Abstract
Background
Although lymph node (LN) metastasis (LNM) of papillary thyroid carcinoma (PTC) is common, routine prophylactic LN dissection (LND) is still controversial. The purpose of this study was to investigate risk factors for recurrence of PTC with clinically node-negative lateral neck to determine the utility of intraoperative LN biopsy.
Materials and methods
This study involved 185 patients with pathologically confirmed PTC and clinically node-negative lateral neck. All patients underwent thyroidectomy with or without ipsilateral or bilateral central LND after intraoperative central LN biopsy. Routine lateral neck LND was not performed. Clinicopathologic and intraoperative findings and post-treatment recurrences were recorded. Univariate and multivariate analyses with Cox-proportional hazards model were used to identify factors associated with recurrence.
Results
During a follow-up of 50–96 months, six (3.2 %) patients had recurrences in lateral cervical LNs at a median 28 months (range 7–57 months) after surgery. Overall, 2- and 5-year RFS rates were 98.4 and 96.7 %, respectively. Univariate analyses revealed that tumor size (P = 0.005), bilaterality (P = 0.033), T4 disease (P < 0.001), and intraoperative diagnosis of central LNM (P = 0.001) were significantly predictive of recurrence. Multivariate analyses showed that T4 disease (P = 0.049) and intraoperative diagnosis of central LNM (P = 0.027) were independently predictive of recurrence.
Conclusions
Prophylactic lateral neck LND is not advocated for PTC with clinically node-negative lateral neck. Intraoperative LN biopsy may help identify patients at risk for recurrence and those who would benefit from LND.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, accounting for approximately 85 % of all thyroid cancers in areas of sufficient iodine intake [1]. PTC shows an excellent prognosis, with 10-year overall survival rates after surgery greater than 90 %, and only a few patients dying of recurrences or metastases [1, 2]. However, PTC metastasizes to regional lymph nodes (LNs) in approximately 30–80 % of patients [3, 4]. The metastatic pattern of PTC consists of an orderly progression to the lymphatic basin, firstly to the central neck and subsequently to the lateral neck compartment [5, 6]. Cervical LN metastasis has no major impact on survival in low-risk patients but is an important factor for locoregional recurrence [7]. Despite this high rate of PTC metastasis, prophylactic LN dissection has significant risks and minimal impact on survival [4, 8, 9].
Little is known about the clinical implications of elective lateral LN dissection (LLND) in PTC, although many studies have analyzed the implications of central LN dissection (CLND). Prophylactic LLND, defined as the removal of jugular LNs at cervical levels II–IV, revealed that 57.5 % of patients with PTC had metastatic LNs in the lateral compartment [10]. Although therapeutic LLND, along with imaging and biopsy of the lateral neck, has been recommended, the utility of prophylactic LLND remains unclear, regardless of imaging or palpation findings [11, 12]. No studies have shown any survival benefit or reduction in recurrence when prophylactic surgery is performed [9, 11]. Therefore, American Thyroid Association (ATA) has not recommended prophylactic LLND for patients with clinically node-negative lateral neck [11]. However, the accuracy of ultrasound (US) and computed tomography (CT) for diagnosing nodal metastasis may be as low as 27 % [10]. The failure to detect N1b disease may lead to false stage assignment as stage III or lower rather than stage IV, which may cause postoperative recurrence. Furthermore, few randomized controlled trials have assessed the necessity and extent of neck dissection in PTC and its prognostic implications.
The aim of this study was to investigate the risk factors for recurrence of PTC in patients with clinically node-negative lateral neck, as determined by image-guided fine needle aspiration (FNA) and intraoperative LN biopsy of the lateral neck. This would help determine the utility of intraoperative LN biopsy.
