Abstract
Male body size affects access to mates in many animals. Attributes of sexual signals often correlate with body size due to physiological constraints on signal production. Larger males generally produce larger signals, but costs of being large or compensation by small males can result in smaller males producing signals of equal or greater magnitude. Female choice following multiple male traits with different relationships to size might further complicate the effect of male body size on access to mates. We report the relationship between male body size and pheromone signaling, and the effects on female mate search and courtship in the sea lamprey (Petromyzon marinus). We predicted that pheromone production in the liver and the liver mass to body mass ratio would remain constant across sizes, resulting in similar mass-adjusted pheromone release rates across sizes but a positive relationship between absolute pheromone release and body mass. Our results confirmed positive relationships between body mass and liver mass, and liver mass and the magnitude of the pheromone signal. Surprisingly, decreasing body mass was correlated with higher pheromone concentrations in the liver, liver mass to body mass ratios, and mass-adjusted pheromone release rates. In a natural stream, females more often entered nests treated with small versus large male odors. However, close-proximity courtship behaviors were similar in nests treated with small or large male odors. We conclude that small males exhibit increased release of the main pheromone component, but female discrimination of male pheromones follows several axes of variation with different relationships to size.
Significance statement
Large males often produce signals that are more attractive to females. We determined the relationship between male body size and pheromone signaling in the sea lamprey (Petromyzon marinus). We predicted that larger males, who have a larger pheromone-producing organ, would have a larger pheromone signal and that females, who prefer larger pheromone signals, would prefer the odor of large males. Surprisingly, we found that mass-adjusted pheromone release rates increase with decreasing male body mass due to higher rates of pheromone synthesis and a slightly larger liver proportional to total body mass. In a natural stream, small male odors attracted more females but elicited similar courtship behaviors compared to large male odors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrasexual and intersexual selection shape male body size in many animals (Andersson 1994). Large males often realize higher fitness due to higher fecundity, better access to resources, and various other mechanisms. Direct and indirect benefits of mating with large males maintain female preferences for large males in many taxa (Andersson 1994). However, large size can be costly (Blanckenhorn 2000) and, as a result, small males have mating advantages in some contexts (Wiklund and Fagerström 1977; Zonneveld 1996; Bisazza and Pilastro 1997; Morbey and Ydenberg 2001; Raihani et al. 2006). Trade-offs between body size and other traits linked to fitness can also result in disruptive selection leading to alternative male tactics (Gross 1996). Because of the effects male size has on fitness, many animals use signals that confer information about body size to competitors and possible mates.
Male body size constrains production of many sexual signals. The sizes of organs that produce signals often increase with increasing body size (Gerhardt 1994; Hughes 1996; Blaul and Ruther 2012), and, as a result, signal attributes accurately indicate body size (Bee et al. 1999; LeMaster and Mason 2002; López et al. 2006; Mager et al. 2007; Fugère et al. 2011; Blaul and Ruther 2012; Pisanski et al. 2016). Size-related constraints on signal production generally benefit large males, but small males in some species can produce signals more effectively than large males (Gibson and Bradbury 1985; Gratson 1993). Furthermore, males in some species can produce signals that inaccurately portray size to deceive receivers (Bee et al. 2000; Hughes 2000) or more effectively communicate under the constraints associated with small size (Carazo and Font 2014). Evaluation of mates and competitors using multiple components (Candolin 2003) with potentially different functions and relationships with size (Bro-Jørgensen and Dabelsteen 2008) further complicates the effect of male body size on access to mates. Many reports describe the presence or absence of a positive correlation between body size and qualities of auditory or visual signals, but deviations from the predicted positive relation of body size to signal quality are rare and the interaction between body size and signals acting through other sensory modalities remains poorly understood.
