVS [22]. However, to our knowledge the sensitivity of AVS has not been assessed as a function of left and right side cases, despite anatomical differences.
The location of AVS has been subject to recent debate, with the development of super-selective segmental adrenal venous sampling [23]. Most authors use the Mayo clinic’s technique as reference, where sampling is performed on the convergence between inferior diaphragmatic vein and left adrenal vein [24].
This demonstrates the importance of a review of the adrenal venous anatomy. An inventory of variations that can distort this exam by underestimating the rate of adrenal hormones can help to understand the rate of false negative AVS on the left side.
We build on this work by undertaking an exhaustive clinical study on cadavers to establish a more precise understanding of the small venous variations. The main objective of this study is to find direct anastomosis between the adrenal vein and the azygos system on the left side. The secondary objectives are establishing the presence of a connection between inferior phrenic vein, the anatomical variability of the left adrenal vein and the incidence of a RLAT.
Methods
Study subjects
This is an anatomical descriptive, monocentric, prospective study about anatomical variations of this area.
This study was performed on 44 European formalin human cadavers between 55 and 102 years old (mean age 82.0 years), and a size included between 130 and 185 cm (average: 165 cm). There were 22 women and 22 men. The characteristics of the population are summed up in the Table 1.
Embalming consisted in injecting first 45,000 ml of formalin diluted to 1.15% in a solution containing lanolin then 4500 ml of formalin diluted to 1.44% in the right carotid artery after death. Afterward, corpses were stored at 3.6 °C. All dissections were performed in the LADAF (Laboratoire d’Anatomie des Alpes Françaises/French Alps Anatomical Laboratory), between November 2014 and October 2015.
Dissection protocol
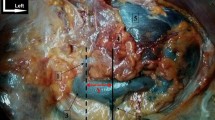
Dissections have been protocoled to maximize the reproducibility. The small intestine and the colon were removed as well as the duodeno-pancreatic block, the stomach and the spleen, to provide comfortable access to the retroperitoneum. Subsequently, we dissect upward to the left renal vein. Vessels were exposed by dissection and surrounding fat and connective tissue removed. Then, the left renal vein was dissected to its insertion with the inferior vena cava, and then the adrenal vein on its anterior face was highlighted (Fig. 1). Subsequently, the posterior side of the gland was dissected to look for direct anastomosis with the azygos system. The adrenal gland on all its faces was dissected too, in order to establish the presence of a secondary adrenal vein. The posterior face of the left renal vein was carefully explored in order to find the RLAT or trunk of Lejars. IPV was also largely dissected to the anterior face of the left diaphragmatic crus. Small capsular vessels starting from the gland have not been dissected except in cases of nonnegligible size. Finally, the anatomical piece was removed from the body (Fig. 2). A vein was considered as an anastomosis with the azygos system if it crosses the psoas major muscle through its anterior surface or if it passes posterior to it.
Once a vessel is established as terminating far from the classical area of venous drainage, it was exempted from further dissection. The volume of these connections was estimated by the external diameter of these vessels; a more precise way to visualize it was not possible in our protocol. The authors acknowledge that a better method to explore these connections may exist; however, we consider it of relatively small clinical interest to pursue such a difficult dissection of a small vessel with external diameter under 1 mm, in a remote part in the middle of a muscle.
At the end of the dissection, we registered the following measures and data: presence of an anastomosis between LAV and azygos system, distance from the gland to the junction with IPV, length and diameter from junction IPV to the renal vein, presence of a RLAT, length from the IVC to adrenophrenic trunk, length of the renal vein, diameter of the renal vein at the junction with the adrenophrenic trunk and at the junction with the IVC, diameter of the RLAT, length of the renal vein between IVC to RLAT and, between IVC and gonadic vein. Finally, we measured the adrenal gland and reported any pathological aspect.
