Abstract
Background
The purpose of this study was to determine the prevalence of hypomagnesemia in patients undergoing thyroidectomy and evaluate the relationship of hypomagnesemia with transient and severe hypocalcemia.
Materials and methods
This was a prospective observational study of 50 patients undergoing thyroidectomy. Blood samples were collected pre- and postoperatively for calcium, albumin, magnesium, phosphorous and parathormone (PTH). Signs, symptoms of hypocalcemia and volume of intravenous fluids used perioperatively were documented. The statistical analysis was performed using STATA I/C 10.1.
Results
Preoperatively, twelve patients (24 %) had hypomagnesemia and one (2 %) hypocalcemia. On the first postoperative day, hypomagnesemia was seen in 70 % and hypocalcemia in 30 %. A similar trend was observed in the fall and rise of postoperative calcium and magnesium values (p = 0.41). Severe hypocalcemia was present in three patients (6 %). All three patients had a very low postoperative PTH (<2 pg/ml). Among them, two patients (66 %) had hypomagnesemia and their hypocalcemia responded to intravenous magnesium correction. Significant risk factors for postoperative hypocalcemia include a higher volume of fluid used perioperatively and low postoperative PTH (<8 pg/ml) (p = 0.01 and 0.03, respectively).
Conclusion
Preoperative hypomagnesemia (24 %) was prevalent in this cohort of patients. Postoperative hypomagnesemia is a common event (70 %) following total thyroidectomy, and magnesium levels tend to mimic the calcium levels postoperatively. The cause of hypocalcemia post-thyroidectomy in this study is mainly a factor of parathyroid function and fluid status. Severe hypocalcemia is a rare event, and hypomagnesemia is associated in the majority of these patients. The role of magnesium correction to alleviate severe hypocalcemia needs to be further studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total thyroidectomy has been advocated as the procedure of choice to reduce short-term and long-term recurrence in malignant and benign disease. It requires skilled capsular dissection to avoid nerve and parathyroid injury. It is generally a safe procedure, but hypocalcemia is the most frequent postoperative complication. Hypocalcemia may be permanent or temporary. Permanent hypocalcemia is a crippling complication, fortunately reported to be infrequent (0–3.5 %) [1]. Transient hypocalcemia on the other hand is more frequent with an incidence ranging from 0.3 to 65 % in the literature, and the response to treatment is unpredictable [2–4]. The risk factors for the development of hypocalcemia following total thyroidectomy have been extensively investigated in the literature but there is limited experience on the role of hypomagnesemia in this context. Hypomagnesemia has long been associated with hypocalcemia in chronic disease states. Hypomagnesemia has been postulated to result in decreased production/secretion of parathormone (PTH), end organ resistance to PTH and decreased production of vitamin D all of which results in hypocalcemia [5–12]. In our experience with post-thyroidectomy hypocalcemia (unpublished data), we observed a subset of patients in whom the serum calcium does not rise, despite adequate supplements of oral elemental calcium (1 gm thrice daily) and vitamin D (calcitriol 0.5 μg twice daily). We termed this as “severe hypocalcemia”. Hypomagnesemia was present in 75 % of these patients. In the literature, there are only four studies that have investigated the effect of post-thyroidectomy hypomagnesemia on the development of hypocalcemia [13–16]. All four studies detected a significant fall in magnesium levels following thyroidectomy. Wilson et al., Garrahy et al. and Hammerstad et al. have additionally reported a significant association between postoperative hypomagnesemia and hypocalcemia (p = 0.04, 0.005 and 0.015, respectively) [13–15].
We conducted this prospective study to determine the prevalence of hypomagnesemia among patients undergoing thyroidectomy and to evaluate if postoperative hypomagnesemia is a risk factor for the development of post-thyroidectomy temporary and severe hypocalcemia.
