Abstract
Background
The role of active scar prevention in postoperative scar management is important. Botulinum toxin type A (BTXA) has been shown to improve postoperative scars in the past decades. The aim of this systematic review and meta-analysis was to evaluate the effectiveness and safety of BTXA injection for scar prevention.
Methods
The authors searched the databases of Medicine, Embase, the Cochrane Library, Web of Science, and CINAHL from inception through November 2018 for randomized controlled trials (RCTs) reporting the use of BTXA in scar prevention. The outcomes were the visual analogue scale (VAS) score, Vancouver Scar Scale score, scar width, patient satisfaction and adverse events.
Results
A total of nine RCTs were identified in this systematic review and meta-analysis. The VAS score was significantly higher in the BTXA group than in the control group (weighted mean difference (WMD) = 1.32, 95% confidence interval (CI) = 1.06–1.58, P < 0.00001). The Vancouver Scar Scale score was significantly lower in the BTXA group (WMD = − 1.25, 95% CI = − 2.23 to − 0.26, P = 0.01). The scar width was also significantly smaller in the BTXA group (WMD = − 0.18, 95% CI = − 0.24 to − 0.12, P < 0.00001). There was a significant difference in terms of patient satisfaction between the BTXA group and the control group (relative risk (RR) = 1.38, 95% CI = 1.09–1.74, P = 0.007). Only two studies reported complications, and other studies reported no complications during the follow-up period.
Conclusions
This systematic review and meta-analysis demonstrates that BTXA injection can reduce scar width in wounds and improve the overall appearance of postoperative scars and suggests that BTXA may be a safety therapy for scar prevention.
Level of Evidence II
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scars are defined as the product of the excessive growth of benign fibres that remain after the healing of a wound and are inevitable following surgery or trauma [1]. They can cause pain, itching, and other uncomfortable symptoms, leading to functional, cosmetic, and psychological morbidity. There are a number of therapeutic strategies for scars currently, but none of them are entirely satisfactory [2]. Early management of postoperative scars is more likely to produce a better aesthetic appearance and require fewer treatments [3, 4].
Botulinum toxin type A (BTXA), a neurotoxic protein, can induce chemodenervation by inhibiting the release of acetylcholine at the neuromuscular junction, causing muscular paralysis lasting approximately six months [5]. The tension that acts on the wound edges is a major factor that determines the final cosmetic appearance of a scar [6]. Since BTXA can reduce tension on the wound by preventing underlying muscle contraction during the healing phase, BTXA has been used successfully to prevent hypertrophic scar development in wounds.
Although some systematic reviews of BTXA for the prevention of hypertrophic scars have been reported, they were conflicting and had obvious deficiencies. Several new high-quality blinded, randomized clinical trials of BTXA have been performed recently. Thus, we conducted a systematic review and meta-analysis of all randomized trials published to date to determine whether BTXA is an effective and safe method for scar prevention.
Methods
Search Strategy
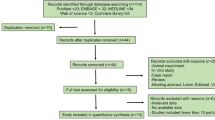
This systematic review was registered (CRD42018118640) with the international prospective register of systematic reviews (PROSPERO), and we have prepared this report to adhere to the standards of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [7]. The electronic databases of Medline, Embase, the Cochrane Library, Web of Science, and CINAHL were searched for articles published from database inception until November 2018. The search items included “wound”, “wound heal”, “wound healing”, “surg*”, “scar”, “scars”, “scarred”, “scarring”, “keloid*”, “hypertrophic”, “cicatrix”, “botulin*”, “botulinum toxin”, “botulinal toxin”, “botulinum toxin A”, “botulinium”, “botulism toxin”, “botulinum”, “botulism toxins”, “azzalure”, “bocouture”, “BoNT”, “BoNT A”, “serotype A”, “botox”, “botulin A”, “botox*”, “botulin toxin a”, “BTXA”, “dysport”, “dyslor”, “evabotulinum*”, “evabotulinum”, “onabotulinumtoxin”, “abobotulinumtoxin”, “incobotulinum”, and “incobotulin*”. A description of the search process is shown in Fig. 1.