Data analysis
For each dissection, the main information about the body was manually collected. All dissections were recorded as drawings and photographs. A reflex digital camera EOS 650D Canon was used to take pictures. Lengths were measured with a supple surgical rule graduated in centimeters. We used the computer program Mesurim Pro [25] to get precise measures and to maximize the reproducibility between each measure. It has been decided not to take into consideration the capsular veins if they were too thin to be dissected.
Inspired by Cesmebasi adrenal venous classification [1], we created an iconography summing up the different adrenal venous anatomy subtypes we expected to see (Fig. 3). Incidence of double renal vein has not been reported because of their minor surgical interest.
Anatomy subtypes of the left adrenal vein from Cesmebasi [1]. a vein joins renal alone; b vein receives IPV and drains into renal; c double adrenal veins, one receives IPV; d left adrenal vein receives LGV (doubled) but not the IPV; e adrenal vein receives one of two IPV, while the other is received by the adrenal gland and drains into the renal; f vein drains directly into IVC (K kidney, A adrenal gland, IPV inferior phrenic vein, LAV left adrenal vein, IVC inferior vena cava, LGV left gonadic vein)
Ethics and human considerations
Human bodies used in this study were donated for medical science by donators themselves. Written and witnessed consent to donate their bodies to science for anatomical and pedagogical purposes was given prior to death. This donation was free, anonymous and regulated by the French funeral legislation.
Results
No direct anastomosis between the adrenal vein and the azygos system was found among our 44 dissections. However, we found two anastomoses (4.5%) with capsular adrenal vessels.
Regarding lumbo-azygo trunk anatomical variations, we observed 35 trunks (79.5%) draining into the left renal vein and 3 (6.8%) into the left gonadic vein (Fig. 4).
Considering anatomical subtypes, we found an inferior phrenic vein ending into main left adrenal vein in 38 cases (86.4%) and into a double adrenal vein in 6 cases (13.6%). It was missing in 6 cases (13.6%).
About anatomical subtypes, IPV drained into an adrenal vein in all the cases to join an adrenophrenic trunk. Concerning the distribution of adrenal vein anatomy, our dissection reports 35 times (84.1%) classic disposition with a single adrenophrenic trunk draining into the left renal vein and 6 times an accessory adrenal draining into the LRV in six cases (13.6%) with an average diameter of 2.5 mm (±0.5 mm), and once (2.3%) not ending into the LRV. Anastomoses had a mean diameter of 2 mm.
A venous cartography of this area has been set, using the average of all the measures manually recorded. The mean length of the LRV was 68.4 mm (±15.3 mm), 21.3 mm (±5.4 mm) wide at its termination into the IVC and 14.5 mm (±3.2 mm) wide at the level of the LAV. LAV’s opening in LRV was on average wide 5.9 mm (±1.6 mm) and 30.8 mm (±6.6 mm) far from the IVC. LAV measured 7.6 mm (±4.7 mm) to the junction with the IPV and adrenophrenic trunk was average 17.5 mm (±7.0 mm). RLAT finished into the LRV 43.2 m (±11.3 mm) far from the IVC was 5.1 mm (±2.4 mm) wide. LGV ended in the LRV on average 40.6 mm (±7.7 mm) far from the IVC, length and width of adrenal gland were, respectively, 49.9 mm (±9.8 mm) and 24.5 mm (±7.9 mm). Finally, the drainage volume of the inferior phrenic vein (IRV) can be assessed by its diameter: 2.95 mm (±0.98 mm).
Discussion
This original study has clinical application in adrenal surgery and interventional radiology. It gives a complete and precise cartography of the adrenal venous pathway, which needed to be clarified.
It is a common fact that adrenal surgery is difficult because of its depth and the narrow connections with major vessels. Currently, in adrenal surgery, the presence of atypical vein is stressful for the surgeon when performing adrenalectomy for phaeochromcytoma. Control of blood loss and hemodynamic stability is crucial for a safe procedure. The fact that no anastomosis with the azygos pathway was found comforts the surgeon that the ligation of the LAV on its end is a safe procedure but that it is not enough to avoid a total release of amines. If we suppose that the diaphragmatic circulation could drain a part of this release, the surgical procedure must go on along the phrenic crus to ligate all the vessels possibly coming from the adrenal gland. Nevertheless, surgeon should not forget the risk of double adrenal vein which has been found in seven cases.