Materials and methods
Study design
From March 2006 to August 2008, this study involved patients with pathologically confirmed, previously untreated PTC and clinically node-negative lateral neck who underwent thyroidectomy with or without ipsilateral or bilateral CLND. Preoperative US and FNA were used to identify primary tumors and LNs suspected of metastases. Patients also underwent intraoperative LN biopsy. Patients were excluded if they had metastatic nodes in the lateral neck before and at surgery (n = 48), recurrent PTCs (n = 26), or other thyroid pathology (n = 41), or were lost to follow within 2 years after surgery (n = 4), or had distant metastases at initial presentation (n = 1) (Fig. 1). A total of 185 consecutive patients were finally included. Tumors were staged according to the American Joint Committee on Cancer (AJCC) staging system (7th ed., 2010) [13]. The Institutional Review Board of our hospital approved this study, and written informed consent was obtained from all enrolled patients.
Flow chart of the study population. Note: Clinically node-negative lateral neck indicates the absence of positive LNs from ultrasonography-guided fine needle aspiration and intraoperative LN biopsy in the lateral neck. LNs lymph nodes, PTC papillary thyroid carcinoma, IO intraoperative, LNM lymph node metastasis, CLND central neck lymph node dissection
Surgery, intraoperative LN biopsy, and pathological examination
The enrolled patients underwent total thyroidectomy (n = 178) or lobectomy and isthmusectomy (n = 7). Lobectomy was typically performed as the initial surgical treatment for patients with low-risk papillary thyroid microcarcinoma (PTMC) and a solitary malignant lesion limited to a single lobe [11]. Any patients who had a solitary PTMC but positive LNs on pathology underwent total or completion thyroidectomy. Of the 185 patients, 101 underwent prophylactic CLND, 48 underwent therapeutic CLND, and 36 received no treatment (Fig. 1). Therapeutic CLND was defined when preoperative or intraoperative histological diagnosis of central compartment lymph node metastasis was made, regardless of macro- or micro-metastasis. All patients underwent preoperative high-resolution US and intraoperative central LN biopsy. Patients suspected of having metastatic nodes in the central compartment underwent therapeutic bilateral CLND. Unilateral CLND was performed on that side for clinically non-metastatic PTC localized to the ipsilateral side or for PTCs with a single positive intraoperative central LN and small metastatic foci. Unilateral CLND included dissection of the prelaryngeal/pretracheal and paratracheal regions ipsilateral to the proven PTC location [14]. Patients who had a solitary PTMC without metastasis, extrathyroid extension or local invasion, and had no familial history of thyroid cancer or previous irradiation of the neck did not undergo unilateral or bilateral CLND.
CLND was performed in a conventional manner, not using typical microdissection methods [15]. Nodal clearance was performed cranially to both superior thyroid arteries and the pyramidal lobe, caudally to the innominate vein, laterally to the carotid sheaths, and dorsally to the prevertebral fascia [4]. Particular attention was paid to ensure the identification of the parathyroid glands, and parathyroid autotransplantation was performed as required, not on principle. The thymus was routinely preserved by separation from the central LNs. Routine LLND was not performed on patients without preoperatively or intraoperatively positive lateral neck nodes. All surgery was performed by the same surgeon (J-L.R.).
All patients underwent intraoperative biopsy of sentinel or non-sentinel LNs. The intraoperative biopsy of LNs did not depend on the preoperative US findings. After retraction of the skin and muscular flap, approximately 0.2 mL of 2 % methylene blue was injected into the parenchyma surrounding the primary tumor. The first blue-stained LN, defined as the sentinel LN, was harvested and sent for both frozen and standard sections [16, 17]. In addition, to increase the intraoperative detection rate of metastatic cervical LNs, the other blue-stained LNs as well as any other central LNs, if suspicious of metastasis, were harvested for histological examination. The presence of metastatic foci from intraoperative LN biopsy was first examined with step serial frozen sections and was confirmed with permanent sections. Patients without apparent sentinel LNs also underwent intraoperative biopsy of one or more non-sentinel LNs close to primary tumors in the central compartment. Patients who had no sentinel LNs in the central compartment often underwent exploration of ipsilateral level IV. However, no other patients underwent routine exploration of LNs in the lateral neck compartment that were not suspected of metastasis by intraoperative palpation or preoperative images. Any patients with metastatic LNs in the lateral compartment that were intraoperatively identified were excluded in analyses because they underwent simultaneous therapeutic LLND.