A multi-component male pheromone guides female mate search and courtship in sea lamprey (Petromyzon marinus) (Buchinger et al. 2015). Sexually mature males signal to females with a pheromone that consists primarily of 7α,12α,24-trihydroxy-5α-cholan-3-one-24-sulfate (3-keto-petromyzonol sulfate, 3kPZS) (Li et al. 2002), a bile alcohol that is synthesized in the liver, released via the gills into the water (Siefkes et al. 2003; Brant et al. 2013), and guides long-distance upstream movements (Siefkes et al. 2005; Johnson et al. 2009; Brant et al. 2016b). Minor components elicit courtship behaviors that include attendance to the area of the nest and nest maintenance (Johnson et al. 2012). Although many animals appear to evaluate mates using odors associated with the major histocompatibility complex (MHC), lampreys do not appear to possess MHC (Flajnik and Kasahara 2010). However, variation in 3kPZS signaling among males is high (Siefkes et al. 2003; Brant et al. 2013; Buchinger et al. 2013) and likely results in differential access to mates as females prefer the higher of adjacent pheromone concentrations (Johnson et al. 2009). Given an expected positive relationship between liver mass and body mass (Delahunty and de Vlaming 1980), and pheromone release and total body mass (Scott and Ellis 2007), larger males conceivably benefit from a larger absolute pheromone signal. However, the sources and consequences of variation in pheromone signaling in sea lamprey, as most vertebrates, remain poorly understood.
Here, we evaluated the effects of size on pheromone signaling in sea lamprey. To test the null hypothesis that larger males benefit from a proportionally larger pheromone signal, we determined the effect of size on 3kPZS production and release in males across a range of sizes, and the consequences of size-related pheromone differences on access to mates using female-choice assays in a natural stream. We predicted that (1) size-adjusted pheromone production and release remain constant across size, resulting in a positive relationship between pheromone release and male size, and, as a result, (2) females prefer the odors of large males to the odors of small males.
Methods
Experimental animals
The U.S. Fish and Wildlife Service Marquette Biological Station provided sea lamprey. Tanks fed with ambient Lake Huron water at the U.S. Geological Survey’s Hammond Bay Biological Station held sea lamprey prior to experiments. All experiments used sexually mature male (spermiated) and female (ovulated) sea lamprey. Sea lamprey were held in streams to induce maturation, which was determined by expression of gametes following gentle manual pressure.
Pheromone signaling
Pheromone release was determined for 87 spermiated males ranging from 360 to 555 mm in total length and 63–345 g in body mass (length = 467.45 ± 4.29 mm, mass = 208.13 ± 6.18 g, mean ± SE). Individual males were held in 5 L of aerated deionized water for 2 h, after which 50 mL of water was collected, spiked with 5 ng 5-deuterated 3kPZS ([2H5] 3kPZS) as an internal standard, and frozen at − 20 °C. Pheromone production was determined for a subset of 56 of the males sampled for pheromone release ranging from 380 to 555 mm in length and from 100 to 345 g in mass (length = 475.1 ± 4.94 mm, mass = 222.34 ± 7.5 g, mean ± SE). Immediately after pheromone collection, males were euthanized using an overdose of tricaine methanesulfonate (MS222; Sigma-Aldrich, St. Louis, MO, USA), the hepatosomatic index (HSI; %) determined ([liver mass / total mass] × 100), and liver and gill tissues stored at − 80 °C. Tissue samples were spiked with 500 ng [2H5] 3kPZS, and pheromones were extracted using described methods (Brant et al. 2013). 3kPZS and the immediate precursor 3α,7α,12α,24-tetrahydroxy-5α-cholan-24-sulfate (petromyzonol sulfate, PZS) were quantified using an established method of ultra-high performance liquid chromatography with tandem mass spectrometry (Li et al. 2011). The relationship between HSI and mass was evaluated using Spearman’s rank correlation, and the effect of mass estimated using linear regression in R (R Core Team 2014). Data were square root transformed to meet model assumptions, except 3kPZS release rates per total mass and tissue concentrations of 3kPZS which were square root [n + 1] and natural logarithm transformed, respectively.
