Abstract
Background
The use of alloplastic and allogenic nasal implants is widely popular in rhinoplasty. However, the use of these materials is accompanied by a risk of infection and extrusion. Traditionally, management of these complications is performed in a dual-staged fashion. First, the implant is removed and infection is controlled, then a delayed reconstruction is performed. However, scarring and soft tissue contracture make a delayed reconstruction challenging, and optimal aesthetic outcomes are difficult to achieve. This study was designed to evaluate the outcomes of immediate nasal reconstruction following removal of an infected nasal implant.
Methods
A retrospective chart review was performed of all patients who had infected nasal implants and underwent simultaneous removal and immediate nasal reconstruction with autologous cartilages (n = 8). Data collected included patient age, race, pre-operative presentation, intraoperative surgical maneuvers, and post-operative outcomes and complications. Post-operative results were used to measure success of the single-staged method.
Results
Follow-up ranged from 12 to 156 months with mean 84.4 months of the eight patients who were evaluated in the study, none had any major post-operative complications that required revision or reconstruction. All of the patients had marked improvement in nasal form and function. Six of the eight (75%) patients reported excellent aesthetic outcomes; two (25%) requested revisional surgeries for aesthetic concerns.
Conclusion
Low complication rates and excellent aesthetic outcomes are possible in immediate autologous reconstruction following removal of an infected nasal implant. This is an alternative approach that obviates the inherent problems of a traditional delayed reconstruction.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266..
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloplastic and allogenic nasal implants are frequently utilized in rhinoplasty to achieve dorsal augmentation, correction of contour irregularities, and airway improvement [1]. The most common implants are made from silicone (SiO2), porous high density polyethylene (pHDPE, Medpor), expanded polytetrafluoroethylene (ePTFE, Gore-Tex), and acellular allogeneic human cadaver dermis (AlloDerm) [1]. These synthetic implants are popular due to their relative ease of use and due to their long-lasting results. However, the introduction of foreign material into the nose also confers the risk of infection or extrusion. The rate of failed nasal silicone implants, indicated by infection and/or extrusion, ranges from 4 to 36% [2].
Traditionally, the management of infected nasal implants is multi-staged [3]. First, the infected implant is removed and the compromised tissue is irrigated with copious amounts of an antibiotic solution. Simultaneous treatment with systemic antibiotics is also standard. No reconstruction is performed and a waiting period of four to six months is observed. This allows for the infection to clear and for the inflammatory response to subside. A delayed reconstruction is then performed utilizing standard reconstruction techniques with autologous cartilage [4,5,6].
The staged approach has noteworthy drawbacks. Inherently, removal of the implant will cause the soft tissue envelope to contract and develop scar tissue. This can lead to obvious nasal deformity that causes patients to be socially distressed during the waiting period in between surgeries. Even once reconstruction takes place, the patient will likely have a displeasing aesthetic outcome. The lack of pliable tissue makes reconstruction itself complicated and limits the mouldability of the nasal structure.
As a result of these challenges and concerns, the senior author developed a treatment plan for infected nasal implants, incorporating immediate autologous reconstruction. This resolves the aforementioned downfalls of a staged approach while also preventing repeat infection and extrusion of a synthetic implant. Performing immediate reconstruction preserves the soft tissue envelope, thereby improving functional and aesthetic outcomes. The material used for restoration of dorsal height loss following implant extraction needs to be biocompatible, long lasting, and have a natural look and feel [1, 7]. Thus, autologous cartilage is the preferred option that provides indelible results with a lower risk of immune response elicitation [8, 9]. This review was designed to demonstrate the safety, efficacy, and success of this single-stage approach.