The CLND specimens were marked, separated, and sent for pathological examination, along with thyroid specimens. Pathologic specimens were stained with hematoxylin–eosin and viewed by light microscopy. The size, number, and location of each primary tumor and the presence of extrathyroidal extension and lymphovascular invasion of the primary tumor were carefully assessed. Histological examinations were performed by a board-certified pathologist (G.G.) with 20 years of experience in thyroid pathology. The size and number of LNs harvested, and the number, size, and extracapsular spread (ECS) of positive LNs were reported.
Postoperative complications and follow-up
All patients underwent pre- and postoperative laryngoscopic examination and monitoring of serum total calcium. Hypocalcemia was defined as total calcium <8.0 mg/dL, corrected for serum albumin concentration. Permanent hypocalcemia was defined as low total calcium concentration requiring calcium supplementation for more than 6 months after surgery.
Patients with pT1b–T4 or pN1-staged PTC underwent postoperative radioactive iodine (I131) ablation using 30–150 mCi and TSH suppression thyroxine therapy, except for those who had undergone thyroid lobectomy. All patients were followed postoperatively for the identification of recurrent diseases by regular clinical and US examinations, measurement of serum thyroglobulin concentrations, and whole-body iodine scanning after surgery.
Statistical analysis
Statistical analysis was performed with SPSS, version 21.0 for Windows (IBM, Armonk, NY, USA). Continuous variables were expressed as median (range) or mean ± standard deviation (SD), and categorical variables as numbers and percentage. The χ 2 test was used to investigate differences between categorical data related to postoperative complications, and the t test was used to compare means of continuous variables; all the tests were two-sided. The primary endpoint was to identify factors prognostic of recurrence-free survival (RFS). Time periods were calculated from the date of surgery to the date of any recurrence or to the last clinical follow-up. Recurrence was defined as the presence of histologically confirmed recurrent tumors at local, regional and/or distant sites. RFS curves were calculated using the Kaplan–Meier method and compared using the log-rank test. A Cox-proportional hazards model was used to evaluate factors prognostic of RFS by univariate and multivariate analyses; tests were based on the likelihood ratio statistic, and the estimated hazard ratio (HR) and 95 % confidence interval (CI) were calculated. Variables with P < 0.05 on univariate analyses were selected for inclusion in the multivariate model. Receiver operating characteristic (ROC) curve analysis was used to determine the area under the curve (AUC) in order to estimate RFS according to a specific variable and to determine the tumor size cutoff value predicting RFS. All tests were two-sided and differences were considered statistically significant at P < 0.05.
Results
Patient characteristics and pathology
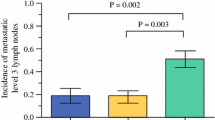
The 185 study patients consisted of 39 men and 146 women, of median age 47 years (range 18–87 years). Patient characteristics are summarized in Table 1. The mean size of proven PTC was 1.2 cm (range 0.3–5.5 cm). Multifocal and bilateral tumors were identified in 60 (32.4 %) and 32 (17.3 %) patients, respectively, with macroscopic and microscopic extracapsular extension in 86 (46.5 %) and 109 (58.9 %) patients, respectively. Tumors stages were T1 in 71 patients (38.4 %), T2 in 2 patients (1.1 %), T3 in 106 patients (57.3 %), and T4a in 6 patients (3.2 %), with 155 (83.8 %) patients having MACIS scores <6.0. Pathologic central LN metastases were observed in 67 (45.0 %) of the 149 patients who underwent CLND (Fig. 1). Methylene blue-stained sentinel LNs were observed in the central compartments of 157 (84.3 %) of the 185 patients, with 44 (23.8 %) positive on intraoperative frozen sections. Four patients were intraoperatively diagnosed with central non-sentinel LNs. Therefore, a total of 48 patients were intraoperatively diagnosed with central LN metastasis (Fig. 1). Nineteen (28 %) of 67 patients with central LN metastasis received no proper diagnosis by intraoperative LN biopsy. The median total LNs harvested and involved in the central neck were 9 (range 3–34) and 1 (range 0–10), respectively. The median size of metastatic tumors was 3.4 mm (range, 0.5–15 mm). The mean size of neck metastatic foci was larger in patients with intraoperative diagnosis of central LN metastasis than in those without it (4.1 ± 2.5 vs. 2.0 ± 1.1 mm, P = 0.001). Bilateral paratracheal metastases were found in 4 of the 39 (10 %) patients who underwent bilateral CLND.