Behavioral assays
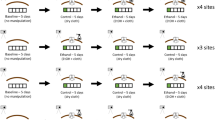
In-stream behavioral assays evaluated the responses of ovulated females to large versus small male odors. Behavioral assays were conducted on 27 June 2015–3 August 2015 in the upper Ocqueoc River, MI, USA. Sea lamprey infested the Ocqueoc River in the mid-1900s (Applegate 1950), but are now restricted from the upper stretches by a barrier. The site and method used replicate previous studies on sea lamprey pheromone communication (Johnson et al. 2009, 2012; Brant et al. 2016a).
Briefly, female responses to odors applied to side-by-side spawning nests were monitored visually and with a passive integrated transponder (PIT, Oregon RFID; www.oregonrfid.com) array. Each female was outfitted with a 23-mm PIT tag and a unique combination of two colored streamer tags. A single trial involved approximately 10 females, depending upon availability. Groups of females were held 45 m downstream of the constructed nests in 0.5-m2 acclimation cages at least 8 h prior to being used in a trial. Immediately prior to an experiment, odors were applied to each of the two constructed spawning nests using a peristaltic pump (Masterflex L/S; www.coleparmer.com). Each nest was made to resemble a sea lamprey spawning nest with a shallow cavity surrounded by rocks (Johnson et al. 2015) and was surrounded by a 1-m2 PIT antenna. The two adjacent nests were 1 m apart. Twenty liters of odor was applied to each nest at 167 mL min−1. After 30 min of odor application, the group of females was released and observed for 1.5 h. We recorded the first nest that each female entered, the number of visits to each nest, the duration of time a female spent in each nest, and the number of nesting behaviors exhibited in each nest. Females remained in the stream after being used in a trial and would occasionally revisit nests in subsequent trials, but unique PIT tag codes allowed us to include individuals only in the analysis of their respective trial. Females that remained in the proximity of our assay are unlikely to have influenced the behavior of focal females as sea lamprey move independently of one another (Siefkes et al. 2005). We could not record data blind because our study involved observations of focal animals in the field and we had too few personnel to have some dedicated to recording female behavior and others dedicated to preparing odors.
In two separate series of experiments, we compared female responses to (1) high versus low odor concentrations and (2) large versus small male odors. Odors for experiments were collected by holding a total of 42 spermiated males in 3 L of water for 2 h (small: n = 21, 120.31 ± 6.27 g, 379.48 ± 7.99 mm; large: n = 21, 269.75 ± 7.59 g, 492.95 ± 4.84 mm; mean ± SE). Males were held individually in buckets. Odors were collected in three groups; one set of 30 males and, due to unforeseen needs for additional odors, a second and third set of eight and four males, respectively. Odors from equal numbers of males (large = 15, 4, and 2; small = 15, 4, and 2) were combined across both size groups (experiment 1; mixture) or combined according to male size (experiment 2; large versus small), and were stored at − 20 °C. A 10-mL sample was collected for 3kPZS quantification, which followed the methods described above.
Experiments directly compared two odor treatments by applying each into one of two side-by-side nests. Experiment 1 compared the mixture to 0.1× mixture to demonstrate that the assay could detect female movement towards a higher over lower odor concentration (N trials = 7, n females = 76). Experiment 2 compared the odor of large males to the odor of small males (N trials = 12, n females = 127). Over the 2 h of experiment, 300 mL mixed into 19.7 L was applied for the 0.1× mixture treatment, and 3 L mixed into 17 L was applied for the large, small, and mixture treatments. As male odors were collected in 3 L of water for 2 h, a 3-L per 2-h application rate matches the odor that would be released by one male on the nest for the duration of an experiment.
