Abstract
Background
Postoperative facial scarring can be a significant psychological burden for patients to carry after surgery, often resulting in prolonged mental health dysfunction. Currently, there is no established method to prevent facial scar formation; however, there are several methods to prevent facial scar hyperplasia and improve scar quality. Botulinum toxin A (BTA) has been widely used due to its properties of muscle paralysis and known success in plastic surgery and cosmetology. This meta-analysis aimed to evaluate the efficacy of BTA in preventing postoperative facial scar hyperplasia and improving scar quality.
Methods
PubMed, MEDLINE, EMBASE, web of science, and Cochrane libraries were searched for randomized controlled trials (RCTs) (published before May 2021) wherein BTA was used for the treatment of facial scars. The efficacy and safety of BTA were evaluated by the following scales: the Vancouver Scar Scale (VSS), Visual Analog Scale (VAS), Observer Scar Assessment Scale (OSAS), Patient Scar Assessment Scale (PSAS), and Stony Brook Scar Evaluation Scale (SBSES); the BTA effect on scar width and complications was also assessed.
Results
Ten RCTs involving 114 cases were included. Through quantitative analysis, the BTA injection group had a higher VAS score, lower VSS score, lower OSAS score, and smaller scar width. However, no significant difference was noted in the incidence of postoperative complications between the two groups.
Conclusions
This meta-analysis demonstrated that BTA can safely improve the appearance of postoperative facial scars by significantly inhibiting scar hyperplasia and improving scar quality.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative scars are unpleasant, especially on the face or any other conspicuous area, and preventing them is a key point of emphasis in plastic surgery. Facial scars not only disfigure the appearance but can also cause dysesthesia with itching, pain, and dysfunction resulting from scar contractures [1]. These adverse effects are physically inconvenient and often present a real psychological burden for the patient [2]. Tension is an important factor affecting wound healing and the postoperative scar appearance on the face. Wound tension is generated by elastic retraction of the dermis and movement of the musculature in the deep layers [3]. Persistent tension may prolong the inflammatory phase during wound healing and increase the risk of scar hyperplasia [4]. Simultaneous contraction of the deep muscles may lead to persistent microtrauma and hypertrophic scar formation [4].
Due to the physical and psychological adverse effects attributed to facial scarring, ongoing research efforts have been devoted to inhibiting scar formation [1]. For example, several methods, such as lasers [5], have been used to inhibit scar proliferation and improve the appearance of facial scars. However, despite the use of such methods, finding a safe and effective method for preventing scars has not yet been realized [1]. Botulinum toxin studies have been encouraging and in recent years, the clinical applicability of botulinum toxin type A (BTA) has been gradually expanded, and it may now be an effective method for anti-scar treatment [6, 7].
Botulinum toxin is characterized by seven serotypes, with type A most commonly used in plastic surgery [8]. In 1973, Alan Scott first administered botulinum toxin to the lateral rectus muscle in a monkey [9]. BTA has been used to treat muscle dysfunction disorders, such as strabismus and blepharospasm, and also can be used cosmetically to relieve dynamic facial wrinkles [10]. The neurotoxin produces a marked effect by inhibiting the release of acetylcholine [10]. BTA inhibits scar hyperplasia by temporarily paralyzing the muscles adjacent to the wound and reducing the wound tension [11]. Furthermore, at the cellular level, BTA can impact cell growth and differentiation as well as cell signaling during scar formation [3]. In vitro studies have proved that BTA directly suppresses fibroblast-to-myofibroblast differentiation [3], and it is known to prevent transforming growth factor beta 1 (TGF-β1) expression, which is considered to be an important factor affecting scar hyperplasia [7, 12, 13]. Therefore, the aforementioned results provide a theoretical basis for BTA use in the management of postoperative scar formation [14].
There have been several randomized controlled trials (RCTs) investigating the BTA effect on scar hyperplasia inhibition [15,16,17]. However, there is a lack of systematic and comprehensive evaluation on the effectiveness of BTA in inhibiting scar hyperplasia and improving postoperative appearance. Therefore, we systematically reviewed relevant RCTs and performed a quantitative analysis to provide a comprehensive evaluation of the efficacy of BTA in preventing postoperative facial scars.
Methods and Materials
The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards [18]. Our meta-analysis was registered on the INPLASY (INPLASY202170077).