Univariate and multivariate analyses of risk factors for recurrence
All patients were regularly followed for a median 72 months (range 50–96 months) after surgery. Six (3.2 %) patients had recurrences at a median of 28 months (range 7–57 months) after surgery, five in the lateral neck alone and one in the central and lateral neck. ROC curve analyses showed that the AUC of tumor size was 0.827 (95 % CI 0.709–0.945, P = 0.007), with a cutoff value for tumor size of 1.25 cm. Univariate analysis revealed that tumor size (P = 0.005), bilaterality (P = 0.033), advanced tumor (T4, P < 0.001), and intraoperative diagnosis of central LN metastasis (P = 0.002) were significantly associated with recurrence (Table 2). Multivariate analyses showed that T4 disease (HR 6.32, 95 % CI 1.01–39.80; P = 0.049) and intraoperative diagnosis of central LNM (HR 11.59, 95 % CI 1.32–101.86; P = 0.027) were independent factors predictive of recurrence (Table 3; Fig. 2).
Surgical morbidity and follow-up
Of the 185 patients, 34 (18.4 %) had temporary hypocalcemia and two (1.1 %) had permanent hypocalcemia (Table 4). The temporary hypocalcemia rate was significantly higher in patients who underwent bilateral CLND than in those who underwent unilateral CLND and those who did not undergo CLND (2/36 vs. 21/110 and 11/39, P = 0.039). Permanent vocal fold paralysis occurred in five patients, with significant invasion of the unilateral recurrent laryngeal nerve by tumors. Temporary vocal fold paralysis was observed in two patients, both of whom recovered within 3 months; this rate did not differ in patients with and without CLND (0/36 vs. 2/143, P = 0.479). Two patients showed postoperative bleeding and one had minimal chyle leakage.
Assessment of the six patients with recurrence included level II, III, or IV of the lateral neck ipsilateral to the primary tumor in five patients, and ipsilateral levels II and IV and the thyroidectomy bed in one patient. All six patients with tumor recurrences underwent modified radical neck dissection on the ipsilateral side. Five of these patients remain alive without tumor recurrence at the last follow-up; the sixth patient died without evidence of diseases at age 94 years.
Fourteen patients who underwent lobectomy for a non-metastatic, low-risk PTMC did not receive postoperative radioactive I131 ablation. Other remaining patients received I131 ablation of median 80 mCi (range 30–150 mCi). Most patients underwent TSH suppression thyroxine therapy, and four patients with lobectomy refused the therapy.
Discussion
This study examined the risk factors for recurrence in 185 patients with PTC and clinically node-negative lateral neck who underwent thyroidectomy with or without CLND. All patients underwent preoperative US, FNA, and intraoperative central LN biopsy. Although the incidence of central LN metastases was high (45 %) in these patients, only 3.2 % experienced postoperative recurrences, mostly in the lateral neck. Although the lateral neck is the second most common site of PTC metastasis, few studies have assessed elective LLND and its prognostic implications. The efficacy of prophylactic LLND for patients without clinically node-negative lateral neck remains unclear [11, 12], although our data suggest that prophylactic LLND is not necessary due to the relatively low recurrence rate. The expectant management of the lateral neck appears to be reasonable in patients with clinically node-negative lateral neck.