Materials and methods
A prospective observational study of fifty patients undergoing total thyroidectomy between 1st October 2012 and 30th September 2013 was conducted at the department of Endocrine Surgery, Christian Medical College, Vellore, Tamil Nadu, India. All adult patients undergoing total thyroidectomy for benign or malignant disorders of the thyroid were included in the study.
Pre- and postoperative blood samples were collected for calcium, albumin, magnesium, phosphorous and PTH. Postoperative blood samples were collected between 5 and 6 a.m. on all days following surgery till discharge and a fasting sample at the first out-patient review. The definitions used in this study were as follows—Hypocalcemia: corrected calcium <8.0 mg/dl, hypomagnesemia: S. magnesium (total) <1.8 mg/dl, hypophosphatemia: S. phosphorous <2.5 mg/dl and hypoparathyroidism: PTH <8 pg/ml. The biochemical estimation of serum calcium, magnesium, phosphorous and albumin was performed using photometric method (modular P800 automated analyser—Roche), and iPTH (intact parathormone) was estimated by chemiluminescence (Adiva centaur centre—Siemens). The volume of intravenous fluids used (in millilitres) as well as the presence or absence of symptoms and signs (Chvostek’s and Trousseau’s) of hypocalcemia was documented. The symptoms included tingling/numbness in the face and extremities, typically perioral, fingers and toes; cramping sensations in the muscles of the arms, legs and abdomen. Chvostek’s sign was elicited by tapping the cheek in the region of the anterior border of the masseter (Chvostek’s II) which is more sensitive than the Chvostek’s I elicited by tapping antero-inferior to the tragus. Contraction of the facial muscles indicated a positive test. The result was graded as absent, sluggish or brisk. Trousseau’s sign was evaluated by inflating the sphygmomanometer cuff to 20 mmHg above the systolic blood pressure for one minute, during which time induction of a carpal spasm indicated a positive test. The result was documented as being positive or negative. These signs and symptoms were elicited between 7 and 8 a.m. on all postoperative days till discharge by the same person.
Normocalcemic patients were discharged on the second postoperative day. Hypocalcemic patients received calcium supplementation as per the post-thyroidectomy hypocalcemia treatment protocol depicted in Table 1. Hypocalcemic patients who despite supplementation with calcium carbonate 3 gm and calcitriol 1mcg daily for at least 24 h continued to have falling corrected calcium levels were said to have “severe hypocalcemia”. In these patients, if total serum magnesium was <1.8 mg/dl (hypomagnesemia), intravenous (IV) magnesium sulphate 2 gm was infused thrice daily for 2 days. Patients with hypomagnesemia whose calcium levels marginally improved or remained stable on calcium and vitamin D supplements were not treated with intravenous magnesium.
The statistical analysis was performed using STATA I/C 10.1. The descriptive statistics were performed using mean with standard deviation or frequency with percentages. Categorical data were summarized using frequency along with percentages. χ 2 square test was used to assess the association between variables. Postoperative hypocalcemia and hypomagnesemia were binary outcome variables, and therefore, a logistic regression was performed for univariate and multivariate analysis. A significant association was defined with a (p) value of less than 0.05.
Results
Patient characteristics, surgical details and pathology are depicted in Table 2.
Pre- and postoperative serum calcium and magnesium
Preoperative hypocalcemia was present in only one patient whose corrected calcium was 7.8 mg/dl. In four patients (8 %), the corrected calcium values were between 8 and 8.29 mg/dl. The remaining forty-five patients (90 %) had corrected calcium within our laboratory reference range. Twelve patients (24 %) had hypomagnesemia preoperatively (range 1.62–1.78 mg/dl). Fifteen patients (30 %) developed hypocalcemia (corrected calcium range 6.9–7.9 mg/dl), and thirty-five patients (70 %) developed hypomagnesemia (range 1.10–1.78 mg/dl) following surgery.