Selection Criteria
Studies were selected according to the following inclusion criteria: (1) publications evaluating the use of BTXA for preventing postoperative scars; (2) interventions involving injection of BTXA with normal saline or no treatment as a control treatment; (3) publications evaluating the clinical improvement outcomes and associated adverse effects; (4) randomized controlled trials (RCTs); and (5) publications restricted to those published in the English language. Studies were excluded according to the following criteria: (1) studies evaluating the use of BTXA in the treatment of hypertrophic scars and keloids; (2) case reports, reviews, letters, and commentaries; (3) animal studies; and (4) duplicate records.
Data Extraction
Two independent reviewers (JSZ and CH) independently extracted data from the eligible studies according to the inclusion and exclusion criteria, and data were extracted from these studies using a standard form that included first author, publication year, region, location of the scars, participants, wounds, type of BTXA/placebo, dose of BTXA, average duration of follow-up, and other relevant information.
Quality Assessment
Two independent reviewers (YW and JW) evaluated the methodological quality of each included study using the assessment tool from the Cochrane Handbook. Disagreements were resolved by negotiation. All included trials were classified into the following categories: low risk, high risk and unclear.
Statistical Analysis
The statistical analyses were performed using RevMan Version 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Continuous outcomes were pooled by the inverse variance method, and weighted mean differences (MDs) with 95% confidence intervals (CIs) were used. Dichotomous outcomes were analysed with the Mantel–Haenszel method, and relative risks (RRs) with 95% confidence intervals (CIs) were calculated to estimate the pooled effects. A value of P < 0.05 was used as the level of significance. Heterogeneity among the studies was calculated using the I2 statistic. When I2 < 50%, a fixed effects model was applied; otherwise, a random-effects model was used. Sensitivity analysis was performed to explore the impact of an individual study by deleting one study at a time. Owing to the limited number (< 10) of included studies, publication bias was not assessed.
Results
A total of 5926 articles were identified from the databases. After removal of 934 duplicate articles, 4992 potential articles were left to screen by reading the titles and abstracts. Of these, the full texts of 71 articles were reviewed, and 62 articles were excluded after assessment of the full texts. Nine studies, all of which were RCTs, were included in the systematic review. Table 1 provides the characteristics of the reviewed articles.
Intervention Measures
All the studies used BTXA as the intervention, while the concentration, dose, volume, injection time and course were varied. According to the summary (Tables 1, 2), most wounds were located on the face, except one study evaluated wounds located on the neck, and another study evaluated wounds located on the chest. One study failed to state the dosage of BTXA; the minimum effective dose was 6 U, and the maximal dose was no more than 80 U per participant. For the dose estimation of BTXA, Gassner et al. [15] described a dose of 30 U for 2–4 cm forehead wounds (concentration: 75 U/ml). Hu et al. [4] reduced the dose to 10 U for 1 cm scar (concentration: 50 U/ml) for facial wounds. For three-month-old infants with a unilateral cleft lip, Chang et al. [11] calculated the dose of BTXA according to the baby’s weight (1 U/kg). All BTXA treatments were dissolved in normal saline, and the concentration ranged from 10 to 75 U/ml. Three of nine studies reported that BTXA was used immediately after surgery, one study reported that BTXA was used 10 days before surgery, and in other studies, BTXA injection was performed from 1 to 12 days postoperatively. All the studies used BTXA one time except for one study [9], which used BTXA two times. The four brands of BTXA were from the USA, China, and Korea.