Materials and Methods
A retrospective chart review was performed on eight patients with infected nasal implants who underwent single-stage surgical source control and immediate nasal reconstruction (Figs. 1, 2, 3, 4, 5, 6, 7 and 8). All cases were performed by the senior author between September 2009 and February 2022. Patients included in this study were all patients presenting with infected nasal implants requiring removal and who had adequate skin coverage for an autologous reconstruction. Patients excluded were those with grossly infected nasal implants demonstrating uncontrolled draining purulence that did not clear with antibiotic therapy or those with necrotic skin envelopes that showed skin loss (Fig. 9). Recorded demographics included age, sex, race, details of previous rhinoplasty, and initial chief complaint (Table 1).
a–d 32-year-old female with a history of an Alloderm implant placed one year prior to presentation, presenting with a wound and abscess on the left medial canthus/nasal sidewall and a repeat papular lesion 4 months later. The patient also presented with a septal deviation, internal valve collapse, and nasal deviation. e–h 1 year after removal of infected AlloDerm implant and bone debridement with immediate placement of DCF graft to fill in the deficit. Septoplasty was performed and spreader and columellar grafts were placed at the same time
a–d 46-year-old female first presented with an 8 mm × 3 mm deep open wound of the nasal dorsum due to extrusion of suspected GoreTex or Medpor implant. e–h 1 year after implant removal and reconstruction with DCF graft. Rib cartilage was used for spreader grafts, lateral crural strut grafts, and a columellar strut graft
a–d 47-year-old female presented with an impending glabella extrusion of an infected dorsal implant. Patient was with a history of airway obstruction due to completely collapsed left internal and external valves and very constricted right internal and external valves. The septum was severely left deviated with a notable posterior septal perforation. The external nose was consistent with short nose deformity—overly rotated tip and nostril retraction, worse on the left side. e–h 1.5 years after immediate removal of the infected implant and capsulectomy, septoplasty, spreader grafts, lateral crural strut grafts, a columellar strut graft, and a DCF graft to address the saddle nose deformity. Scar tissue retraction of lining did not allow for use of a caudal septal extension graft, but, excellent tip de-rotation and nostril lengthening was achieved with use of composite graft
a–d 28-year-old female presented with a silicone L-strut implant with acute pain and redness through her dorsum. e–h 1 year following removal of the silicone implant and reconstruction of the dorsum with a DCF graft, septal extension graft, and columellar strut. The lateral cruras were also reconstructed. Composite ear grafts were harvested and used for nostril lengthening. Finally, alar base reduction was performed
a–d 33-year-old female with a history of an L-strut similar implant presented with a chronic infection of the implant extruding through her tip. e–h 2 years after implant removal and DCF reconstruction. Additional lateral crural strut grafts and a columellar graft were also placed. The tip wound was treated with debridement and local wound care
a–d 34-year-old female presented with a silicone implant causing the tip to begin to appear increasingly rotated, but she did not want removal of the implant at that time. 5 months after the initial consult, she returned to the clinic with a severely shortened nose—overrated and underprojected tip and a lowered radix. The implant had extruded through the anterior tip, causing erythema and cellulitis of the surrounding skin. Post-op after implant removal and reconstruction with a DCF graft. Patient also had tip reconstruction and lengthening with a caudal septal extension graft, columellar strut graft, lateral crural grafts, and composite ear graft. Visible scarring was still present on the right nostril and the nose was not of sufficient length of the patient’s liking. e–h Patient had a minor revision surgery 2.5 years later to address these aesthetic concerns via scar tissue subcision and placement of composite ear graft
a–d 32-year-old TGF with a history of a previous Medpor implant presenting with inflammatory scarring to the skin and subsequent visible deformities. The dorsal graft was also pushing her tip inferiorly. During the primary surgery, as much Medpor as possible was removed, but some had to be left behind as it would require a skin resection which the patient did not consent to. e–h 3 years following partial removal of the Medpor implant and DCF reconstruction, as well as a columellar strut, lateral crural strut grafts, and fascia tip graft
a–d 36-year-old male with an infected silicone implant extruding through the vestibular skin of the right nostril. He also had a cadaveric columellar strut in place. The nose was beginning to take on saddle nose deformity. e–h 13 years post-op from removal of implant and DCF reconstruction, in addition to a composite ear graft
a, b This patient is an exemplary figure of someone who is not a suitable candidate for immediate reconstruction. These images were taken the first day she presented at our clinic, in dire need of emergency infection control. She had an I-shaped silicone implant that extruded through her glabella and was causing severe pustules and erythema of her nose. Salvage of the skin envelope was performed with immediate antibiotic therapy, removal of the implant, and debridement in a controlled operating room setting
Under the principles of the Declaration of Helsinki, it was determined that patient involvement in this study was of minimal risk and burden. Thus, no IRB approval was obtained. Patients included in this study have consented to release and use of their photographs and data for academic study.