Postoperative symptoms and signs of hypocalcemia
Twenty-five patients (50 %) had clinical features of hypocalcemia postoperatively. 21/25 patients (84 %) had either symptoms or signs present. Among these patients, hypomagnesemia was present in 19/21 (90.5 %), whereas hypocalcemia was present in 11/21 (52.4 %) patients. Four patients (16 %) had both symptoms and signs present, and both hypocalcemia and hypomagnesemia were present in all four patients. Hypomagnesemic patients were at a higher risk of developing clinical features of hypocalcemia (p = 0.06).
Risk factors for postoperative hypocalcemia
Univariate analysis of the risk factors for postoperative hypocalcemia in this study is shown in Table 3. The significant risk factors were PTH status and hemodilution.
We used postoperative PTH to assess the degree of parathyroid injury. Twenty patients had low postoperative PTH (<8 pg/ml). Among these, nine patients (45 %) had hypocalcemia. The PTH for these nine patients was very low (<3 pg/ml). The remaining eleven patients were normocalcemic. Surprisingly, 6/30 patients (20 %) with normal PTH were hypocalcemic. Two of these patients developed a positive Chvostek’s sign on postoperative day one alone, while one patient had mild symptoms of hypocalcemia. None of these patients required readmission for hypocalcemia. The risk of developing hypocalcemia was four times greater (95 % CI 1.11–15.06) in patients with low postoperative PTH than in those with a normal postoperative PTH (p = 0.034). Additionally in the postoperative period as the calcium decreased, phosphorous increased suggesting the presence of hypoparathyroidism (Fig. 1). Postoperative low PTH was a significant indicator for the development of postoperative hypocalcemia on multivariate analysis as well (p = 0.05).
The volume of fluids used perioperatively varied from 1500 to 4000 ml, averaging 2330 ml. Forty two percent received 2500 ml of intravenous fluids in the perioperative period. When analysed as a continuous variable, a significant association between the volume of fluids used and the presence of postoperative hypocalcemia was observed (p = 0.03). Univariate analysis revealed that patients who received more than 2500 ml of fluids were at nine times greater risk (95 % CI 1.57–56.96) of developing hypocalcemia compared to those who received less than 2500 ml. On multivariate analysis, as well, these patients were at significantly greater risk of developing postoperative hypocalcemia (p = 0.01).
The factors of age greater than 40 years, female gender, hyperthyroidism and the extent of surgery were not significantly associated with the development of postoperative hypocalcemia in this study (p = 0.84, 0.65, 0.12 and 0.64, respectively).
Risk factors for postoperative hypomagnesemia
Univariate analysis of the risk factors for postoperative hypomagnesemia in this study is shown in Table 4. Gender was the only significant risk factor. The risk of development of postoperative hypomagnesemia was almost seven times greater (95 % CI 1.71–27.46) for females when compared to males on univariate analysis (p = 0.007). This risk fell to five times (95 % CI 0.85–30.76) on multivariate analysis which was close to significance (p = 0.075).
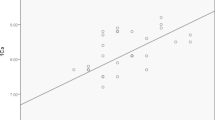
All other factors evaluated were not significant. Among the twenty patients with low postoperative PTH, as many as sixteen (80 %) had hypomagnesemia; however, hypomagnesemia was also high (19/30, 63.3 %) in patients with normal PTH. Despite the boxplot (Fig. 2) illustrating lower magnesium levels among patients with low postoperative PTH than those with normal PTH, this was not significant (p = 0.21). The volume of perioperative fluids used, age greater than 40 years or the extent of surgery were significantly associated with the development of postoperative hypomagnesemia (p = 0.1, 0.11 and 0.10, respectively). There were five patients with hyperthyroidism in this cohort and all five were hypomagnesemic. Hence univariate analysis could not be performed to compare hyperthyroidism and development of postoperative hypomagnesemia. Chi-square test showed no significant association between these two factors (p = 0.12).
The hypocalcemia–hypomagnesemia relationship
Among the fifteen patients with hypocalcemia, twelve (80 %) had associated with hypomagnesemia. Though a similar trend of fall in calcium and magnesium below the normal range in the immediate postoperative period and normalization by end of the first week was observed (Fig. 1), this was not significant (p = 0.41).