Such data could also help the radiologist and be used safely as a reference thanks to the protocoled dissection and multiple measurements. One suggestion of this study is that adrenal venous sampling on the left side by the current technique could lead to false negatives, because of the dilution caused by the diaphragmatic venous flow, the frequency of the double adrenal vein and the connections of capsular veins with the azygos system. In the opinion of the authors, a selective or super-selective venous sampling is a more reliable technique and could increase sensibility of AVS.
However, this study may present some weaknesses. Monkhouse [10] described anastomosis with azygos system through capsular adrenal veins. In our experience, very small capsular vessels have not been dissected. It has been chosen not to take the into account because, considering hormones release, these superficial and tiny vessels are insignificant in the drainage of the gland as a whole, but they may likely provide alternative drainage pathways in case of blockage of the main veins. This is a questionable point, as the small vessels surrounding the adrenal gland in the connective tissue could potentially get connections with the azygos system. But it seemed logical to disregard these vessels because of the impossibility to get a reproducible protocol and to distinguish them from nervous plexus on cadavers.
It is important to state that we perform these dissections on healthy adrenal glands. But it is known that phaeochromocytoma is a richly vascularized tumor because of its angiogenic capability [19, 26]. Connections between adrenal vein and parietal vessels could be created in those conditions. It deserves further exploration.
Moreover, lack of power may be a weakness of this article. Nonetheless, it is one of the largest series with a strict methodology and a large panel of measurements. The results match with the findings of previous RLAT anatomical studies, and this helps to characterize it as a frequently occurring, but not omnipresent structure, although the rate (86.3%) in our study is superior to the Lejars (77%), Auvert (84%) and Monkhouse (74%) studies [10, 11].
The inferior phrenic vein drained into an adrenal vein in 100% of cases, and we did not report drainage of the IPV directly into the LRV. These results are not consistent with the findings of Loukas [17], who found a connection between LAV and IPV in only 25% of cases and in the renal vein in only 15% (Fig. 5). This difference could be explained by the two different protocols. In fact, IPV was not always dissected up to its origin on the diaphragm.
In conclusion, no anastomosis between left adrenal vein and the azygos system has been found in this study. The inferior phrenic vein has been found to drain to the left adrenal vein or, in case of a double vein to one of the two adrenal veins. We provide a precise mapping of the left adrenal vein variability. Our findings show a consistent prevalence of double adrenal vein (13.6%), and a RLAT has been described in 86.3% cases, which is comparable with the findings of the literature. Our analysis helps to highlight three questionable points that deserve further examinations. IPV relationships are discordant in the literature. Based on anatomical observations on healthy left adrenal glands, the ligation of the adrenal vein solely is not enough to entirely interrupt the adrenal vein drainage. Finally, the dilution caused by diaphragmatic venous flow, the presence of a double adrenal vein and the capsular connections with the azygos system should be taken into account for adrenal vein sampling in further clinical studies.