Search Strategy
PubMed, MEDLINE, EMBASE, web of science, and Cochrane libraries were searched for all RCTs (published before May 2021) wherein BTA was used for the treatment of postoperative facial scars. The search terms were “face”, “cheek”, “chin”, “eye”, “forehead”, “mouth”, “nasolabial fold”, “nose”, “scar”, “scars”, “scarred”, “scarring”, ‘‘cicatrix’’, “Botulinum Toxin”, “Botulinum Toxin, Type A”, “Clostridium Botulinum Toxins”, “Toxin, Botulinum”, “randomized controlled trial”, “controlled clinical trial”, “randomized”, “placebo”, and “randomly”. Studies were listed with key information using Microsoft Word 2017 (Microsoft Corp., Redmond, WA, USA) by two investigators.
Inclusion and Exclusion Criteria
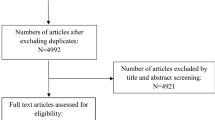
All original published RCTs describing the use of BTA in preventing postoperative facial scars were included. The search was not conducted with any language or regional restrictions, and if a foreign language article was found, an English version of the article was sought or translated into English. Duplicate studies, animal experiments, in vitro studies, case reports, review articles, editorials, meeting abstracts, letters or viewpoints, studies that included fewer than 10 participants, and studies with full text or date not available were excluded (Fig. 1).
Data Extraction and Quality Assessment
Data were extracted from the included articles by two reviewers (S.Y. and M.R.J.) independently. Data were collected on the following parameters: country, age, sex, scar location, BTA concentration, injection site and time, control group reagent, outcome measures, complications, and follow-up time. Two authors applied the Cochrane Handbook tool to independently perform a quality assessment of the literature of the included studies. In case of disagreement, it was reassessed by another author (Z.S.).
Statistical Analysis
Review manager 5.4 was used to analyze the data and perform the meta-analysis. The mean differences (MDs) value or standardized mean differences (Std. MDs) value was calculated by the inverse variance method for continuous variables, and the Mantel-Haenszel method and odds ratio (OR) were applied for dichotomous variables. Combined values were expressed with 95% confidence intervals (CIs), and differences were considered statistically significant if the result was p < 0.05. The heterogeneity of each included study was evaluated using I2. A fixed effect model was used if the calculated result was <50%; otherwise, a random effect model was used. Begg’s test and funnel plots were used to evaluate publication bias in the meta-analysis. A symmetric graph indicated that publication bias may not exist. Conversely, an asymmetric graph indicated possible publication bias or systematic difference between large-sample and small-sample studies. Begg’s test with a rejection region of p = 0.05 was used to evaluate the existence of publication bias. Sensitivity analyses were performed by removing each included study one by one, changing the inclusion criteria, or excluding a certain type of study to identify the impact of the corresponding study.
Results
A total of 114 case studies were retrieved after the initial search, and 44 remained after inclusion criteria application. Upon further review of these, 16 case studies ultimately met the criteria. These 16 articles were read carefully in full text, and 10 of them were finally included in this study [1, 19,20,21,22,23,24,25,26,27]. The literature characteristics of the included studies are summarized in Table 1. The 10 articles contain a total of 344 cases with approximately 19–59 cases per study. All the enrolled patients were evaluated for postoperative scars. Eight studies [1, 19,20,21,22,23, 25, 26] applied 0.9% normal saline as the control group, while no special treatment for the control group in the two other studies was noted [24, 27]. The most commonly used BTA concentration was 25 u/mL [1, 19, 20, 24], two studies [22, 23] used BTA at a concentration of 50 u/mL, and the remaining four studies used concentrations of 10 u/mL [27], 40 u/mL [26], 75 u/mL [21], and 100 u/mL [25], respectively. The follow-up interval for all the studies was at least 6 months. Seven studies set the Vancouver Scar Scale (VSS) [19, 20, 22,23,24,25, 27] and the Visual Analog Scale (VAS) as the outcome measures [1, 19, 20, 22, 23, 26, 27]. Five [19, 20, 22, 24, 25] of the RCTs reported using scar width as the results index. In addition, two [1, 27] RCTs reported the use of the Patient and Observer Scar Assessment Scale (POSAS), and only one [1] study reported the use of the Stony Brook Scar Evaluation Scale (SBSES).
