Abstract
Introduction
An inverted nipple can cause significant functional and psychologic disturbance to women. The holy grail of any surgical technique to correct this is to restore adequate nipple projection and at the same time, try to preserve lactation and nipple sensation. We describe our experience using an inferior dermal nipple-areolar interposition flap to correct the inverted nipple alongside with selective release of the lactiferous ducts of the nipple.
Materials and Methods
We have employed this technique successfully in 97 cases of inverted nipples in 60 patients with follow-up periods of up to 2 years. Twenty-three of them had unilateral inversion, and 37 of them had bilateral nipple inversion.
Results
The appearance of the nipple was good to excellent. Seventy to 80% of the initial postoperative nipple projection at the end of 1 year was maintained. Postoperative complications included stitch abscess in one patient (n = 1) and an epidermal cyst in another (n = 1). Nipple sensation was preserved in 100% of cases. There was no recurrence of inversion in any of the nipples.
Discussion
By identifying the root cause of inverted nipples in each individual case, and selectively targeting them, we minimize surgical morbidity with a simple technique that avoids any form of traction or compression of the nipple and minimizes the risk of altered nipple sensation.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these evidence-based medicine ratings, please refer to the table of contents or the online instructions to authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inverted nipples are a common condition, affecting at least 2% of the female population [1]. Apart from being a functional problem during breast-feeding, it also causes significant psychologic and emotional disturbance. They may arise from congenital or acquired causes such as mastitis, or previous surgery [1, 2]. Depending on the severity of nipple inversion, they have been graded as follows [3, 4]. Grade I refers to nipples that are easily everted manually and maintain their projection. Grade II inverted nipples describe those that can be everted but will not remain so. Grade III nipples can only be everted with difficulty and with surgical correction.
While restoring adequate nipple projection is the goal in corrective procedures, the maintenance of lactiferous ducts and the preservation of nipple sensation are important for optimal results following treatment. This is easier said than done as illustrated by the number of different techniques to correct it. In this article, we present a technique developed on the basis of a clear understanding of the pathomechanics of nipple inversion in each individual case which has given us satisfactory correction of inverted nipples in our practice.
Patients and Methods
In a retrospective review of our data from 2001 to 2006, 60 patients (59 females and 1 male) with nipple inversion underwent corrective surgery. Of the patients in our practice, 23 of them had unilateral inversion and 37 of them had bilateral nipple inversion, giving a total of 97 cases of corrected nipple inversions. Patients’ ages ranged from 21 to 54 years (mean age of 37 years). All patients were followed up at 3 months, 6 months, 1 year, 18 months and 2 years. They were evaluated for loss of nipple projection, altered nipple sensation and recurrence of inversion and surveyed regarding the ability to breast-feed. Measurements of nipple height were taken at initial follow-up at 3 months and measured again at 2 years as a percentage to determine height loss.
The causes of nipple inversion were varied and are given in Table 1. These were as follows—congenital, hypoplastic, post-infective, post-mammaplasty and surgically recalcitrant cases. In 18 patients with congenital inverted nipples, nipplet usage had been futile previously.
This technique follows three important principles which are the (1) selective division of short and hypoplastic ducts and dense connective tissue, (2) provision of dermal support under the nipple and (3) reduction in the base width of the nipple. All patients were advised that breast-feeding may not be feasible post-correction, and all were seeking cosmetic correction alone.
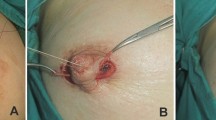
Following infiltration with 1% lignocaine with 1:200,000 adrenaline, the tethering forces in grade I and II inverted nipples became more evident as compared to the grade III (non-retractile) inverted nipples, acting therein as an indicator of the severity of inversion. A vertical ellipse is marked along the height of the nipple–areola at the 6 o’clock position (Fig. 1a). The nipple is then retracted with skin hooks, and a traction suture is placed at the summit of the nipple (Fig. 1b).
