Abstract
Introduction
Recent studies have revealed that Aquafilling gel used for breast augmentation causes complications. In this study, we investigated which surgical approach should be used to treat these complications.
Materials and Methods
This observational cohort study analysed the data of 31 women suffering from complications after breast enlargement with Aquafilling injection who were treated at our department in 2016–2021. Patients underwent either conservative or radical surgery. GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) was used for data processing.
Results
Approximately 88.89% of patients after conservative surgery required reoperation, while only 22.73% of patients treated radically needed revision surgery. Every patient who underwent an attempt to remove the gel via needle prior to admission required surgery. Seventy-five per cent of them had positive culture swabs, whereas only 26.09% of patients who did not undergo needle aspiration had positive cultures. A positive correlation between the volume of injected filler and the number of symptoms was observed.
Conclusions
In addition to irrigation and drainage, Aquafilling removal should include infiltrated tissue excision during primary surgery. Moreover, needle aspiration of the filler is ineffective, and it may lead to a gel infection. Furthermore, the more filler is injected, the higher the number of complications observed.
Level of Evidence IV This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast augmentation remains one of the most common operations worldwide, with a shown impact on self-esteem improvement and sexual well-being [1,2,3,4,5,6,7,8]. On the one hand, breast enhancement with classic silicone implants has an established position and is the most frequently performed cosmetic surgical procedure in the US [9]. On the other hand, less-invasive methods are constantly sought and advertised as methods with a short recovery time, without general anaesthesia and visible scars [10,11,12,13].
One alternative for implants is liquid tissue fillers. Although they became extremely popular with the rise of the aesthetic medicine field, their use is an old concept [12, 14]. The experiments with paraffin, vegetables and mineral oils, and liquid silicone ended similarly with rapidly increasing complications such as chest wall tissue necrosis, sepsis, thrombosis, and even death [14].
Another injectable material introduced in Ukraine in the late 1980s under the name Interfall consisted of polyacrylamide hydrogel (PAAG) [14,15,16]. Polyacrylamide is a nondegradable synthetic polymer composed of acrylamide monomers. Hydrogel consists of approximately 2.5% polyacrylamide suspended in 0.9% sodium chloride solution [17]. Despite its nonbiodegradability, PAAG is believed to be a stable, nontoxic and nonallergenic agent and an ideal substitute for silicone breast implants [14, 17]. Unfortunately, polyacrylamide began to cause massive and different complications over time [15,16,17,18,19,20,21].
In 2012, a new filler for soft tissue contouring appeared under the tradename Aquafilling Bodyline (Biomedica. spol, s,r,o, Czech Republic). According to the manufacturer's declaration, it was supposed to be degradable and to consist of 96–98% solution of 0.9% sodium chloride and 2–4% a substance described as cation copolyamide; however, chemical analysis showed that Aquafilling contains polyacrylamide particles [22]. The procedure required only local anaesthesia and therefore could be performed at the outpatient treatment room. The hydrogel was injected via a 2–3 mm incision within the inframammary fold and, according to the official instructions by the manufacturer, was placed between the mammary gland and pectoralis muscle.
Since its introduction, a growing number of adverse reactions after Aquafilling have been reported. Ultrasonography and magnetic resonance imaging revealed that the gel can scatter through the tissues as well as infiltrate the muscle fibres [23]. Subsequent Aquafilling complications occurred, including pain, wounds, fluid discharge, inflammation, and gel migration [24, 25]. New reports of the adverse effects of Aquafilling have been published; nevertheless, there are still no guidelines on how to approach these conditions and how to treat them effectively.
In this paper, we compare two different surgical gel removal techniques and assess the efficacy of prior needle aspiration for Aquafilling. Moreover, we evaluated whether the volume of injected filler influences the clinical findings in such patients.
Materials and Methods
Data were obtained from 31 female patients admitted to the Plastic Surgery Department due to complications following breast augmentation with Aquafilling gel. The collected information included the volume of injected filler, time from the injection to symptom onset and type and number of complications. Table 1 shows the characteristics of the study group. The sources of data were both the past medical history of each patient and the current medical examination. Furthermore, patients were asked if any attempt to remove the gel prior to admission was made.
Gel migration within the body was evaluated with ultrasonography (USG) and magnetic resonance imaging (MRI). Intraoperative visual assessment was used to distinguish scattered gel infiltration from distinctive gel container formation. During the procedure, gel swab tests were performed for culture. Histopathological examination of the removed tissue was performed.
Initially, patients were treated with conservative surgery (narrow skin incision near the gel concentration, massive saline and betadine irrigation through a cannula, followed by a few days of drainage); however, due to the persistence of symptoms, we moved towards more radical treatment.