Types of Outcome Measures
Visual Analogue Scale (VAS) Score
The VAS is a subjective measure for scar evaluation, and scores range from 0 (worst possible) to 10 (best possible) [10]. Five studies [4, 10,11,12, 14] were included in the VAS score meta-analysis. Ziade et al. [14] described raw VAS data, so we converted the raw data to the mean and the standard deviation. There was no heterogeneity among the included studies (Chi2 = 2.54, P = 0.64, I2 = 0%), and a fixed effects model was used. The VAS score was significantly higher in the BTXA group than in the control group (weighted mean difference (WMD) = 1.32, 95% CI = 1.06–1.58, P < 0.00001, Fig. 2a). In the Gassner et al. [15] study, the results were interpreted with the median VAS scores, and the score was 8.9 in the BTXA group and 7.2 in the control group. The results indicate that BTXA was associated with a more favourable appearance than the control treatment when used in the prevention of postoperative scarring.
a Forest plots of mean difference in VAS score comparing BTXA with control. b Forest plots of mean difference in VSS score comparing BTXA with control. c Forest plots of mean difference in width of scar comparing BTXA with control. d Forest plots of relative risks in patient satisfaction comparing BTXA with control
Vancouver Scar Scale Score
The Vancouver Scar Scale score is probably the most widely known scar scale that consists of four components (pigmentation, vascularization, pliability and scar height); the total score ranges from 0 to 13, with 0 representing normal skin [16]. Data about the Vancouver Scar Scale scores were available in five studies [4, 8, 9, 11, 12]. In these included studies, obvious heterogeneity was observed (Tau2 = 0.91; Chi2 = 16.96, df = 4 (P = 0.002); I2 = 76%), so a random-effects model was employed. Pooled results detected a significant difference between the two groups (WMD = − 1.25, 95% CI = − 2.23 to − 0.26, P = 0.01, Fig. 2b), suggesting that BTXA injection achieved an improved scar appearance compared with the control treatment.
Width of Scar
The scar width was investigated in five studies [4, 8, 9, 11, 12]. Among these five studies, two articles [11, 12] reported two points of scar width each, and we used all these records in the analysis. Due to the substantial heterogeneity (Chi2 = 13.84, P = 0.03, I2 = 57%), a random-effects model was used for this meta-analysis. There was a significant difference in scar width between the BTXA group and the control group (WMD = − 0.18, 95% CI = − 0.24 to − 0.12, P < 0.00001, Fig. 2c).
Patient Satisfaction
Participants rated their satisfaction with scar improvement as very satisfied, satisfied, slightly satisfied, and unsatisfied. We defined the percentages of subjects who were “very satisfied”, “slightly satisfied” and “satisfied” as the effective rate of patient satisfaction. In total, two articles [8, 13] reported patient satisfaction. The heterogeneity (Chi2 = 1.81, P = 0.18, I2 = 45%) was limited, so a fixed effects model was adopted. The results revealed that there was a significant difference between the two groups regarding patient satisfaction (RR = 1.38, 95% CI = 1.09–1.74, P = 0.007, Fig. 2d).
Adverse Events
Seven of the nine included studies reported that no complications were observed, and only two studies reported adverse events. In one study [8], no serious complications except local pain (17.6%, 3/17) and pruritus (5.9%, 1/17) occurred after BTXA injection, and the symptoms quickly disappeared without special treatment. Another study [14] reported one complication in the “toxin” group, and the same dosage of BTXA was injected on both sides of the zygomaticus minor (ZM) and the levator labii superioris alaeque nasi muscle (LLSAN) to immobilize a wound on the philtrum. Then, an asymmetrical smile was observed on day 7 postoperatively.
Trial Quality Assessment
The nine studies were all randomized, blinded, controlled trials. Most trials had a low risk of performance or detection bias in six categories. A summary of the risk of bias is shown in Fig. 3.
Sensitivity Analysis
We performed a sensitivity analysis by omitting one study at a time. The results showed that the outcomes did not differ markedly, and the meta-analysis had strong reliability.