Analysis of Outcome Indicators
VAS Comparison
VAS is the simplest scar scale in management and use, and the value range of this index is 0 to 10, where “0” is the worst and “10” is the best. The observer needs to make a subjective judgment on the scar. It is related to the prognosis of the facial scar; however, it does not describe the specific scar characteristics [28]. VAS was used in seven RCTs involving 336 cases to evaluate the final scar outcomes between the experimental and control groups. The results of the quantitative analysis showed that the facial scar with BTA injection had a higher VAS score than the control group (MD = 1.10, 95% CI = 0.89 to 1.30, p < 0.00001) with an acceptable heterogeneity (I2 = 47%) (Fig. 2).
VSS Comparison
VSS is the earliest scar assessment scale first used for the therapeutic evaluation of burn scars. VSS evaluates the overall condition of a scar in a scored form by observing the pigmentation, vascularity, pliability, and height [29]. A total of seven studies involving 291 case-control comparisons reported VSS scores, and some heterogeneity between the included studies was found through preliminary analysis (I2 = 59%); therefore, the random effects model was used for analysis. The results indicated that the BTA injection group had lower overall VSS scores (Std. MD = − 0.64, 95% CI = − 1.03 to − 0.25, p = 0.001). We subsequently attempted to assess the source of overall heterogeneity by subgroup analysis of the four subitems of the VSS. Analysis of two RCTs with a detailed comparison of each subitem revealed that there was a significant difference in scar pliability between the experimental and control groups (MD = − 0.26, 95% CI = − 0.45 to − 0.07, p = 0.008), while no statistical difference in the other three subitems was noted (Fig. 3).
a The forest plot showed that the BTA injection group had a lower overall Vancouver Scar Scale (VSS) score than the control group. b To examine the cause of heterogeneity in the overall VSS scores, we performed subgroup analyses of the individual subscores of VSS and found that scar pliability was significantly improved in the experimental group compared to that in the control group
POSAS Comparison
A total of two eligible studies involving 69 cases used the POSAS for its outcome measure assessment. The BTA injection group showed a lower Observer Scar Assessment Scale (OSAS) score than the control group, and there was a significant difference between the two groups (MD = − 0.83, 95% CI = − 1.33 to − 0.34, p = 0.001), with no significant heterogeneity (I2 = 0%) (Fig. 4). However, with Patient Scar Assessment Scale (PSAS), no significant statistical difference between the two groups was noted.
Scar Width Comparison
The degree of scar proliferation after wound healing can be reflected in the final scar width [30]. Five studies reported detailed follow-up results of this outcome measure. Two of them [19, 20] measured and compared the scar width of two points, and one of them [20] measured the scar width using two different methods. The pooled results showed that the scar width of the BTA injection group was smaller than that of the control group (MD =− 1.05, 95% CI =− 1.27 to− 0.83, p < 0.00001), with no significant heterogeneity (I2 = 0%) (Fig. 5).
SBSES and Adverse Events Comparison
SBSES is an objective indicator of scar evaluation that focuses more on the assessment of scar appearance [31]. The SBSES score was used as the outcome measure in only one study, and no statistically significant conclusion was made. We analyzed four studies [21, 23, 25, 27] on reported adverse reactions. The pooled results showed that the BTA injection appeared safe. Our fixed effects model indicated no significant association between BTA injection and complications (pooled relative risk, 0.99, 95% CI, 0.22 to 4.53, p = 0.99), with no significant heterogeneity (I2 = 0%) (Fig. 6).
Risk of Bias, Publication Bias, and Sensitivity Analysis
All the studies used for the analysis were assessed for risk of bias according to the Cochrane Handbook, and the results indicated that these studies were all low risk (Fig. 7). Publication bias was examined using the funnel plot (Fig. 8) and Begg’s test. No significant publication bias was found in this meta-analysis. Funnel plots were largely symmetrical, and the results of Begg’s test showed that the p values were > 0.05. The sensitivity analysis suggested that the results of this meta-analysis were reliable.