Radical surgery required a wider incision, usually within the inframammary fold (or slightly higher if patient does not wish to undergo a silicone implant breast augmentation in the future—due to the breast size decrease following gel removal). Furthermore, in addition to saline and betadine irrigation of the wound, an excision of macroscopically affected tissues was made (focused on white, “milky” tissue infiltrated by the Aquafilling and necrotic lesions).
If the symptoms relapsed, a revisional surgery was performed. The reoperation rate was determined for both techniques. GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) was used for data processing. Statistical significance was determined using Fisher’s exact test where available. Values of p < 0.05 were considered significant. Pearson’s correlation coefficient was assessed. Clinical flowchart was created with https://www.biorender.com.
Results
All patients from the study group (n = 31) were symptomatic. The majority of patients (74.19%) suffered from more than one complication simultaneously. The symptoms observed are presented in Table 2. Gel migration into the axillary region was the most frequent complication (61% of the study group). Inframammary gel migration was observed less often (45%). Other common symptoms included breast pain (48%), upper extremity pain (48%), and breast deformity (45%). Figure 1 depicts a patient from the study group suffering from left inframammary gel migration and breast asymmetry before (A) and after surgical Aquafilling removal (B).
A Patient from the study group prior to gel removal surgery. Breast asymmetry as well as left inframammary gel migration can be observed. There was a major gel container in the right breast. B Postoperative view of the same patient. The gel container was removed from the right breast through the submammary incision, and necrotic tissues were radically dissected. In the left breast, we had to make a lateral incision as well as another incision below the inframammary fold due to Aquafilling migration
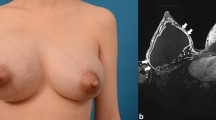
In 12 patients (38.71%), a distinctive gel container was encountered, whereas gel scattered through the tissues occurred in 19 cases (61.29%). Figure 2 shows an ultrasonography image of a distinctive gel container (A) as well as its subsequent removal (B). Tissue infiltrated by scattered gel was observed intraoperatively as white, “milky” lines (Fig. 3A) and necrotic lesions (Fig. 3B). No correlation between the type of gel migration and the need for revision surgery was observed.
There was a correlation between the amount of gel administered and the number of symptoms occurring (Pearson’s r = 0.6751, p value < 0.0001). A higher volume of Aquafilling rendered more complications (Fig. 4). There was no correlation between the amount of filler and the time from injection to onset. Moreover, the number of symptoms as well as the injection-onset timeframe was not influenced by the age of the patients.
Eight out of 31 patients (25.81%) underwent an attempt to aspirate the gel through a needle in an outpatient clinic. These procedures were ineffective because all of them eventually required surgical Aquafilling removal. The positive culture incidence was higher in this group than in patients who did not attempt to remove the gel prior to admission (75–26.09%, respectively, p = 0.0316, OR = 8.5). These data are shown in Fig. 5. In the whole study group (n = 31), positive culture results were obtained by 12 patients (38.71%). Among them, there was one case of Candida parapsilosis, whereas the rest were caused by bacteria (the most common was Staphylococcus).
Overall, 13 patients out of 31 (41.94%) required a reoperation. Conditions that rendered it necessary were as follows: relapse of initial symptoms, fistula, abscess, and recurrent container formation. The likelihood of revisional operation was significantly higher among patients after conservative surgery than among those approached more radically (88.89–22.73%, respectively, p = 0.0012, OR = 27.2). Figure 6 depicts the reoperation rate among the study group, depending on the type of initial treatment. Furthermore, there was no evidence of carcinogenesis in histopathological examination.
Discussion
The fact that all of the patients presented to our department were symptomatic may indicate that the awareness of Aquafilling toxicity is still very low in society, even though there is strong evidence of its dangerous side effects. The filler can scatter through the tissues and infiltrate muscle fibres, causing pain, inflammation, and gel migration [23, 24]. It can reach even distal parts of the extremities [25]. Our study supports these findings, as similar complications were observed in our patients (Table 2, Fig. 1). Cheng et al. stated gravity as the first reason for gel migration and muscle activity as the second reason [20]. In our opinion, the third reason may be gradual progressive ischaemia and necrosis of the surrounding tissues (Fig. 3B), as often skeletonized and clotted vessels were visible macroscopically.
Furthermore, Chalcarz et al. discovered that Aquafilling causes an inflammatory response independent of visible symptoms [26]. The negative effects of hydrogel injection might be the consequence of chemical changes in the gel structure over time, as studies have shown that polyacrylamide is harmless to the body, but the acrylamide monomer released from polyacrylamide contains toxins. Animal studies have shown that acrylamide reproductive toxicity and nervous toxicity can lead to gene mutation and tissue changes [27]. However, there is no evidence of an Aquafilling cancerogenic effect in histopathological tissue examination [28]. Additionally, in our study, we did not find any sign of carcinogenesis in the excised material.