Discussion
In 2000, BTXA was first used for scar prevention in a primate model and has shown to be effective in improving the eventual cosmetic appearance of facial scars [17]. Subsequent human trials with long-term follow-up have also suggested the safety and efficacy of this treatment. We know that any treatment strategies need to be based on good clinical evidence, and meta-analyses and systematic reviews are considered to provide the best available evidence. BTXA injection is one potential preventive treatment for scars, and it is necessary to systematically evaluate the efficacy and safety of this treatment to supply evidence for clinical strategies. Our included eligible studies were all RCTs, which are often viewed to have the highest level of scientific evidence and can generally produce credible and robust conclusions. Some trials have taken the split-scar study, which offered strong evidence on the efficacy and safety of BTXA in scar prevention.
The evidence from this meta-analysis suggests that BTXA provides a clinically significant benefit for scar prevention compared with control treatment in terms of the VAS score (WMD = 1.32, P < 0.00001), Vancouver Scar Scale score (WMD = − 1.25, P = 0.01), width of the scar (WMD = − 0.18, P < 0.00001) and patient satisfaction (RR = 1.38, P = 0.007). These outcomes consistently favoured the BTXA group compared with the control group across studies except with regard to the Vancouver Scar Scale score; Chang et al. [11] and Hu et al. [4] both observed that there was no significant difference in the Vancouver Scar Scale scores between the experimental and control groups. Hu et al. [4] assumed that this result may be due to the traditional use of this scar assessment for evaluating burn scars. However, in the other three studies [8, 9, 12], there was a statistically significant difference between the two groups in the Vancouver Scar Scale scores. Kim et al. [13] chose the modified Stony Brook Scar Evaluation Scale (SBSES) to assess the scar preventive effects of BTXA. The modified SBSES was more suitable and its sensitivity was higher than that of the initial SBSES, and the results showed that BTXA injection was effective in modulating thyroidectomy scars. In the study by Lee et al. [9], the authors measured quantitative colour differences between the scar and surrounding normal skin with the Commission International d’Eclairage L*a*b* colour coordinates; the results showed that less scar discoloration was noted among patients treated with BTXA injections than among control group participants. More scientific scar assessment methods should be applied in future research. Overall, our analysis indicated that BTXA injection was associated with a narrower scar and a more satisfactory appearance of the surgical scar than the control group.
The molecular mechanism of BTXA in scar prevention is still not completely understood. Some experimental studies have revealed that BTXA can delay fibroblast growth by inhibiting the cell cycle and is able to decrease the expression of transforming growth factor-β1 (TGF-β1) during wound healing [18, 19]. In addition, BTXA has been proven to inhibit fibroblast-to-myofibroblast differentiation in vitro [20]. These studies provide theoretical support for the clinical application of BTXA for scar prevention.
Regarding the safety of BTXA, only 5 patients suffered from complications, including three patients with local pain, one patient with pruritus, and one patient with muscular weakness, all of whom recovered. No serious adverse events or systemic reactions associated with BTXA were reported in the included literature. However, injection dose, time and course were varied in the included studies. The dose of BTXA injection administered should achieve adequate muscle paralysis in a safe manner and eventually lead to a cosmetic appearance, but due to wide individual differences and the different sizes of muscles around wounds, it is difficult to determine a unified standard dose of BTXA for scars or wounds in different areas of the body. Some studies [4, 15] describe dose estimation methods that can be used as a reference. Due to the molecular properties of BTXA, most researchers believe that BTXA should be injected in the very early phase of wound healing (i.e., prior to or immediately following wound closure). There was no consensus on the course of injections in studies that described the details of the BTXA injection process. The majority of the researchers designated several sites of injection at a distance of 5–30 mm on either side of the wound. To better understand the use of BTXA, we still need to design research programs to evaluate a safe and effective dosage, timing and procedure.
In short, this systematic review suggests that it is safe and effective to use BTXA for scar prevention. Although this review has offered a systematic and scientific evaluation of BTXA for scar prevention, several limitations of our study must be noted. First, the sample size in several articles was small. Second, our meta-analysis could not differentiate wound types or wound locations. Third, this meta-analysis could not consider the effect of different ethnicities, varying skin types and ages. Fourth, the injection dose, time and course were varied, also introducing bias. Finally, as only English literature was included, publication bias was also present.