We use mixed-effect linear and logistic regression models in R to evaluate the effect of odor female behavior. Specifically, we evaluated the effect of odor on (1) first nest choice, (2) whether or not females visited a nest at some point during the trial, with a random effect of fish identity, (3) the number of times females visited a nest during the trial, with a random effect of fish identity, (4) the duration of time a female remained in a nest, with a random effect of fish identity, and (5) whether or not females moved rocks on the nest, which is a typical nest maintenance behavior (Johnson et al. 2015), with a random effect of fish identity. In each model, we tested for nest biases (left versus right) and included a random effect of trial. Time data were square root transformed to meet model assumptions. All analyses used the lmer and glmer functions in R package lme4, type II sums of squares, and α = 0.05.
Results
Small males exhibit increased pheromone signaling relative to body mass
3kPZS release rates adjusted by body mass, and pheromone concentrations in tissues decreased with increasing male body mass (Table 1, Fig. 1). Release of 3kPZS and pheromone concentrations in tissues were similar to previous reports (Table 2; Brant et al. 2013). Total body mass did not predict absolute 3kPZS release. Liver mass positively correlated with total mass (Spearman’s rank, ρ = 0.82, P < 0.001) and absolute 3kPZS release increased with total liver mass (Table 1), but relative liver mass (HSI) negatively correlated with male mass (Spearman’s rank, ρ = − 0.24, P = 0.029), and 3kPZS release adjusted for the main effect of liver mass decreased with increasing total mass. Tissue concentrations of 3kPZS and its precursor PZS decreased with increasing total body mass.
Small male odors attract more females but elicit similar courtship behaviors
Only females that moved upstream and entered a nest were included in the analysis (experiment 1: n = 27, experiment 2: n = 45). Fish that did not move up to a nest died during the experiment (experiment 1: n = 4, experiment 2: n = 14), moved downstream (experiment 1: n = 16, experiment 2: n = 6), or remained in or near the release cage (experiment 1: n = 29, experiment 2: n = 62).
Experiment 1 confirmed that females prefer higher concentrations of male odor. First nest choice was biased towards the right nest (χ 2 1 = 10.43, P = 0.001), but was higher for the nest treated with the full mixture than the nest treated with 0.1× mixture (χ 2 1 = 32.67, P = <0.001; N trials = 7; n fish = 27; Fig. 2). The logistic regression model for first nest entry in experiment 1 would not converge when a trial was included as a random effect, hence a model without the trial effect was used. More females eventually visited nests treated with the full mixture than nests treated with the 0.1× mixture (χ 2 1 = 8.87, P = 0.003; Fig. 3) and visited the nest treated with the full mixture more often than the nest treated with 0.1× mixture (χ 2 1 = 38.43, P < 0.001). Retention data were unavailable for five females that lost PIT tags during the trials. Females that entered a nest spent a longer duration of time in the right nest (χ 2 1 = 5.90, P = 0.015), but spent more time in the nest treated with the full mixture compared to the nest treated with 0.1× mixture (χ 2 1 = 13.68, P < 0.001; Fig. 3). Eleven of 25 females that entered nests treated with the full mixture moved rocks (range = 1–63), but none of the 13 females that entered nests treated with 0.1× mixture moved rocks (Fig. 3).