Previous studies assessed the risk factors for lateral cervical LN metastasis in patients who underwent prophylactic LLND for PTC with clinically node-negative lateral neck [10, 18–20]. A recent report showed that the LN metastasis rate in 603 consecutive patients with clinically negative neck who underwent bilateral CLND and LLND was 23 %, including 8 % with occult lateral neck metastasis; thus, in these patients, metastatic LN in the lateral neck was significantly associated with LN metastasis [18]. Another recent study reported that the LN metastasis rate, as confirmed by intraoperative frozen biopsy, in 62 patients with PTC who underwent prophylactic LLND after ipsilateral central metastasis was 55 %, with primary tumor size and an increased number of positive bilateral central LNs being risk factors for occult lateral LN metastasis [19]. A Japanese group utilized an aggressive surgical protocol, including thyroidectomy, CLND, and prophylactic LLND, in 1,231 PTC patients without preoperatively detectable lateral LN metastasis [20]. Lateral LN metastasis was detected in 40.5 % of these patients and was significantly associated with male gender, age ≥55 years, tumor size >3 cm, and massive extrathyroid extension. A systemic review of LN metastasis in PTC showed that the frequency of occult lateral LN metastasis was dependent on T stage, with an overall metastatic rate of 57.5 % [10]. Although these studies reported risk factors for occult lateral LN metastasis in patients who underwent prophylactic LLND, few studies have assessed the risk factors for lateral nodal recurrence in patients who did not undergo routine LLND. Although the surgical approach we used was less aggressive than in previous studies [18–20], we observed a low recurrence rate, perhaps due to the routine use of postoperative radioactive iodine therapy.
We investigated the risk factors for recurrence in PTC patients without prophylactic LLND, emphasizing the clinical importance of intraoperative diagnosis of metastatic LNs, whether or not they were sentinel LNs. Univariate analysis showed that primary tumor size, tumor bilaterality, massive extrathyroidal extension (T4a), and intraoperative diagnosis of LN involvement were significant predictors of recurrence, with T4 disease and intraoperative diagnosis of LN involvement being independently associated with recurrence on multivariate analysis. Local PTC invasiveness has been shown to be prognostic [20], but the prognostic implications of intraoperative central LN biopsy had not been prospectively evaluated. More frequently, intraoperative LN biopsy detected larger metastatic foci in lateral than in false-negative LNs. Most false-negative nodes contained metastatic foci ≤4.0 mm in size, which may not significantly affect recurrence after surgery and postoperative iodine ablation therapy. Intraoperative LN biopsy may be used to select patients who would benefit from compartment neck dissection, thus reducing unnecessary LLND and its possible morbidity in these patients. Minimizing the risks of comorbidities is of great importance in PTC patients with excellent prognoses [21], whereas aggressive surgery may lead to more complications without additional benefits. Extension of the surgical field and tissue peeling using a more aggressive neck dissection may increase postoperative pain, neck and shoulder movement limitations, as well as sensory abnormalities [22, 23].
Although this study had the advantages of prospective data collection, analysis, and follow-up, it had several limitations. Our patients did not undergo routine LLND for PTC with clinically node-negative lateral neck. Randomized controlled trials of prophylactic LLND may be precluded by the considerable additional cost and morbidity. However, the present study is the first to show a significant association between intraoperative central LN diagnosis and recurrence. Sentinel LN biopsy is not currently often used but may be helpful in providing this additional prognostic information. In addition, this may guide in selecting the patients who need to undergo central LN dissection to remove metastatic diseases in the central compartment. It remains unclear, however, whether improvements in the detection of small microscopic metastases in the central neck lead to better prediction of post-treatment recurrence.
In conclusion, our results indicate that routine prophylactic LLND is unnecessary in patients with PTC with clinically node-negative lateral neck. The size, bilaterality, and presence of T4 disease in the primary tumor, as well as the intraoperative diagnosis of metastatic nodes in the central compartment, were risk factors for recurrence. Intraoperative LN biopsy may be the reliable methods of selecting patients at risk for recurrence and those who would benefit from neck dissection. Our findings may require confirmation in prospective studies of patients randomized to intraoperative LN biopsy or neck dissection.