Conclusion
This systematic review and meta-analysis, which summarized the results of RCTs, demonstrated that early BTXA injection could significantly reduce scar width and improve the overall appearance of surgical scars. There were no serious complications associated with BTXA for the prevention of hypertrophic scar development in wound healing. BTXA injection could be a preventive method for unsightly scars. Further large-scale RCTs are needed for further confirmation.
References
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49(1):35–43
Berman B, Maderal A, Raphael B (2017) Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg 43(Suppl 1):S3–S18
Block L, Gosain A, King TW (2015) Emerging therapies for scar prevention. Adv Wound Care (New Rochelle) 4(10):607–614
Hu L, Zou Y, Chang SJ, Qiu Y, Chen H, Gang M, Jin Y, Lin X (2018) Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg 141(3):646–650
Pirazzini M, Rossetto O, Eleopra R, Montecucco C (2007) Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev 69(2):200–235
Barnes LA, Marshall CD, Leavitt T, Hu MS, Moore AL, Gonzalez JG, Longaker MT, Gurtner GC (2018) Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care (New Rochelle) 7(2):47–56
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Li YH, Yang J, Liu JQ, Xie ST, Zhang YJ, Zhang W, Zhang JL, Zheng Z, Hu DH (2018) A Randomized, placebo-controlled, double-blind, prospective clinical trial of botulinum toxin type A in prevention of hypertrophic scar development in median sternotomy wound. Aesthet Plast Surg 42(5):1364–1369
Lee SH, Min HJ, Kim YW, Cheon YW (2018) The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthet Plast Surg 42(2):530–537
Zelken J, Yang SY, Chang CS, Chang CJ, Yang JY, Chuang SS, Chen HC, Hsiao YC (2016) Donor site aesthetic enhancement with preoperative botulinum toxin in forehead flap nasal reconstruction. Ann Plast Surg 77(5):535–538
Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK (2014) Botulinum toxin to improve results in cleft lip repair. Plast Reconstr Surg 134(3):511–516
Chang CS, Wallace CG, Hsiao YC, Chang CJ, Chen PK (2014) Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 9(12):e115690
Kim YS, Lee HJ, Cho SH, Lee JD, Kim HS (2014) Early postoperative treatment of thyroidectomy scars using botulinum toxin: a split-scar, double-blind randomized controlled trial. Wound Repair Regen 22(5):605–612
Ziade M, Domergue S, Batifol D, Jreige R, Sebbane M, Goudot P, Yachouh J (2013) Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg 66(2):209–214
Gassner HG, Brissett AE, Otley CC, Boahene DK, Boggust AJ, Weaver AL, Sherris DA (2006) Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc 81(8):1023–1028
Vercelli S, Ferriero G, Sartorio F, Cisari C, Bravini E (2015) Clinimetric properties and clinical utility in rehabilitation of postsurgical scar rating scales: a systematic review. Int J Rehabil Res 38(4):279–286
Gassner HG, Sherris DA, Otley CC (2000) Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 105(6):1948–1953 (discussion 1954–1955)
Zhibo X, Miaobo Z (2008) Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J Plast Reconstr Aesthet Surg 61:1128–1129
Xiao Z, Zhang F, Lin W, Zhang M, Liu Y (2010) Effect of botulinum toxin type A on transforming growth factor beta 1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthet Plast Surg 34:424–427
Jeong HS, Lee BH, Sung HM, Park SY, Ahn DK, Jung MS, Suh IS (2015) Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg 136(2):171e–178e
Author information
Authors and Affiliations
Contributions
JSZ and CH contributed to the literature search and data extraction. YW and JW were responsible for assessing the bias and performing the data analysis. FZ revised the manuscript. All the authors have approved the publication of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Zhu and Yin Wang should be considered as first authors.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, J., Zhang, J. et al. Effectiveness and Safety of Botulinum Toxin Type A Injection for Scar Prevention: A Systematic Review and Meta-analysis. Aesth Plast Surg 43, 1241–1249 (2019). https://doi.org/10.1007/s00266-019-01358-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-019-01358-w