Ovulated females were more likely to enter nests first when baited with male odor at higher concentrations (left) and small male odor (right). m: mixture of odors collected from large and small males. 0.1×: a 10-fold dilution of the same mixture of odors collected from large and small males. l: odor collected from large males. s: odor collected from small males. Numbers within bars represent the number of females whose first nest choice was the nest treated with either odor. l = number of females the chose a given treatment when applied to the left nest, r = number of females that chose a given treatment when applied to the right nest. P values were determined using mixed-effects logistic regression. Error bars represent one standard error
Ovulated females did not exhibit different courtship responses to odors from large or small males. a Females visited more often nests treated with male odor at higher concentrations (left) but equally often nests treated with small versus large male odor (right). Numbers within bars indicate the number of females that visited each odor at some point during the trial over the total number of females that visited either nest. b Females spent more time at nests treated with male odor at higher concentrations (left) but equal durations of time at nests treated with small versus large male odor (right). Numbers within bars represent the number of females that entered the nest and for which the duration of time they remained in the nest was recorded over the total number that entered the nest and for which the duration of time they remained in the nest was recorded. c Females were more likely to move rocks in nests baited with male odor at higher concentrations (left) but equally to move rocks in nests baited with small and large male odor (right). Numbers within bars represent the number of females that moved rocks over the number of females that entered a nest. m: mixture of odors collected from large and small males. 0.1×: a 10-fold dilution of the same mixture of odors collected from large and small males. l: odor collected from large males. s: odor collected from small males. Error bars represent one standard error. P values were determined using mixed effects logistic and linear regression
The results from experiment 2 indicated that small male odor attracts more females than large male odor but elicits similar courtship behaviors. First nest entry was biased towards the left nest (χ 2 1 = 5.09, P = 0.024), but was higher in the nest treated with small male odor compared to the nest treated with large male odor (χ 2 1 = 4.07, P = 0.044; N trials = 12; n fish = 45; Fig. 2). Time data from the first two trials comparing large male odor and small male odor were lost due to PIT equipment failure. Females were equally likely to visit nests treated with either odor at some point during the trial (χ 2 1 = 0.48, P = 0.49; Fig. 3). Females visited the left nest more often than the right (χ 2 1 = 10.11, P = 0.002), but did not visit nests treated with either odor more often (χ 2 1 = 0.97, P = 0.328). Females that entered a nest did not stay significantly longer at nests treated with the odor of either large males or small males (χ 2 1 = 3.74, P = 0.053; Fig. 3) and were equally likely to move rocks in nests treated with either odor (χ 2 1 = 1.24, P = 0.265; range = 1–28; Fig. 3).
Quantification of 3kPZS in odors used for behavioral assays yielded no difference in concentration between large and small male odors (large = 173.93 ± 27.53 ng/mL; small = 159.38 ± 23.22 ng/mL; X ± SE; t = 0.40, df = 40, P = 0.69, two-tailed t test), and confirmed small males released 3kPZS at a higher mass-adjusted rate (large = 0.97 ± 0.154 μg/g/h; small = 1.92 ± 0.244 μg/g/h; X ± SE; t = − 3.28, df = 40, P = 0.002, two-tailed t test).
Discussion
Given that body size is an important fitness character in many taxa (Andersson 1994), we determined the effect of male size on pheromone signaling and the consequences of size-related differences in male pheromones on female behavior in a natural stream. We found (1) a negative relationship between mass-adjusted pheromone signaling and total body mass, (2) that females moved towards the odor of small males over large males, and (3) that females exhibited similar courtship behaviors in nests treated with large and small male odors. Our results offer the first evidence, to our knowledge, for increasing investment in a chemical signal with decreasing size, and rare insight into the effect of complex interactions between size and signals on the various levels of female choice.
Dual mechanisms of higher pheromone synthesis and a proportionally larger liver allow small males to produce a larger pheromone signal that likely increases mating opportunity. Pheromone concentrations in tissues, possibly driven by higher expression of biosynthetic enzymes that synthesize bile acids (Brant et al. 2013), and the hepatic somatic index increased with decreasing body mass. The mechanisms of slightly larger liver mass and higher pheromone production independent of liver mass are further supported by our observation that release of 3kPZS increased with increasing liver mass and decreased with increasing body mass after accounting for the main effect of liver mass. Based upon our data, higher pheromone production and proportionally larger livers translate into absolute 3kPZS release rates that are approximately equal for small (1st quartile) and large (3rd quartile) males. In contrast, if relative release rates remained constant across sizes, small males would release approximately 2× less absolute 3kPZS. We suggest that the increased release of 3kPZS by small males results in ecologically relevant concentration differences. Female sea lamprey orient towards a higher concentration of 3kPZS when presented at only 2× the concentration of an adjacent source (Johnson et al. 2009). Indeed, our female-choice experiments indicate that the pheromone signal of small males matches that of large males, and even attracts more females.