Three patients (6 %) developed severe hypocalcemia with very low postoperative PTH (<2 pg/ml). Two of these patients (66 %) were hypomagnesemic (Mg of 1.07 and 1.14 mg/dl) and had persistent symptoms of hypocalcemia. Following intravenous magnesium correction, hypocalcemic symptoms resolved and serum calcium rapidly normalized. These numbers were too small for statistical analysis.
Discussion
Preoperative serum calcium and magnesium
Five patients had calcium levels below our laboratory reference range prior to surgery. Among them, only one patient developed postoperative hypocalcemia (corrected calcium 7.6 mg/dl). This patient had associated mild symptoms which resolved in 2 days with calcium supplementation. In the study by Wilson et al., preoperative calcium level below their laboratory reference range was detected in only one patient. This patient developed mild symptoms of hypocalcemia following surgery, though calcium levels were normal and the symptoms resolved without calcium supplementation [13]. Other authors have depicted normal preoperative calcium levels in all patients [15–17].
Literature on the prevalence of hypomagnesemia among the normal population is scarce. In this study, 12 patients (24 %) were found to be hypomagnesemic prior to surgery. All the twelve patients had mild hypomagnesemia with values between 1.6 and 1.79 mg/dl. To the best of our knowledge, this is the first documentation of the prevalence of hypomagnesemia among this subset of patients in India. This prevalence lies between the estimates of 4.6 and 14.5 % in Iranian and German population and 43 % among pregnant women in Haryana [18–20]. The study from Haryana in India also found a significant association between parity and hypomagnesemia, women with parity of more than two being at a higher risk of developing hypomagnesemia than nulliparous women [20]. The higher prevalence of hypomagnesemia observed in our study as well as the one from Haryana may be secondary to nutritional factors.
There are several reports of hypomagnesemia following chronic proton pump inhibitor (PPI) usage [21–24]. PPIs impair absorption of magnesium by the intestinal epithelial cells by inducing inhibition of transient receptor potential melastatin 6 and 7 channels [25]. In India, these medications are commonly dispensed over the counter. These factors were not assessed in our study.
Postoperative symptoms and signs of hypocalcemia/hypomagnesemia
Wilson et al. showed that both hypocalcemia and hypomagnesemia were significantly associated with symptoms (p = 0.015 and 0.006, respectively), and patients were more likely to be symptomatic when both cations were low (p = 0.019) [13]. Szubin et al. in a study of forty patients undergoing total thyroidectomy detected that serum magnesium levels decreased slightly in all patients postoperatively and stated that this could exert an unrecognized effect on serum calcium levels. They concluded that magnesium levels need to be monitored postoperatively and corrected if below normal [26]. Jones et al. described a patient with primary hyperparathyroidism who developed symptomatic hypocalcemia (S. calcium—6 mg/dl) on the tenth postoperative day following surgery. This was unresponsive to oral and intravenous calcium correction. She was detected to be hypomagnesemic (S. magnesium—0.7 mg/dl), and following intravenous magnesium correction, there was dramatic improvement in her symptoms and gradual normalization of serum calcium [27]. The present study also suggests that hypomagnesemia correlates closely with the clinical features of hypocalcemia (p = 0.06). Among the three “severe hypocalcemic” patients, two were hypomagnesemic and the persistent hypocalcemic symptoms in these patients were relieved only after intravenous magnesium correction. Hence the correction of hypocalcemia without concurrent normalization of magnesium may prolong the clinical manifestations of hypocalcemia in a subset of patients.