Infection Control and Surgical Techniques
Patients who presented with infected but not extruding dorsal implants, i.e. without a wound, were treated with broad-spectrum oral antibiotics pre-operatively. These were patients with milder symptoms, i.e. foul smell, pain, redness, were given clindamycin and/or amoxicillin. If a staphylococcus infection was suspected, the patient was administered a combination of bactrim, rifampicin, and clindamycin. Seven days was sufficient for the erythema to resolve and allow for the patient to be taken to the operating room. Any wounds caused by implant extrusion were cultured. Infection control was accomplished by IV antibiotic treatment that was guided by culture results and infectious diseases specialist consultation.
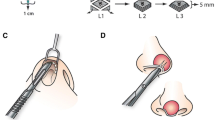
Intraoperative antibiotics were patient specific, administered adjuvantly to the founded pre-operative regimen. A transcolumellar inverted V-shaped incision was used to access and remove the infected nasal implant. Standard open rhinoplasty techniques were applied and capsulectomy was performed. Great care was taken to remove the entire capsule to release the contracted nose and minimize chronic bacterial burden of the biofilm without causing further damage to the soft tissue and skin. The degree of scarring was assessed; the entire length of the dorsum and tip skin are vulnerable to scar tissue collection where the foreign material resided and demanded release and excision of fibrotic tissue. The granulated tissues were gently debrided, and the nose was frequently irrigated with copious betadine and antibiotic solution consistent with treating the microorganism discovered during the pre-op evaluation. After obtaining source control, attention was then brought to reconstructing the nose and restoring dorsal height.
From the senior author’s extensive practice revising catastrophic noses, he biases autologous rib cartilage for reconstruction. Even if septal cartilage has not been harvested in the revision setting, neither septal nor ear cartilage provides the same strength and durability as rib cartilage. The sturdy integrity of rib grafts is critical especially when the structure needs to withstand the contractile forces and scarring in these cases. For restoration of dorsal height, patients received a diced cartilage-fascia (DCF) graft composed of autologous rib cartilage and temporalis or rectus fascia (see techniques below). Preliminary, the platform upon which the DCF graft lay needed to be stable. Within this patient set, there was no noted septal weakening; L-strut modulation was only performed if the patient had a deviation. Infection was prone to traumatic scarring of the airway mechanism, so additional rib cartilage grafts were used to reconstruct the middle vault and valves using spreader grafts. Regularly used were caudal septal extension and strong columellar strut grafts to elongate the nose, de-rotate the tip, and stabilize the tip projection. Composite ear grafts were needed to lengthen contracted nostrils to a more reasonable, aesthetically pleasing position (see technique below).
When the implant had extruded, the macerated tissue edges of the wound were freshened and left open with the fascia graft reconstruction deep to the wound. Wound care was performed to help the wound close over the fascia graft, which typically occurred rapidly. Suture closure was avoided to prevent purulent infection. The wound was treated with xeroform gauze dressing changed every 3–5 days or as needed. Post-operatively, patients were prescribed 14 days of patient specific, directed antibiotics.
Costal Cartilage Harvest
Rib harvest is performed by first identifying the desired rib location. The fifth through ninth ribs are viable options for harvest; however, the eighth rib is preferred for its length and volume of material. A 2–3 cm skin incision is made over the desired rib and the fibres of the external oblique are bluntly separated. The length of rib to be harvested is identified and dissected free in a subperiosteal fashion. Doyenne retractors are placed circumferentially around the rib and a #15 blade is used to vertically transect the rib directly over the retractors. After the rib has been harvested and prior to closure, the wound is filled with saline and a Valsalva manoeuvre is performed to confirm that there is no pneumothorax. Perichondrium, muscle fascia, and scarpa’s fascia are closed in layers using a 3-0 PDS suture. Skin is closed with a 3-0 Monocryl suture.