Our research revealed that the form of gel distribution (distinctive containers or scattered through the tissue) does not influence the timeframe between the injection and symptom onset or the number of complications. However, there was a correlation between the volume of injected filler and the number of symptoms (Fig. 4). It seems that the more gel is administered, the more complications can be observed. The reason for this may be simply the volume of hydrogel, or it could be related to the number of injections. When a large amount of filler is injected, it may require multiple deep injections with blind manipulation, which can result in bleeding and uneven gel spreading [29].
Since Aquafilling is administered via a needle, one-fourth of our patients underwent an attempt to remove the gel by needle aspiration in an outpatient clinic prior to admission to our department. This indicates that this technique is ineffective because the symptoms did not subside, and all patients eventually required surgical treatment. To determine if other patients were treated successfully with needle aspiration and therefore were not referred to our department, we contacted clinics where these attempts were made. They did not report any effective needle removal; however, this is a limitation of our study because such a successful attempt could have been made somewhere else. Nevertheless, there is no evidence of Aquafilling needle aspiration effectiveness in the literature. Similar attempts with polyacrylamide hydrogels were proven ineffective [30].
Moreover, a considerably higher gel infection rate was found in patients after needle aspiration than in patients who were referred directly for surgical treatment (Fig. 5). The reason for this could be that external bacteria were administered during the needle injection process. This theory is supported by the observed pathogen heterogeneity. After analysis of the antibiograms, our first-choice antibiotic was ciprofloxacin.
There are different approaches of Aquafilling removal described in the literature, from more conservative irrigation and drainage procedures to subcutaneous mastectomy if the mammary gland is infiltrated [31]. Nomoto et al. reported that Aquafilling can be removed with a waterjet-assisted liposuction device; however, it is less efficient when the gel infiltrates the muscle or mammary gland [32]. Overall, available methods rely either on gel rinsing or breast tissue excision; therefore, they can be divided into conservative and radical surgical approaches. Nevertheless, there is no clear guideline as to when to apply these techniques.
In this study, we compared the effectiveness of conservative and radical surgical gel removal. In the initial cases, our interventions were limited to incisions, emptying of filler cysts, irrigation, and drainage. The number of complications and necessary reinterventions was very high. Therefore, we have changed the approach to a more radical surgical treatment. In addition to the evacuation of gel containers, we began cutting out radically changed tissues to the possible extent during the primary operation. We observed a significantly lower reoperation rate among the patients who were treated radically than among those who underwent conservative surgery (Fig. 6). This indicates that emptying the containers is not enough to alleviate the symptoms and that a radical approach is more beneficial for these patients. Without tissue excision, Aquafilling cannot be removed completely, and the symptoms will reoccur. Clinical flowchart of the radical surgical approach is shown in Fig. 7.
Conclusions
We showed that in Aquafilling breast augmentation cases, radical surgical treatment is superior to conservative surgical approach. Aquafilling removal should include infiltrated tissue excision during primary surgery, in addition to massive saline and betadine irrigation of the wound and drainage. Moreover, attempts to evacuate the gel through a needle are ineffective, and they may lead to gel infection. However, due to the small study group, correlation between needle aspiration and infections needs further investigation. Symptomatic patients after Aquafilling breast augmentation should be referred directly to the surgical theatre, whereas treatment of asymptomatic patients needs further research. It seems that a higher filler volume increases the likelihood of complication occurrence.
References
Kaoutzanis C, Winocour J, Unger J, Gabriel A, Maxwell GP (2019) The evolution of breast implants. Semin Plast Surg 33(4):217–223
Asimakopoulou E, Zavrides H, Askitis T (2020) Plastic surgery on body image, body satisfaction and self-esteem. Acta Chir Plast 61(1–4):3–9
Crerand CE, Infield AL, Sarwer DB (2007) Psychological considerations in cosmetic breast augmentation. Plast Surg Nurs 27(3):146–154
Solvi AS, Foss K, von Soest T, Roald HE, Skolleborg KC, Holte A (2010) Motivational factors and psychological processes in cosmetic breast augmentation surgery. J Plast Reconstr Aesthet Surg 63(4):673–680
Alderman A, Pusic A, Murphy DK (2016) Prospective analysis of primary breast augmentation on body image using the BREAST-Q: results from a nationwide study. Plast Reconstr Surg 137(6):954e–960e