No publication bias exists in the included studies, including the evaluation of BTA inhibition of scar hyperplasia by the indicated outcome measures (VAS, VSS, OSAS, and scar width). a No significant publication bias was found when the BTA effect was evaluated by VAS. b No significant publication bias was found when the BTA effect was evaluated by VSS. c No significant publication bias was found when the BTA effect was evaluated by OSAS. d No significant publication bias was found when the BTA effect was evaluated by scar width
Discussion
Scar formation occurs after the body is traumatized and then the skin protects the wound through nascent fibrin, resulting in wound healing by resolution rather than regeneration [19]. Wound healing includes three phases: inflammatory, proliferative, and remodeling phases. In the first two phases, granulation tissue and extracellular matrix are produced under regulation in a certain order [32]. However, under the influence of external factors, this order may be broken, adversely affecting the balance between the production and degradation of these substances; this leads to the production of hypertrophic scars and possibly keloids [4]. Insufficient reduction of the tension of the incision and poor nutritional status are both external factors that break this balance [32]. In addition, tissue microtrauma caused by repeated activity or displacement of damaged tissue will intensify the inflammatory response and cellular metabolic activity in the incision area, resulting in increased extracellular collagen levels, increased glycosaminoglycan deposition, and hypertrophic scar formation [33]. Facial expressions are produced by the contraction of the facial muscles, and since these muscles are superficial without bony insertions, the muscle movement causes tension across the adjacent skin and subcutaneous tissue [19]. When the direction of the wound is perpendicular to the underlying facial muscle, the muscular contraction produces tension at the incision edge, which increases the risk of a widened scar and hypertrophic scar formation [14].
Botulinum toxin is a potent neurotoxin that acts as a transient muscle paralytic that can be extracted from Clostridium botulinum into six different serum serotypes. Among the different serotypes, type A (BTA) and type B (botulinum toxin B) are used to treat different disorders [34], and BTA has been used for neurological and skin appendage disorders [35]; it has also been used to improve the appearance of facial lines [36]. Since 2000 [14], efforts have been made to explore and elucidate the effect of facial scar hyperplasia inhibition and improving postoperative appearance [24]. The mechanism of BTA for inhibiting scar hyperplasia includes three main characteristics: (1) reduce tension [11], (2) inhibit fibroblast proliferation and differentiation and promote their apoptosis [37], and (3) inhibit the expression of TGF-β1 [7]. Wound tension is one of the key factors associated with scar hyperplasia [38, 39]. Multiple surgical techniques have been used to reduce the tension around the wound. BTA temporarily paralyzes the adjacent muscles, reducing muscle activity and tension [40]. No current molecular mechanism clearly illustrates that BTA can inhibit scar hyperplasia; however, it reportedly can inhibit proliferation and promote fibroblast apoptosis [37]. Fibroblasts can secrete collagen and TGF-β1, and both are important factors affecting scar formation [37, 41]. Damaged skin tissue secretes inflammatory mediators that activate melanocytes [42]. It has been reported that BTA can reduce this activation by inhibiting local infiltration of those inflammatory cells [41]. It has been postulated that BTA inhibits scar hyperplasia mainly in the early stage of scar formation by reducing fibroblast proliferation and differentiation. Therefore, early postoperative injection of BTA enhances scar hyperplasia inhibition and improves the appearance of the facial scar [8].
There are various types of scar treatments, and effectively evaluating their results is something that should not be ignored in clinic practice. Historically, the therapeutic effect of scar treatment was assessed by descriptive clinical observation and camera comparison. This article attempted to analyze the treatment efficacy of BTA injection via scar assessment scales. In our meta-analysis, five scales were used to evaluate scar status as a study outcome measure. VAS is the simplest scar assessment scale, which can be used for diverse scar assessments, and VSS and POSAS were originally designed to evaluate burn scars; SBSES was developed to evaluate scars of surgical incisions with a focus on cosmetic appearance [43]. VAS is widely used for scar assessment as a subjective evaluation method [44]. Due to the characteristics of the scale, such as sensitivity, reproducibility, and accessibility, VAS was more suitable to evaluate simple facial scars [1]. Our analysis showed that the results of this scar scale were statistically significantly different between the BTA injection group and control group, which suggested that BTA has favorable effects on facial scar formation. VSS consists of the following components: pigmentation, vascularity, pliability, and scar height [43]. Through the analysis of the seven studies [19, 20, 22,23,24,25, 27], we found that the overall score of VSS in the BTA injection group was lower than that in the control group. However, there was medium heterogeneity among the studies (I2 = 59%). Upon further analysis, we found that the heterogeneity may be caused by the differences of the four VSS subscores; therefore, subgroup analysis was performed based on the four subscales. However, due to incomplete data, subgroup analysis was only performed in two RCTs. Through the review of the results of the included literature and qualitative analysis, we found that among these subscores, the most significantly improved subscore was pliability. These results indicate that BTA injection can significantly reduce wound tension and inhibit fibroblast proliferation, thereby improving the overall VSS score [22].