Our results indicate that female discrimination of male pheromones follows several axes of variation that differ in their relationship with male size. Complex signals consist of distinct components that can have different underlying functions and face different selective pressures (Candolin 2003; Bro-Jørgensen 2010). In sea lamprey, 3kPZS guides long-distance mate search and attraction to a male’s nest, while additional components elicit female courtship behaviors in close proximity to a male (Johnson et al. 2009, 2012). We observed that females were more likely to enter first nests treated with the odor of small males over the odor of large males, but attended nests treated with either odor an equal number of times and similar durations of time, and were equally likely to exhibit nesting behaviors. Hence, the relation of male size to pheromone signaling differed between one or more of the components that mediate mate search by females and the components that mediate the courtship behaviors by females in close proximity to males. Furthermore, female entry into nests treated with small male odors over large male odors, despite each having a similar concentration of 3kPZS, indicates that an additional pheromone component mediates nest entry and exhibits a pattern of variation different from 3kPZS. Phylogenetic comparisons also indicate that multiple components of the pheromone blend have evolved differently; sea lamprey are attracted to odors of species that do not release 3kPZS, suggesting that minor components may be conserved (Buchinger et al. 2013, 2017). The observed preference for small male odors despite similar concentrations of 3kPZS is unlikely the result of stress odors released by large males because females would presumably have spent less time and exhibited fewer nesting behaviors rather than only choosing small male odors initially. Taken together, our results indicate complex and meaningful patterns of signal variation, but invite further research to unveil the ultimate function of pheromone variation in guiding female mate choice.
Our results do not directly implicate deceit by small males. Dishonest signaling implies that the receiver’s response to a signal is in error (Carazo and Font 2014). Female sea lamprey must actively use 3kPZS to evaluate male size and prefer to mate with large males for increased pheromone signaling by small males to be deceptive. While chemical signals guide mate choice in many taxa (Johansson and Jones 2007) and can be deceptive (Christy and Rittschof 2010; Ng et al. 2014), female preference for large males in sea lamprey, although conceivable, is not yet established. Large males may be able to better defend nests against intruders (C.O. Brant, personal communication 2015), and construct larger nests that likely retain more eggs and therefore support higher egg survival (Smith and Marsden 2009; Johnson et al. 2015). However, females might gain no benefit from mating with large males other than reduced costs of mate search resulting from a larger pheromone signal, given a positive relationship between size and signal magnitude. Hence, our results might represent small males compensating for their size with higher relative investment into pheromone signaling, with no underlying sexual conflict. Given the positive relationship between body mass and liver mass, and liver mass and pheromone release rate, we suggest that small males increase investment into 3kPZS signaling rather than the alternative that large males decrease investment into 3kPZS signaling. However, decreased 3kPZS signaling in large males, perhaps the result of higher energetic costs, is also possible, as is a benefit to females mating with small males. More information on the benefits females gain through mate choice will allow a better understanding of the ultimate mechanisms underlying the observed pattern of signal variation across male size.
In conclusion, we present evidence that increasing mass-adjusted pheromone signaling with decreasing body mass has distinct effects on female mate search and courtship in sea lamprey. The large amount of variance unexplained by size requires continued investigations on the determinants of pheromone release. A better understanding of mate choice in sea lamprey will reveal whether females are deceived into perceiving small males as large, or if differences in pheromone signaling are the result of compensation by small males, or a cost for large males. Regardless, our results offer rare insight into the complexities of animal communication through a chemical perspective, which, compared to other senses, is rarely taken in sexual selection research (Coleman 2009), especially in vertebrates (Symonds and Elgar 2008).