Papadopulos NA, Kovacs L, Krammer S, Herschbach P, Henrich G, Biemer E (2007) Quality of life following aesthetic plastic surgery: a prospective study. J Plast Reconstr Aesthet Surg 60(8):915–921
Figueroa-Haas CL (2007) Effect of breast augmentation mammoplasty on self-esteem and sexuality: a quantitative analysis. Plast Surg Nurs 27(1):16–36
Penaud A, De Mortillet S (2013) Evaluation of the psychological benefits of breast augmentation for aesthetic purposes. Results of a multicenter prospective study of a series of 181 patients. Ann Chir Plast Esthet 58(1):10–17
American Society of Plastic Surgeons National Clearinghouse of Plastic Surgery Procedural Statistics (2018) Plastic surgery statistics report
Graf RM, Closs Ono MC, Pace D, Balbinot P, Pazio ALB, de Paula DR (2019) Breast auto-augmentation (mastopexy and lipofilling): an option for quitting breast implants. Aesthetic Plast Surg 43(5):1133–1141
Van Slyke AC, Carr M, Carr NJ (2018) Not all breast implants are equal: a 13-year review of implant longevity and reasons for explantation. Plast Reconstr Surg 142(3):281e–289e
Hedén P, Sellman G, von Wachenfeldt M, Olenius M, Fagrell D (2009) Body shaping and volume restoration: the role of hyaluronic acid. Aesthet Plast Surg 33(3):274–282
Reichenberger MA, Biedermann N, Germann G (2011) Ästhetische mammaaugmentation [Aesthetic breast augmentation]. Chirurg 82(9):782–788
Peters W, Fornasier V (2009) Complications from injectable materials used for breast augmentation. Can J Plast Surg 17(3):89–96
Chen B, Song H (2016) Management of breast deformity after removal of injectable polyacrylamide hydrogel: retrospective study of 200 cases for 7 years. Aesthet Plast Surg 40(4):482–491
Jin R, Luo X, Wang X et al (2018) Complications and treatment strategy after breast augmentation by polyacrylamide hydrogel injection: summary of 10-year clinical experience. Aesthet Plast Surg 42(2):402–409
Ono S, Ogawa R, Hyakusoku H (2010) Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg 126(4):1349–1357
Luo SK, Chen GP, Sun ZS, Cheng NX (2011) Our strategy in complication management of augmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg 64(6):731–737
Cheng NX, Wang YL, Wang JH, Zhang XM, Zhong H (2002) Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthet Plast Surg 26:375–382
Cheng NX, Xu SL, Deng H et al (2006) Migration of implants: a problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthet Plast Surg 30(2):215–225
Leung KM, Yeoh GP, Chan KW (2007) Breast pathology in complications associated with polyacrylamide hydrogel (PAAG) mammoplasty. Hong Kong Med J 13(2):137–140
Roh TS (2016) Position statement of Korean Academic Society of Aesthetic and reconstructive breast surgery: concerning the use of Aquafilling® for breast augmentation. Arch Aesthet Plast Surg 22(01):45–46
Arslan G, Celik L, Atasoy MM, Celik L, Cubuk R (2017) Complication of non-US guided procedure of aquafilling breast gel. Med Ultrason 19(2):236–237
Jung BK, Yun IS, Kim YS, Roh TS (2018) Complication of AQUAfilling® gel injection for breast augmentation: case report of one case and review of literature. Aesthet Plast Surg 42(5):1252–1256
Gierej P, Radziszewski M, Miłoński P, Noszczyk B (2022) Distal hand migration of polyacrylamide gel after breast augmentation: a case report and review of the literature. Indian J Plast Surg 56(2):178–181
Chalcarz M, Żurawski J (2021) Injection of Aquafilling® for breast augmentation causes inflammatory responses independent of visible symptoms. Aesthet Plast Surg 45(2):481–490
Xu C, Cao M, Bao B, Li H, Yin D (2012) Tissue degeneration 7 years after breast augmentation with injected polyacrylamide hydrogel (PAAG). Aesthet Plast Surg 36(1):160–162
Chalcarz M, Żurawski J (2022) The absence of early malignant changes in women subjected to Aquafilling breast augmentation on the basis of E-cadherin and N-cadherin immunohistochemical expression. Cent Eur J Immunol. 47(4):350–356
Xiong C, Chen Y, Xu Y, Jiang W, Yin X, Chen D, Gong X, He T, An Y, Han Y (2023) A review of complications of polyacrylamide hydrogel injection. Chin J Plast Reconstruct Surg 5(2):86-95
Qiao Q, Wang X, Sun J et al (2005) Management for postoperative complications of breast augmentation by injected polyacrylamide hydrogel. Aesthet Plast Surg 29(3):156–162
Choi WJ, Song WJ, Kang SG (2023) Complications and management of breast augmentation using two different types of fillers: a case series. Arch Aesthet Plast Surg. 29(1):41–45
Nomoto S, Ogawa R (2021) Removal of Aquafilling using body-jet: a waterjet-assisted lipsuction device. Plast Reconstr Surg Glob Open 9(6):e3451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose and received no financial support.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gierej, P., Woźniak-Roszkowska, E., Radziszewski, M. et al. Treatment of Complications After Minimally Invasive Breast Augmentation with Aquafilling Gel. Aesth Plast Surg 47, 2322–2329 (2023). https://doi.org/10.1007/s00266-023-03648-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03648-w