VSS and VAS have some limitations. They are dependent on clinicians and do not focus on real patient discomfort. To compensate for these limitations, POSAS has been used for scar assessment, and it is the first scale to integrate the views of the observer and patients [45]. PSAS included six items on a 10-scale assessment, and OSAS has five items on a 10-scale assessment. Compared to the actual scar, these scales pay more attention to reflect the subjective experience. However, the scale items of PSAS consist of pain and pruritus, which are uncommon with facial scarring [43]. Our results showed that there were significant differences between the BTA injection group and the control group; some inadequacies cannot be ignored in using these scales to evaluate facial scars. These scales were commonly used to assess healing in severe and complex wounds with tissue loss; they may have limited value for postoperative facial scarring where there was no significant tissue loss with optimum local conditions [46, 47]. In contrast, SBSES scale was more suitable for the evaluation of simple facial scars because it focuses more on the aesthetic appearance [43]. The scale contains six items with the possible category scores being 0 or 1 [48]. However, only one of the included studies [1] evaluated facial scarring using this scale; therefore, we were unable to make an effective analysis and draw meaningful conclusions.
In addition to the scale assessment, five studies [19, 20, 22, 24, 25] evaluated the role of BTA in scar hyperplasia by comparing the scar width at the 6-month follow-up. Upon analysis, we found that the scar width of the experimental group was smaller at the 6-month follow-up, which further confirmed that BTA could reduce the incision tension, inhibit fibroblast proliferation and differentiation, and then improve the appearance of the facial scar. In addition, biopsy results further confirmed the role of BTA in inhibiting scar hyperplasia. Masson trichrome stain showed that the control group had a denser collagen fiber deposition than the BTA group [1]. The action time of BTA is up to 6 months, similar to a normal wound healing process. This means that BTA has sustained muscle tone relief, which may explain the superior effect of the BTA injection group at 6 months. In our studies, the follow-up time of one study [27] was extended to 1 year, thereby creating a treatment time window wherein BTA effectively inhibits scar hyperplasia. The process by which BTA works, either by delay or disruption of scar formation, may be important to elucidate with the ultimate goal of long-term scar reduction.
The injection protocol of BTA was varied among the 10 included studies. First, the total injection dose and the injection concentration of the BTA were not frequently the same. In the 10 included RCTs, the total injection dose of the BTA was diverse due to the different divisions of the injected facial subunit, with its total dose ranged from 5 to 80 U. Different dose of BTA produces different diffusion rates, which in turn results in different inhibitory scar hypertrophic effects [49]. A recent split-scar experiment has proven that high-dose BTA can better inhibit scar hyperplasia than low-dose BTA [50]. Another important parameter in the BTA injection scheme was the injection concentration. The most common BTA injection concentration in the literature included in this meta-analysis was 25 U/mL. Different from the injection dose, a high concentration of BTA may inhibit proliferation, suppress keratinocyte migration, and reduce angiogenesis, thereby affecting wound healing [51]. Second, the injection time of BTA was also different in each study. Most studies injected BTA between the immediate postoperative period and 7 days postoperatively [1, 19,20,21,22,23,24, 27]; however, two studies injected BTA preoperatively [25, 26]. Although injection times vary, most of the findings suggested that early BTA injection can effectively improve postoperative scar outcomes [52]. The injection time, doses, and BTA concentrations were not identical among studies, and no standardized injection protocol was noted. More high-quality RCTs with large-sample sizes are needed to investigate the optimal injection protocol of BTA for preventing postoperative scar hyperplasia and guide clinical applications.
This meta-analysis has some limitations. First, the total sample size of the study is insufficient, and all the studies did not perform a comparative assessment of all the outcome measures between the experimental and control groups. Second, the difference in the concentration, dose and time of the BTA injection, and patient demographics increases the heterogeneity of the meta-analysis. Third, the scars located in different aesthetic areas of the face, muscle activity, and response to BTA were variable in different areas, which may lead to diverse results.