a Preoperative marking of the drawbridge flap. b Traction sutures placed at the summit of the nipple to evert it. c De-epithelization of the planned drawbridge flap. d Elevation of the nipple portion of the dermal flap as the ‘drawbridge.’ e Lowering of the dermal drawbridge and securing it across the base of the nipple. f Closure of the secondary defect of the ‘drawbridge’ flap creating a narrower nipple cone and increased projection
An ellipse is then de-epithelized with its maximum transverse dimension (25% of the areolar circumference) at the nipple–areola junction (Fig. 1c). The nipple component of the ellipse is then incised to raise a dermal flap from the tip of the nipple down to its base and lowered down in the manner of a drawbridge (Fig. 1d). This allows clear visualization of the nipple anatomy and good access to the inverting lactiferous ducts which are selectively divided under loupe magnification until all the inverting forces are completely released. Usually, only some of the ducts require complete division, thereby preserving some lactiferous ducts.
The dermal ‘drawbridge’ flap is then interposed between the lactiferous ducts and sutured to the opposing side at the 12 o’clock position to form a dermal bridge, supporting the overlying nipple. The drawbridge flap is then secured with a non-absorbable bolster suture (Fig. 1e).
The secondary defect formed as a result of the vertical ellipse is then closed primarily with the suture line extending from the tip of the nipple to the areolar margin. This reduces the nipple base width, pushes the remaining volume of the nipple into its vertical dimension and enhances its projection without the use of a purse-string suture, which could potentially strangulate the blood supply of the nipple (Fig. 1f). A foam dressing designed to protect and maintain the projection of the nipple is then applied and kept for 2 weeks. The bolster suture is removed at 2 weeks (Figs. 2, 3 and 4).
Results
The outcome was satisfactory in all cases. The appearance of the nipple was good to excellent. At the end of 1 year, 75.4% of the initial postoperative nipple projection was maintained on average. Postoperative complications included stitch abscess in one patient (n = 1) and an epidermal cyst in another (n = 1). There were no cases of hematoma. The scars were inconspicuous, and nipple sensation was preserved in 100% of cases. Lactation, although possible, was not reported in the duration of this study. There was no recurrence of inversion in any of the nipples (Table 2).
Discussion
Inverted nipples were first reported by Sir Cooper in 1840 [5]. The first surgical correction of this disorder was described by Kehrer in 1888 [6]. Since then, various surgical techniques have been proposed. Knowledge of the embryology, anatomy and histology of the nipple–areola complex is important in understanding the pathogenesis of the inverted nipple. The nipple appears as a pit in the ectoderm during the 10th week of intrauterine life, and it is only at or soon after birth that mesenchymal proliferation under the pit produces protrusion of the nipple, with further elevation occurring at puberty [7]. The lack of this mesenchymal proliferation is thought to be a major factor in producing the inverted nipple. Histological sectioning of normal nipple has shown that the thickness of dense connective tissue beneath the nipple is approximately double that found beneath the areola. In the inverted nipple, however, there is no difference between the thickness of the connective tissue beneath the areola and the nipple, thus suggesting that normal nipple projection is due to the difference in tissue bulk beneath the nipple itself [1]. The nipple is vascularized by both a subdermal plexus and a deeper intra-glandular plexus running alongside the lactiferous ducts with intimate anastomoses between the two. In relation, the sensory innervation of the nipple consists of a deep and superficial plexus [8].
The underlying pathophysiology of our approach is that nipple inversion is caused by the following: (1) contracted lactiferous ducts, either as the primary cause in cases of infection/inflammation or due to lactiferous duct fibrosis secondary to nipple subsidence, (2) inadequate dermal support at the base of the nipple, as in congenital nipple inversion, and (3) an excessively broad nipple cone as a result of the tethering effects of the first two etiologies.
Current surgical methods to correct the inverted nipple can be classified into three groups based on the above pathologic mechanisms. The first is to create tightness at the neck of the inverted nipple [9,10,11]. The second is to add bulk beneath the nipple after sacrificing its ductal system [12,13,14]. The third method uses duct saving, partial areolar excision, myotomy of areola–mammillary bundles and maintenance of divided retaining tissue by buried sutures [11, 15,16,17]. Broadbent and Woolf stated that performing only transection of the tethering fibrous tissue that causes inversion of the nipple is insufficient and that it is necessary to bolster the connective tissue beneath the nipple [12]. This connective tissue under the nipple can be reconstructed using tissue from the areola [11, 13, 14], from the mammary glandular tissue [12, 18], or by the use of a purse-string suture [19, 20]. All these procedures cause disturbance of lactation and areolar deformities. Extensive dissection and manipulation of the breast tissue can cause fibrosis which can be a potential cause of recurrence.