Fascia Harvest
Several incisions can be utilized for harvesting the deep temporalis fascia (DTF). An incision 1 cm above and 0.5 to 1 cm posterior to the root of the helix may be made (although long hair makes this design challenging). The DTF can also be harvested using a temporal brow lift incision, an incision just above the root of the helix at the hairline (for men who wear crew cuts), or a posterior helical incision using an endoscope. Dissection is then taken directly down to the deep temporalis fascia. Regardless of the approach, it is of utmost importance to harvest a graft large enough to cover the area over the dorsum. This usually requires a graft that is 4 cm in diameter or larger. Closure of the incision on the scalp is typically performed by reapproximating the superficial temporal fascia with an absorbable suture and then stapling the hair-bearing scalp skin. Routine skin closure with sutures can be used if the pretrichial incision is used [10].
Rectus fascia is also a viable option and seems to be the obvious option when harvesting costal cartilage to spare the patient another donor site incision scar. Rectus fascia can be harvested through a subcostal incision which is typically used to harvest rib 7, 8 or 9. It is easily exposed and harvested through this incision, thus saving the patient the incision over the temporalis [11]. While both DTF and rectus fascia are effective in reducing graft demarcations and enveloping diced cartilage for DCFs, it should be noted that the rectus fascia is much more stout than DTF. Therefore, the patient’s skin thickness is an important consideration in fascia graft selection.
Creating the DCF
The diced cartilage and fascia graft is created by harvesting deep temporalis fascia or rectus abdominis fascia. A typical fascia graft for this type of construct is ideally 3–4 cm in diameter. The cartilage used may be rib, ear or septal cartilage that is diced into pieces smaller than 1 mm on an edge and preferably 0.5 mm on the edge to homogenize the texture. The cartilage volume in each graft depends on the amount of projection needed and typically would mimic the dorsal implant. The cartilage is placed in the fascia graft and then closed on each edge. The shape and volume, again, would be designed to mimic the dorsal implant that was previously removed. 4-0 plain gut suture is used to suture the fascia to itself resulting in the closed structure. The needle and suture are left attached at the cranial end to transcutaneously fix the DCF graft through the skin of the radix.
Chondrocutaneous Composite Ear Harvest
For patients with a short, contracted nose, a composite graft is frequently required for reconstruction of the nasal lining. A composite graft that includes skin and cartilage is harvested from the concha cymba and the concha cavum. The incision is placed on the anterior side of the concha cymba and cavum and the harvest includes an ellipse of anterior skin and attached cartilage. The posterior skin has enough skin laxity to require only minimal undermining prior to closure with a 5-0 Prolene suture. The composite graft is then placed in the intercartilaginous position.
Results
This series included five patients of Asian descent and three Caucasian patients, with ages ranging 28–47 years. Five patients had Silicone implants, two had Medpor implants and one had an AlloDerm implant. Follow-up ranged from 12 to 156 months with a mean 84.4 months. No complications were noted. Complication was defined as loss of reconstruction due to infection and/or graft warping and resorption requiring a return to the operating room. Though one patient developed a columellar infection of a suture abscess 5 months post-op, it only required minimal management with an in-office incision and drainage. The infection resolved with oral antibiotics and did not compromise the reconstruction. Two patients did return to the operating room, but only to further enhance their aesthetic result. Overall, there was a 100% success rate, as all patients’ noses maintained stable structure and satisfactory nasal function. There was no noted graft warping or resorption through patient follow-up periods, either, and notably no donor site morbidity. Table 2 shows the post-operative outcomes and sequelaes.
Discussion
Alloplast and allogenic rhinoplasty continue to be a pervasive straightforward operation, but the ease comes at the price of a high chance of complication and infection. Management of infected nasal implants is uniquely challenging due to acute and chronic inflammation, soft tissue injury, loss of structure of the nose, and scarring [4, 5, 7, 12]. The approach to the problem is typically multi-staged, but this leads to creation of a dead space and soft tissue contraction. Subsequently, there will be scar formation that complicates the delayed reconstruction and compromises the final aesthetic results.