References
Cesmebasi A, Du Plessis M, Iannatuono M et al (2014) A review of the anatomy and clinical significance of adrenal veins. Clin Anat N Y N 27:1253–1263. doi:10.1002/ca.22374
Auchus RJ, Michaelis C, Wians FH et al (2009) Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg 249:318–321. doi:10.1097/SLA.0b013e3181961d77
Kahn SL, Angle JF (2010) Adrenal vein sampling. Technol Vasc Interv Radiol 13:110–125. doi:10.1053/j.tvir.2010.02.006
Webb R, Mathur A, Chang R et al (2012) What is the best criterion for the interpretation of adrenal vein sample results in patients with primary hyperaldosteronism? Ann Surg Oncol 19:1881–1886. doi:10.1245/s10434-011-2121-5
Kempers MJE, Lenders JWM, van Outheusden L et al (2009) Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 151:329–337
Pirvu A, Naem N, Baguet JP et al (2014) Is adrenal venous sampling mandatory before surgical decision in case of primary hyperaldosteronism? World J Surg 38:1749–1754. doi:10.1007/s00268-014-2461-y
Anson BJ, Caudwell EW (1948) The anatomy of the para-renal system of veins, with comments on the renal arteries. J Urol 60:714–737
Clemente C (1985) Grey’s anatomy of the human body, 30th edn. Lea & Febiger, Philadelphia
Morre K, Persaud T (2003) The developing human, 7th edn. Saunders, Philadelphia
Monkhouse WS, Khalique A (1986) The adrenal and renal veins of man and their connections with azygos and lumbar veins. J Anat 146:105–115
Auvert J (1967) The left renal vein. Presse Méd 75:1405–1407
Plaisant O, Chung CS, Uhl JF et al (2001) The origin of the azygos venous system, as deduced from an anatomical and radiological study employing a corrosion technique. Eur J Morphol 39:193–201
Johnstone FR (1957) The suprarenal veins. Am J Surg 94:615–620
Cade S (1954) Adrenalectomy for hormone dependent cancers: breast and prostate. Ann R Coll Surg Engl 15:71–107
Gagnon R (1956) The venous drainage of the human adrenal gland. Rev Can Biol Éditée Par Univ Montr 14:350–359
Hidai H (1976) Studies on locality diagnosis of adrenal tumors: anatomical study on adrenal and juxta-adrenal veins in Japanese and its contribution to adrenal vein catheterization method (author’s transl). Nihon Hinyōkika Gakkai Zasshi Jpn J Urol 67:1018–1024
Loukas M, Louis RG, Hullett J et al (2005) An anatomical classification of the variations of the inferior phrenic vein. Surg Radiol Anat SRA 27:566–574. doi:10.1007/s00276-005-0029-0
Rambaud B, Nohra J, Khedis M et al (2007) Retroperitoneal laparoscopic surgery for phaeochromocytoma: analysis of morbidity and haemodynamic instability. Prog En Urol J Assoc Fr Urol Société Fr Urol 17:1319–1323
Scholten A, Cisco RM, Vriens MR et al (2013) Variant adrenal venous anatomy in 546 laparoscopic adrenalectomies. JAMA Surg 148:378–383. doi:10.1001/jamasurg.2013.610
Wu G, Zhang B, Yu C et al (2013) Effect of early adrenal vein ligation on blood pressure and catecholeamine fluctuation during laparoscopic adrenalectomy for pheochromocytoma. Urology 82:606–611. doi:10.1016/j.urology.2013.05.011
Vassiliou MC, Laycock WS (2009) Laparoscopic adrenalectomy for pheochromocytoma: Take the vein last? Surg Endosc 23:965–968. doi:10.1007/s00464-008-0264-7
Asmar M, Wachtel H, Yan Y et al (2015) Reversing the established order: Should adrenal venous sampling precede cross-sectional imaging in the evaluation of primary aldosteronism? J Surg Oncol 112:144–148. doi:10.1002/jso.23963
Satoh F, Morimoto R, Ono Y et al (2015) 8D.04 Clinical benefits of administering super-selective segmental adrenal venous sampling and performing adrenal sparing surgery in the patients with primary aldosteronism. J Hypertens 33(Suppl 1):e114. doi:10.1097/01.hjh.0000467658.23414.b0
Young WF, Stanson AW (2009) What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol 70:14–17. doi:10.1111/j.1365-2265.2008.03450.x
Madre J (2013) Mesurim Pro. Académie d’Amiens
Grise P, Kuhn JM (2003) Feocromocitoma. EMC—Urol 35:1–12. doi:10.1016/S1761-3310(03)72379-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Siebert, M., Robert, Y., Didier, R. et al. Anatomical Variations of the Venous Drainage from the Left Adrenal Gland: An Anatomical Study. World J Surg 41, 991–996 (2017). https://doi.org/10.1007/s00268-016-3817-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3817-2