Risk factors for postoperative hypocalcemia and hypomagnesemia
Hypocalcemia is the most common complication following total thyroidectomy [28, 29]. Improved understanding of surgical anatomy as well as advancements in surgical technique has led to lowered rates of post-thyroidectomy hypocalcemia [30]. The rate of post-thyroidectomy hypocalcemia in this study is comparable to that in the literature—up to 30 % [3, 31]. Among the various causes for post-thyroidectomy hypocalcemia, parathyroid injury/ischemia during thyroidectomy has been established as the most common cause [17]. Consistent with the literature, a strong correlation between low postoperative PTH and the development of post-thyroidectomy hypocalcemia (p = 0.034) was seen in this study as well. The release of antidiuretic hormone in response to surgical stress and consequent retention of water is responsible for hemodilution. Hemodilution leads to alteration of plasma electrolyte concentration resulting in transient postoperative decrease of calcium, magnesium, phosphorous and albumin. Demeester-Mirkine et al. have demonstrated fall in postoperative total and ionized calcium due to hemodilution. [32]. In the present study, the volume of fluids used perioperatively was significantly associated with the development of postoperative hypocalcemia (p = 0.01) despite correction for albumin. From our results, it appears that the serum albumin correction may not fully account for the perioperative dilutional change in calcium levels, and the quantum of IV fluids used may be an independent factor; the mechanism is not clearly understood. Hence, low postoperative PTH and hemodilution were the main factors for the development of postoperative hypocalcemia in this cohort.
A large number of patients were hypomagnesemic following surgery; thirty-five patients (70 %). A similar prevalence of postoperative hypomagnesemia was detected by Wilson et al. in 36/50 patients (72 %) undergoing thyroidectomy. They found hemodilution to play a significant role in the development of hypomagnesemia following thyroidectomy (p = 0.027) [13]. Surprisingly, in this study, the volume of fluids used was not significantly associated with the development of postoperative hypomagnesemia (p = 0.11). However, we have showed a significant association between female gender and the development of post-thyroidectomy hypomagnesemia on univariate analysis (p = 0.007) and a near significant association on multivariate analysis (p = 0.075). Increased parity has been reported to be associated with hypomagnesemia [20]. Though we had not assessed parity, the significant association of female gender and hypomagnesemia could be attributed to increased parity.
PTH stimulates magnesium reabsorption from the kidney and small intestine [33, 34]. PTH also causes release of magnesium from the bone. These actions of PTH result in increasing the magnesium levels. Therefore, a low PTH would result in decreased magnesium levels. In this study, we have documented a low PTH in twenty patients (40 %) postoperatively. Though postoperative PTH and hypomagnesemia were not significantly associated (p = 0.21), magnesium levels were found to be lower in the patients with low postoperative PTH than in patients with normal postoperative PTH.
Hyperthyroid patients have been shown to have decreased serum magnesium concentrations and increased urinary magnesium excretion which could lead to hypomagnesemia [5]. In this study, five patients were hyperthyroid, and though all five were hypomagnesemic, there was no significant association between the two (p = 0.12). The other causes for hypomagnesemia include severe postoperative vomiting and diarrhoea, uncontrolled blood sugars and chronic alcoholism.
The Hypocalcemia–hypomagnesemia relationship
Wilson et al. demonstrated that hypomagnesemia was common in patients undergoing thyroidectomy (72 %) and significantly associated with the development of post-thyroidectomy hypocalcemia [13]. A similar conclusion was made by Garrahy et al. in a retrospective study of two hundred and one patients undergoing thyroidectomy. They also showed that a significant fall in magnesium levels after surgery (p < 0.0001) was significantly associated with the development of permanent hypocalcemia (p = 0.0004) [14]. Hammerstad et al. evaluated the risk factors for the development of hypocalcemia among forty patients with Graves’ disease undergoing total thyroidectomy. They found that patients who developed permanent hypocalcemia exhibited a significant fall in magnesium levels postoperatively at 48 h compared to the preoperative magnesium levels (p = 0.015) [15]. In all the above studies, the lower limit of the reference range for magnesium was 1.7 mg/dl. Sousa et al. evaluated 333 patients undergoing thyroidectomy and found a significant fall in postoperative magnesium levels compared to preoperative levels (p = 0.017) which also varied with the calcium level. However, as the postoperative magnesium levels remained within the reference range (1.6–2.3 mg/dl), they surprisingly concluded that there was no relationship between calcium and magnesium levels in their study [16].