It is not intuitive to reconstruct a portion of the anatomy in the setting of infection. However, in our theory and practice, we show that a single-stage approach is indeed feasible because the infectious burden is mitigated through infection control with antibiotics and removal of the offensive cause of the infection, i.e. the nasal implant. Additionally, the reconstruction is performed with autogenous materials that are capable of resisting repeat infection in this well vascularized setting.
Currently, there is limited literature providing an alternative protocol to obviate these adverse outcomes. Previously, Won and Jin showed that the practice of using autografts in immediate reconstruction of infected nasal implants has safe, predictable, and highly satisfactory results [9]. Their study, consisting of 21 patients, is the largest case series reporting on this technique. During the mean follow-up period of 35 months, only one of their patients developed an infection. Our case series presents eight patients, with a lengthy mean follow-up time of 84.4 months. No patients in this study developed post-op infection or required reconstructive revision, providing further evidence that infected nasal implant removal with immediate autologous reconstruction is a safe and plausible method with favourable results. This single-staged treatment technique addresses infection in a timely manner, while also reconstructing and restoring dorsal height. Equally important, it prevents future soft tissue contraction and scarring which can lead to compromised aesthetic results.
Removal of the infected dorsal implant requires dorsal augmentation with autogenous material for both aesthetic and functional restoration [13, 14]. Utilization of diced cartilage and fascia grafts for restoration of dorsal height has been a highly effective technique, even in extreme cases of dorsal height deficiency. It is also helpful for camouflaging soft tissue irregularities, correcting an over-resected dorsum, augmenting the radix, and correcting a saddle nose deformity, as previously described by the senior author [15]. Immediate reconstruction with DCF after implant removal allows for maintenance of dorsal height and pleasing dorsal aesthetic lines, even in the context of infection. Another benefit of autogenous DCF grafting is its relative ease of post-operative re-modelling or repositioning, if required. Rarely is reoperation for repositioning the graft necessary [8, 16]. In the cases presented here, there were no instances of graft discontent requiring an operative revision.
Ancillary to dorsal augmentation, a thorough and comprehensive nasal analysis may show other structural, functional and aesthetic deficits that must be addressed during the reconstruction. Deficits including internal valve collapse, deviated septum, poor tip support and a short nose can all be addressed with additional autologous cartilage grafts [15]. In this study, we showed that these grafts provided a sound framework resistant to post-infection scarring tendencies that would otherwise cause the nose to over-rotate.
While there are robust advantages to immediate autologous reconstruction, this method has an inherent limitation. The surgeon must mindfully evaluate patient candidacy for a single-staged reconstruction. This is not a viable option when gross purulence does not halt with antibiotic use and/or when the infection has obliterated the skin envelope. Aggressive infection that compromises the vasculature or obstructive oedema and swelling may also be hindering factors. Such cases necessitate delayed reconstruction to first extensively debride the infected tissue and allow time for revitalization and revascularization without any graft replacements.
Prior to performing a reconstruction, whether immediate or delayed, the senior author counsels the patients on an approximate 15% risk of developing an infection after any revision rhinoplasty. Further, while restoring structure and function is paramount, achieving outstanding aesthetics is also of importance. Thus, the patient is informed of the 10–15% possibility of requiring a revisionary procedure to obtain desired aesthetic results.
Conclusion
An infected alloplastic or allogeneic nasal implant may have catastrophic consequences, due to its effects on underlying nasal bone, cartilage, and soft tissue support structures. The surgeon must perform a comprehensive evaluation, manage the infection, and determine surgical corrections. The concept of immediate reconstruction in an infected field can be daunting but is possible with decontamination with antibiotic therapy and removal of the abusive agent. The protocol detailed to reconstruct with autologous materials forestalls future adverse immune response and repeat infection. This study demonstrates that performing a single-staged immediate autologous reconstruction provides aesthetically pleasing and lasting results. All patients included in the study resulted in 100% success rate, with no recurrence of infection or degradation of the reconstruction. This substantiates the safety and efficacy of this treatment option. However, the degree of infection widely varies, so it is critical to determine whether a patient is a suitable candidate for immediate reconstruction. Cases of severe contamination, purulence, and a scant skin envelope require staged reconstruction. Ultimately, the operating surgeon must choose the best course of treatment based on the degree of infection and necrosis to determine the best path to cure.