Conclusion
This literature review and meta-analysis comprehensively evaluated 10 RCTs and found that BTA can effectively prevent scar hyperplasia to improve the postoperative appearance of facial scars. Meanwhile, scar injection with BTA at therapeutic doses was found to be safe by analysis. Therefore, patients undergoing facial surgery can be treated early with BTA injections to prevent scar proliferation and acquire a satisfactory postoperative aesthetic appearance. Further, a comprehensive and systematic evaluation of the effect of BTA on preventing scar hyperplasia is still needed with a larger data volume of clinical controlled experiments.
Reference
Kim SH, Lee SJ, Lee JW, Jeong HS, Suh IS (2019) Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration: a double-blinded, randomized controlled study. Med (Baltimore) 98(34):e16952
Tebble NJ, Thomas DW, Price P (2004) Anxiety and self-consciousness in patients with minor facial lacerations. J Adv Nurs 47(4):417–426
Jeong HS et al (2015) Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg 136(2):171e–178e
Sherris DA, Larrabee WF Jr, Murakami CS (1995) Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am 28(5):1057–1068
Chowdhury B et al (2021) Laser in surgical scar clearance: an update review. J Cosmet Dermatol. https://doi.org/10.1111/jocd.14325,July2,2021
Xiao Z, Zhang F, Cui Z (2009) Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg 33(3):409–412
Xiao Z, Zhang F, Lin W, Zhang M, Liu Y (2010) Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthetic Plast Surg 34(4):424–427
Ibrahim O, Keller EC, Arndt KA (2014) Update on botulinum neurotoxin use in aesthetic dermatology. Semin Cutan Med Surg 33(4):152–156
Scott AB, Rosenbaum A, Collins CC (1973) Pharmacologic weakening of extraocular muscles. Invest Ophthalmol 12(12):924–927
Cocco A, Albanese A (2018) Recent developments in clinical trials of botulinum neurotoxins. Toxicon 147(1):77–83
Sherris DA, Gassner HG (2002) Botulinum toxin to minimize facial scarring. Facial Plast Surg 18(1):35–39
Lu L et al (2005) The temporal effects of anti-TGF-β1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg 201(3):391–397
Reid RR, Roy N, Mogford JE, Zimmerman H, Lee C, Mustoe TA (2007) Reduction of hypertrophic scar via retroviral delivery of a dominant negative TGF-beta receptor II. J Plast Reconstr Aesthet Surg 60(1):64–72
Gassner HG, Sherris DA, Otley CC (2000) Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 105(6):1948–1953
Li YH et al (2018) A randomized, placebo-controlled, double-blind, prospective clinical trial of botulinum toxin type A in prevention of hypertrophic scar development in median sternotomy wound. Aesthetic Plast Surg 42(5):1364–1369
Abedini R, Mehdizade Rayeni N, Haddady Abianeh S, Rahmati J, Teymourpour A, Nasimi M (2020) Botulinum toxin type A injection for mammoplasty and abdominoplasty scar management: A split-scar double-blinded randomized controlled study. Aesthetic Plast Surg 44(6):2270–2276
Elshahed AR, Elmanzalawy KS, Shehata H, ElSaie ML (2020) Effect of botulinum toxin type A for treating hypertrophic scars: a split-scar, double-blind randomized controlled trial. J Cosmet Dermatol 19(9):2252–2258
Moher D et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK (2014) Botulinum toxin to improve results in cleft lip repair. Plast Reconstr Surg 134(3):511–516
Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK (2014) Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 9(12):e115690
Gassner HG et al (2006) Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc 81(8):1023–1028
Hu L et al (2018) Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg 141(3):646–650
Huang RL, Ho CK, Tremp M, Xie Y, Li Q, Zan T (2019) Early postoperative application of botulinum toxin type a prevents hypertrophic scarring after epicanthoplasty: a split-face, double-blind, randomized trial. Plast Reconstr Surg 144(4):835–844
Lee SH, Min HJ, Kim YW, Cheon YW (2018) The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthetic Plast Surg 42(2):530–537
Navarro-Barquín DF et al (2019) Use of the type A botulinum toxin in patients submitted to cheiloplasty to improve results in scarring in patients with nonsyndromic cleft lip and palate. Eur J Plast Surg 42(3):291–294
Zelken J et al (2016) Donor site aesthetic enhancement with preoperative botulinum toxin in forehead flap nasal reconstruction. Ann Plast Surg 77(5):535–538