Much of the recent literature has focused on maintaining the eversion and lack of bulk to the nipple. D’Assumpcao suggested a method where eversion is maintained by resecting quadrilaterals of skin with their short diagonals based upon their nipple–areola junction, thus narrowing the nipple base [21]. Hartrampf and Schneider maintained eversion by passing horizontal mattress sutures through the nipple, while Morris closed the dead space beneath the nipple to preclude retraction [22,23,24,25]. Crestinu and Hamilton both proposed methods that increase nipple bulk by a local advancement flap of fibroductal tissue and maintenance of eversion by suturing the tissue deep beneath the nipple, obliterating the cavity and projecting the nipple forward [17, 18]. Other methods involve local dermal flaps [26, 27], local flaps of breast tissue [12], or free cartilage grafts [28]. The disadvantage of these latter procedures is that breast-feeding will further be impaired and may become impossible if tissue flaps or cartilage grafts are interposed between the cut ends of the ducts. Distraction devices have also been described for such purposes [29, 30].
Alteration of nipple sensation, which can be permanent, is of special concern. This is probably related to the division of the periductal sensory nerves when dividing the lactiferous ducts, in addition to the partial division of the subdermal plexus when making the skin incision. The use of a purse-string suture at the base of the nipple has been described, but is not a physiologically sensible maneuver and, indeed, at times can be a dangerous procedure. A tight knot may result in partial necrosis of the nipple due to a strangulating effect, while a loose one will induce reinversion as the scar tissue retracts.
There are inherent drawbacks with all these described methods: scarring and nipple deformity, incomplete correction and a high recurrence rate (80% if ducts are not divided and 42% if ducts are divided). Other problems include a temporary or permanent change in nipple sensation, vascular compromise and impairment of breast-feeding [31]. In addition, some of the described methods are technically difficult.
The influence of the local anesthetic on the degree of inversion of the nipple can be explained on the basis of the fact that the areola–mammillary muscle allows projection under normal conditions. However, in inverted nipples, this is restricted and reversed by varying degrees of inverting forces. If the muscle is relaxed or paralyzed by any means, this results in pronounced inversion of the nipple. This has been observed in the present study following infiltration of local anesthetic, which causes the transient paralysis of the areola–mammillary muscle. This can also be found in the clinical situation where the patient complains of intermittent inversion, as with the retractile nipple. The intermittent inversion is considered to be due to the relaxation of the areola–mammillary muscle.
This technique deals with inverting forces and provides tissue bulk at the nipple base. The vertical closure of the areola causes tightening of the nipple base without the use of a purse-string suture. It is simple and quick to perform and provides lasting results with minimal complications.
The drawbridge flap approach to correcting nipple inversion is a simple way of treating inverted nipples of any severity with the following objectives, viz. (1) elevating the flap allows for direct visualization of nipple ductal anatomy and selective division of contracted lactiferous ducts, (2) lowering the dermal drawbridge across the base of the nipple further supports the base of the nipple, and (3) closure of the secondary defect created by the drawbridge flap narrows the cone of the nipple and increases its projection.
This technique is simple to perform and provides maintained nipple projection and nipple sensation in the long term.