References
Peled ZM, Warren AG, Johnston P, Yaremchuk MJ (2008) The use of alloplastic materials in rhinoplasty surgery: a meta-analysis. Plast Reconstr Surg. https://doi.org/10.1097/01.prs.0000299386.73127.a7
Choi JY (2020) Complications of alloplast rhinoplasty and their management: a comprehensive review. Facial Plast Surg. https://doi.org/10.1055/s-0040-1717082
Clark JM, Cook TA (2002) Immediate reconstruction of extruded alloplastic nasal implants with irradiated homograft costal cartilage. Laryngoscope. https://doi.org/10.1097/00005537-200206000-00006
Chen DS, Wang TD (2020) Management of dorsal graft and implant infections. Facial Plast Surg. https://doi.org/10.1055/s-0040-1701489
Kim YK, Shin S, Kang NH, Kim JH (2017) Contracted nose after silicone implantation: a new classification system and treatment algorithm. Arch Plast Surg. https://doi.org/10.5999/aps.2017.44.1.59
Winkler AA, Soler ZM, Leong PL, Murphy A, Wang TD, Cook TA (2012) Complications associated with alloplastic implants in rhinoplasty. Arch Facial Plast Surg. https://doi.org/10.1001/archfacial.2012.583
Genther DJ, Papel ID (2016) Surgical nasal implants: indications and risks. Facial Plast Surg. https://doi.org/10.1055/s-0036-1592101
Graw GJ, Calvert JW (2022) Dorsal augmentation. Clin Plast Surg. https://doi.org/10.1016/j.cps.2021.08.003
Won T-B, Jin HR (2012) Immediate reconstruction with autologous cartilage after removal of infected alloplast in revision rhinoplasty. Otolaryngol Head Neck Surg. https://doi.org/10.1177/0194599812459026
Calvert J, Kwon E (2015) Techniques for diced cartilage with deep temporalis fascia graft. Facial Plast Surg Clin North Am. https://doi.org/10.1016/j.fsc.2014.09.005
Cerkes N, Basaran K (2016) Diced cartilage grafts wrapped in rectus abdominis fascia for nasal dorsum augmentation. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000001876
Villanueva K, Martin D, Martinkovich S, Blomain EW (2017) Treatment of a chronically infected nasal silicone prosthesis with continuous antibiotic irrigation and gentamicin-impregnated polymethylmethacrylate beads. JPRAS Open. https://doi.org/10.1016/j.jpra.2017.10.001
Dresner HS, Hilger PA (2008) An overview of nasal dorsal augmentation. Semin Plast Surg. https://doi.org/10.1055/s-2008-1063566
Tanna N, Nguyen KT, Ghavami A, Calvert JW, Guyuron B, Rohrich RJ, Gruber RP (2018) Evidence-based medicine: current practices in rhinoplasty. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000003977
Calvert JW, Patel AC, Daniel RK (2014) Reconstructive rhinoplasty: operative revision of patients with previous autologous costal cartilage grafts. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000000119
Brenner KA, McConnell MP, Evans GR, Calvert JW (2006) Survival of diced cartilage grafts: an experimental study. Plast Reconstr Surg. https://doi.org/10.1097/01.prs.0000195082.38311.f4
Funding
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Calvert is a consultant and paid speaker for Alma Lasers and MTF; however, these relations are not relevant to this manuscript. The authors declare that they have no other conflicts of interest to disclose
Human or Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors
Informed Consent
For this type of study informed consent is not required
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calvert, J.W., Kruayatidee, A., Shakoori, P. et al. Immediate Nasal Reconstruction in Management of Infected Nasal Alloplast and Allografts: A Case Series. Aesth Plast Surg 48, 689–701 (2024). https://doi.org/10.1007/s00266-023-03397-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03397-w