In the present study, though postoperative magnesium levels fell proportionately with the calcium levels, hypomagnesemia was not significantly associated with hypocalcemia (p = 0.41). In the three patients with severe hypocalcemia, two were hypomagnesemic and resistant to calcium and vitamin D therapy. The correction of hypomagnesemia in these two patients resulted in a dramatic normalization of clinical and biochemical hypocalcemia. Hence, a possible role for magnesium correction in severe hypocalcemic patients is proposed and needs to be further studied.
Conclusion
The prevalence of preoperative hypomagnesemia was frequent in this cohort of patients undergoing thyroidectomy and all had mild deficiency. Postoperative hypomagnesemia is seen in the majority of patients following total thyroidectomy, and female patients are at a significantly greater risk of developing the same. Magnesium levels tend to mimic the calcium levels postoperatively. Hypomagnesemia is significantly associated with symptoms and signs of hypocalcemia. The cause of hypocalcemia post-thyroidectomy is mainly a factor of parathyroid function and fluid status. Severe hypocalcemia is a rare event and hypomagnesemia is associated in the majority. We conclude that evaluation for hypomagnesemia should be performed only among the patients who develop severe hypocalcemia following surgery and not routinely before or after thyroid surgery. The role of magnesium correction to alleviate severe hypocalcemia needs to be further studied.
References
Wu J, Harrison B (2010) Hypocalcemia after thyroidectomy: the need for improved definitions. World J Endocr Surg. 2(1):17–20
Kim JH, Chung MK, Son Y-I (2011) Reliable early prediction for different types of post-thyroidectomy hypocalcemia. Clin Exp Otorhinolaryngol. 4(2):95–100
Kirkby-Bott J, Markogiannakis H, Skandarajah A, Cowan M, Fleming B, Palazzo F (2011) Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg 35(2):324–330
McHenry CR, Speroff T, Wentworth D, Murphy T (1994) Risk factors for postthyroidectomy hypocalcemia. Surgery. 116(4):641–647; discussion 647–8
Rude RK, Singer FR (1981) Magnesium deficiency and excess. Annu Rev Med 32(1):245–259
Rude RK (1998) Magnesium deficiency: a cause of heterogeneous disease in humans. J Bone Miner Res 13(4):749–758
Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK (1991) Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab 73(5):1067–1072
Chase LR, Slatopolsky E (1974) Secretion and metabolic efficacy of parthyroid hormone in patients with severe hypomagnesemia. J Clin Endocrinol Metab 38(3):363–371
Suh SM, Tashjian AH Jr, Matsuo N, Parkinson DK, Fraser D (1973) Pathogenesis of hypocalcemia in primary hypomagnesemia: normal end-organ responsiveness to parathyroid hormone, impaired parathyroid gland function. J Clin Invest. 52(1):153–160
Mori S, Harada S, Okazaki R, Inoue D, Matsumoto T, Ogata E (1992) Hypomagnesemia with increased metabolism of parathyroid hormone and reduced responsiveness to calcitropic hormones. Intern Med 31(6):820–824
Freitag JJ, Martin KJ, Conrades MB, Bellorin-Font E, Teitelbaum S, Klahr S et al (1979) Evidence for skeletal resistance to parathyroid hormone in magnesium deficiency. J Clin Invest. 64(5):1238–1244
Rude RK, Oldham SB, Singer FR (1976) Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin Endocrinol (Oxf). 5(3):209–224
Wilson RB, Erskine C, Crowe PJ (2000) Hypomagnesemia and hypocalcemia after thyroidectomy: prospective study. World J Surg 24(6):722–726. doi:10.1007/s002689910116