References
Schwager RG, Smith JW, Gray GF, Goulian D Jr (1974) Inversion of the human female nipple with a simple method of treatment. Plast Reconstr Surg 54:564
Gupta SC (1965) A critical review of contemporary procedures for mammary reduction. Br J Plast Surg 18:328
Han S, Hong Y (1999) The inverted nipple: its grading and surgical correction. Plast Reconstr Surg 104(2):389–395
Scholten E (2000) The classification of inverted nipples. Plast Reconstr Surg 106:737
Cooper AP (1840) On the anatomy of the Breast. Longman, Orme, Green, Brown and Longmans, London
Kehrer FA (1879) Uber Excision des Warzenhofs bei Hohlwerzen. Beitr Exp Geburtshilfe Gynaekol Gizessen 43:170
Vorherr H (1974) Chapter 1. In the breast: morphology, physiology, and lactation. Academic Press, New York
Sarhadi NS, Dunn JS, Lee FD, Soutar DS (1996) An anatomical study of the nerve supply of the breast, including the nipple and areola. Br J Plast Surg 49:156–164
Skoog T (1953) An operation for inverted nipples. Br J Plast Surg 5:65
Lamont E (1973) Congenital inversion of the nipple in identical twins. Br J Plast Surg 26:178
Wolfort FG, Marshall KA, Cochran TC (1978) Correction of the inverted nipple. Ann Plast Surg 1:294
Broadbent TR, Woolf RM (1976) Benign inverted nipple. Trans-nipple-areolar correction. Plast Reconstr Surg 58:673
Elsahy NJ (1976) An alternative operation for inverted nipple. Plast Reconstr Surg 57:438
Teimourian B, Adham MN (1980) Simple technique for correction of inverted nipple. Plast Reconstr Surg 65:504–506
Spina V (1957) Inverted nipple. Plast Reconstr Surg 19:63
Skoog T (1965) Surgical correction of inverted nipples. J Am Med Womens Assoc 20:931
Sowa Y, Itsukage S, Morita D, Numajiri T (2017) Inverted nipple correction with selective dissection of lactiferous ducts using an operative microscope and a traction technique. Aesthet Plast Surg 41(5):1045–1048
Crestinu JM (1987) The inverted nipple: a blind method of correction. Plast Reconstr Surg 79:127
Hamilton JM (1980) Inverted nipples. Plast Reconstr Surg 65:507
Hauben DJ, Mahler D (1983) A simple method for correction of the inverted nipple. Plast Reconstr Surg 71(4):556–559
D’Assumpcao FA, Rosa EMS (1977) Correcting the inverted nipple. Br J Plast Surg 30:249
Hartrampf CR, Schneider WJ (1976) A simple direct method for correction of inversion of nipple. Plast Reconstr Surg 58:678
Morris AM, Rai YS, Lamont PM (1980) A method for correcting the inverted nipple. Br J Plast Surg 33:41
Jeong JH, Park I, Han J, Park JU (2018) Correction of inverted nipples with the double-track sun-cross running suture technique. J Plast Surg Hand Surg 52(2):87–93
Liang W, Zhao Z, Liu S, Gu T (2017) Cross vertical mattress suturing with basilar tightening during the correction of inverted nipple in 30 cases. Aesthet Plast Surg 41(4):782–787
Haesekar B (1984) The application of de-epithelised turn-over flaps to the treatment of inverted nipples. Br J Plast Surg 37:253
Elsahy NJ (1977) An alternative operation for inverted nipple. Plast Reconstr Surg 57(4):438–441
Brent B, Botswick J (1977) Nipple areola reconstruction with auricular tissue. Plast Reconstr Surg 60:353
Feng R, Li W, Yu B, Zhou Y (2018) A modified inverted nipple correction technique that preserves breastfeeding. Aesthet Surg J. https://doi.org/10.1093/asj/sjy119
Yukun L, Ke G, Jiaming S (2016) Application of nipple retractor for correction of nipple inversion: a 10-year experience. Aesthet Plast Surg 40(5):15–707
Terrill PJ, Stapleton MJ (1991) The inverted nipple: to cut the ducts or not? Br J Plast Surg 44:372–377
Acknowledgements
The authors acknowledge the work of Mrs. E M W Mithoff, Consultant Plastic Surgeon, Canniesburn Unit, Glasgow Royal Infirmary, Glasgow, UK. None of the authors have a financial interest in any of the products, devices or drugs mentioned in this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mathur, B., Loh, C.Y.Y. Sensation-Sparing Correction of Inverted Nipples Using the ‘Drawbridge’ Flap Approach. Aesth Plast Surg 43, 348–353 (2019). https://doi.org/10.1007/s00266-018-1260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1260-5