References
Ezaki H, Ebihara S, Fujimoto Y et al (1992) Analysis of thyroid carcinoma based on material registered in Japan during 1977–1986 with special reference to predominance of papillary type. Cancer 70:808–814
Xing M, Alzahrani AS, Carson KA et al (2013) Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501
Shaha AR, Shah JP, Loree TR (1996) Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg 172:692–694
Wada N, Duh QY, Sugino K et al (2003) Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 237:399–407
Sivanandan R, Soo KC (2001) Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg 88:1241–1244
Roh JL, Kim JM, Park CI (2008) Lateral cervical lymph node metastases from papillary thyroid carcinoma: pattern of nodal metastases and optimal strategy for neck dissection. Ann Surg Oncol 15:1177–1182
Lundgren CI, Hall P, Dickman PW et al (2006) Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer 106:524–531
Mazzaferri EL, Doherty GM, Steward DL (2009) The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid 19:683–689
Pereira JA, Jimeno J, Miquel J et al (2005) Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 138:1095–1100
Mulla MG, Knoefel WT, Gilbert J et al (2012) Lateral cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the lateral compartment. Clin Endocrinol (Oxf) 77:126–131
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Stack BC Jr, Ferris RL, Goldenberg D et al (2012) American Thyroid Association (ATA) Consensus Review of the Anatomy, Terminology and Rationale for Lateral Neck Dissection in Differentiated Thyroid Cancers. Thyroid 22:501–508
Edge SB, Byrd DR, Compton CC, et al (eds) (2010) AJCC cancer staging manual. 7th ed. Springer, New York, p 87–96
Koo BS, Choi EC, Yoon YH et al (2009) Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg 249:840–844
Tisell LE, Nilsson B, Mölne J et al (1996) Improved survival of patients with papillary thyroid cancer after surgical microdissection. World J Surg 20:854–859. doi:10.1007/s002689900130
Dzodic R, Markovic I, Inic M et al (2006) Sentinel lymph node biopsy may be used to support the decision to perform modified radical neck dissection in differentiated thyroid carcinoma. World J Surg 30:841–846. doi:10.1007/s00268-005-0298-0
Ji YB, Lee KJ, Park YS et al (2012) Clinical efficacy of sentinel lymph node biopsy using methylene blue dye in clinically node-negative papillary thyroid carcinoma. Ann Surg Oncol 19:1868–1873
Ducoudray R, Trésallet C, Godiris-Petit G et al (2013) Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg 37:1584–1591. doi:10.1007/s00268-013-2020-y
Lim YS, Lee JC, Lee YS et al (2011) Lateral cervical lymph node metastases from papillary thyroid carcinoma: predictive factors of nodal metastasis. Surgery 150:116–121
Ito Y, Higashiyama T, Takamura Y et al (2007) Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg 31:2085–2091. doi:10.1007/s00268-007-9224-y
Hay ID, Bergstralh EJ, Goellner JR et al (1993) Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057
Laverick S, Lowe D, Brown JS et al (2004) The impact of neck dissection on health-related quality of life. Arch Otolaryngol Head Neck Surg 130:149–154
Terrell JE, Welsh DE, Bradford CR et al (2000) Pain, quality of life and spinal accessory nerve status after neck dissection. Laryngoscope 110:620–626
Acknowledgments
This study was supported by Grants (No. 2014-0306) from the Asan Institute for Life Science (J.-L. Roh).
Conflict of interests
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, C.W., Gong, G. & Roh, JL. Intraoperative Diagnosis of Central Compartment Lymph Node Metastasis Predicts Recurrence of Patients with Papillary Thyroid Carcinoma and Clinically Node-Negative Lateral Neck and May Guide Extent of Initial Surgery. World J Surg 39, 194–202 (2015). https://doi.org/10.1007/s00268-014-2800-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2800-z