Garrahy A, Murphy MS, Sheahan P (2014) Impact of postoperative magnesium levels on early hypocalcemia and permanent hypoparathyroidism after thyroidectomy. Head Neck. doi:10.1002/hed.23937
Hammerstad SS, Norheim I, Paulsen T, Amlie LM, Eriksen EF (2013) Excessive decrease in serum magnesium after total thyroidectomy for Graves’ disease is related to development of permanent hypocalcemia. World J Surg 37(2):369–375. doi:10.1007/s00268-012-1843-2
de Sousa A, Salles JMP, Soares JMA, de Moraes GM, Carvalho JR, Savassi-Rocha PR (2010) Evolution of blood magnesium and phosphorus ion levels following thyroidectomy and correlation with total calcium values. Sao Paulo Med J 128(5):268–271
Tredici P, Grosso E, Gibelli B, Massaro MA, Arrigoni C, Tradati N (2011) Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital 31(3):144–148
Syedmoradi L, Ghasemi A, Zahediasl S, Azizi F (2011) Prevalence of hypo- and hypermagnesemia in an Iranian urban population. Ann Hum Biol 38(2):150–155
Schimatschek HF, Rempis R (2001) Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res 14(4):283–290
Pathak P, Kapoor SK, Kapil U, Dwivedi SN (2003) Serum magnesium level among pregnant women in a rural community of Haryana State, India. Eur J Clin Nutr 57(11):1504–1506
Epstein M, McGrath S, Law F (2006) Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 355(17):1834–1836
Broeren MAC, Geerdink EAM, Vader HL, van den Wall Bake AWL (2009) Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern Med 151(10):755–756
Cundy T, Dissanayake A (2008) Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf). 69(2):338–341
Hess MW, Hoenderop JGJ, Bindels RJM, Drenth JPH (2012) Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 36(5):405–413
Perazella MA (2013) Proton pump inhibitors and hypomagnesemia: a rare but serious complication. Kidney Int 83(4):553–556
Szubin L, Kacker A, Kakani R, Komisar A, Blaugrund S (1996) The management of post-thyroidectomy hypocalcemia. Ear Nose Throat J 75(9):612–614; 616
Jones CT, Sellwood RA, Evanson JM (1973) Symptomatic hypomagnesaemia after parathyroidectomy. Br Med J 3(5876):391–392
Christou N, Mathonnet M (2013) Complications after total thyroidectomy. J Visc Surg. 150(4):249–256
Rosato L, Avenia N, Bernante P, Palma MD, Gulino G, Nasi PG et al (2004) Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg 28(3):271–276. doi:10.1007/s00268-003-6903-1
Nair CG, Babu MJC, Menon R, Jacob P (2013) Hypocalcaemia following total thyroidectomy: an analysis of 806 patients. Indian J Endocrinol Metab. 17(2):298
Mishra A, Agarwal A, Agarwal G, Mishra SK (2014) Total thyroidectomy for benign thyroid disorders in an endemic region. World J Surg 25(3):307–310. doi:10.1007/s002680020100
Demeester-Mirkine N, Hooghe L, Van Geertruyden J, De Maertelaer V (1992) Hypocalcemia after thyroidectomy. Arch Surg 127(7):854–858
Vetter T, Lohse MJ (2002) Magnesium and the parathyroid. Curr Opin Nephrol Hypertens 11(4):403–410
Swaminathan R (2003) Magnesium metabolism and its disorders. Clin Biochem Rev. 24(2):47–66
Grant support
This study was supported by Fluid research grant—Christian Medical College, Vellore, and The Tamil Nadu Dr MGR Medical University research grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is registered with Clinical Trials Registry, India (CTRI): CTRI/2013/12/004195.
Rights and permissions
About this article
Cite this article
Cherian, A.J., Gowri, M., Ramakant, P. et al. The Role of Magnesium in Post-thyroidectomy Hypocalcemia. World J Surg 40, 881–888 (2016). https://doi.org/10.1007/s00268-015-3347-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3347-3