Ziade M et al (2013) Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg 66(2):209–214
Sung YT, Wu JS (2018) The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods 50(4):1694–1715
Thompson CM, Sood RF, Honari S, Carrougher GJ, Gibran NS (2015) What score on the vancouver scar scale constitutes a hypertrophic scar? results from a survey of north American burn-care providers. Burns 41(7):1442–1448
Refahee SM et al (2020) Is PRP effective in reducing the scar width of primary cleft lip repair? A randomized controlled clinical study. Cleft Palate Craniofac J 57(5):581–588
Prodromidou A et al (2015) Botulinum toxin for the prevention and healing of wound scars: a systematic review of the literature. Plast Surg (Oakv) 23(4):260–264
Burns JL, Mancoll JS, Phillips LG (2003) Impairments to wound healing. Clin Plast Surg 30(1):47–56
Bae DS, Koo DH, Kim JE, Cho JM, Park JO (2020) Effect of botulinum toxin A on scar healing after thyroidectomy: a prospective double-blind randomized controlled trial. J Clin Med 9(3):868
Bellows S, Jankovic J (2019) Immunogenicity associated with botulinum toxin treatment. Toxins (Basel) 11(9):1–22
Castelão M et al (2017) Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev 12(12):cd003633
Small R (2014) Botulinum toxin injection for facial wrinkles. Am Fam Physician 90(3):168–175
Xiao Z, Qu G (2012) Effects of botulinum toxin type a on collagen deposition in hypertrophic scars. Molecules 17(2):2169–2177
Suarez E, Syed F, Rasgado TA, Walmsley A, Mandal P, Bayat A (2014) Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair Regen 22(5):557–568
Suarez E, Syed F, Alonso-Rasgado T, Mandal P, Bayat A (2013) Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, α2β1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg 131(2):158e–173e
Qiao Z, Yang H, Jin L, Li S, Wang X (2021) The efficacy and safety of botulinum toxin injections in preventing postoperative scars and improving scar quality: a systematic review and meta-analysis. Aesthetic Plast Surg. https://doi.org/10.1007/s00266-021-02196-5,March5,2021
Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW (2009) Effect of botulinum toxin type a on a rat surgical wound model. Clin Exp Otorhinolaryngol 2(1):20–27
Ortonne JP, Bissett DL (2008) Latest insights into skin hyperpigmentation. J Investig Dermatol Symp Proc 13(1):10–14
Vercelli S, Ferriero G, Sartorio F, Stissi V, Franchignoni F (2009) How to assess postsurgical scars: a review of outcome measures. Disabil Rehabil 31(25):2055–2063
Duncan JAL et al (2006) Visual analogue scale scoring and ranking: a suitable and sensitive method for assessing scar quality? Plast Reconstr Surg 118(4):909–918
Bae SH, Bae YC (2014) Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch Plast Surg 41(2):111–115
Nguyen DQ, Potokar T, Price P (2008) A review of current objective and subjective scar assessment tools. J Wound Care 17(3):101–106
Stavrou D, Haik J, Weissman O, Goldan O, Tessone A, Winkler E (2009) Patient and observer scar assessment scale: how good is it? J Wound Care 18(4):171–176
Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE (2007) Development and validation of a novel scar evaluation scale. Plast Reconstr Surg 120(7):1892–1897
Brodsky MA, Swope DM, Grimes D (2021) Diffusion of botulinum toxins. Tremor Other Hyperkinet Mov. https://doi.org/10.7916/D88W3C1M,August2,2021
Chen Z et al (2021) The effect of botulinum toxin injection dose on the appearance of surgical scar. Sci Rep 11(1):13670
Gugerell A, Kober J, Schmid M, Buchberger E, Kamola LP, Keck M (2016) Botulinum toxin A: dose-dependent effect on reepithelialization and angiogenesis. Plast Reconstr Surg Glob Open 4(8):e837
Kim YS, Lee HJ, Cho SH, Lee JD, Kim HS (2014) Early postoperative treatment of thyroidectomy scars using botulinum toxin: A split-scar, double-blind randomized controlled trial. Wound Repair Regen 22(5):605–612
Funding
The authors have nothing to disclose.
The named authors have no commercial interest and financial or material support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yue, S., Ju, M. & Su, Z. A Systematic Review And Meta-Analysis: Botulinum Toxin A Effect on Postoperative Facial Scar Prevention. Aesth Plast Surg 46, 395–405 (2022). https://doi.org/10.1007/s00266-021-02596-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-021-02596-7