References
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Applegate VC (1950) Natural history of the sea lamprey, Petromyzon marinus, in Michigan. Special Scientific Report: Fisheries No. 55. U.S. Department of Interior, Fish and Wildlife Service, Washington, DC
Bee MA, Perrill SA, Owen PC (1999) Size assessment in simulated territorial encounters between male green frogs (Rana clamitans). Behav Ecol Sociobiol 45:177–184
Bee MA, Perrill SA, Owen PC (2000) Male green frogs lower the pitch of acoustic signals in defense of territories: a possible dishonest signal of size? Behav Ecol 11:169–177
Bisazza A, Pilastro A (1997) Small male mating advantage and reversed size dimorphism in poeciliid fishes. J Fish Biol 50:397–406
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Blaul B, Ruther J (2012) Body size influences male pheromone signals but not the outcome of mating contests in Nasonia vitripennis. Anim Behav 84:1557–1563
Brant CO, Chung-Davidson Y-W, Li K, Scott AM, Li W (2013) Biosynthesis and release of pheromonal bile salts in mature male sea lamprey. BMC Biochem 14:30
Brant CO, Huertas M, Li K, Li W (2016a) Mixtures of two bile alcohol sulfates function as a proximity pheromone in sea lamprey. PLoS One 11:e0149508
Brant CO, Johnson NS, Li K, Buchinger TJ, Li W (2016b) Female sea lamprey shift orientation toward a conspecific chemical cue to escape a sensory trap. Behav Ecol 27:810–819
Bro-Jørgensen J (2010) Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol Evol 25:292–300
Bro-Jørgensen J, Dabelsteen T (2008) Knee-clicks and visual traits indicate fighting ability in eland antelopes: multiple messages and back-up signals. BMC Biol 6:47
Buchinger TJ, Li K, Huertas M, Baker CF, Jia L, Hayes MC, Li W, Johnson NS (2017) Evidence for partial overlap of male olfactory cues in lampreys. J Exp Biol 220:497–506
Buchinger TJ, Siefkes MJ, Zielinski BS, Brant CO, Li W (2015) Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool 12:32
Buchinger TJ, Wang H, Li W, Johnson NS (2013) Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc R Soc B 280:20131966
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Carazo P, Font E (2014) ‘Communication breakdown’: the evolution of signal unreliability and deception. Anim Behav 87:17–22
Christy JH, Rittschof D (2010) Deception in visual and chemical communication in crustaceans. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 313–333
Coleman SW (2009) Taxonomic and sensory biases in the mate-choice literature: there are far too few studies of chemical and multimodal communication. Acta Ethol 12:45–48
Delahunty G, de Vlaming VL (1980) Seasonal relationships of ovary weight, liver weight and fat stores with body weight in the goldfish, Carassius auratus (L.) J Fish Biol 16:5–13
Flajnik MF, Kasahara M (2010) Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet 11:47–59
Fugère V, Ortega H, Krahe R (2011) Electrical signalling of dominance in a wild population of electric fish. Biol Lett 7:197–200
Gerhardt HC (1994) The evolution of vocalization in frogs and toads. Annu Rev Ecol Syst 25:293–324
Gibson R, Bradbury J (1985) Sexual selection in lekking sage grouse: phenotypic correlates of male mating success. Behav Ecol Sociobiol 18:117–123
Gratson MW (1993) Sexual selection for increased male courtship and acoustic signals and against large male size at sharp-tailed grouse leks. Evolution 47:691–696
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Hughes M (1996) Size assessment via a visual signal in snapping shrimp. Behav Ecol Sociobiol 38:51–57
Hughes M (2000) Deception with honest signals: signal residuals and signal function in snapping shrimp. Behav Ecol 11:614–623
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Johnson NS, Buchinger TJ, Li W (2015) Reproductive ecology of lampreys. In: Docker M (ed) Lampreys: Biology, Conservation and Control. Springer, Dordrecht, pp 265–303
Johnson NS, Yun S-S, Buchinger TJ, Li W (2012) Multiple functions of a multi-component mating pheromone in sea lamprey Petromyzon marinus. J Fish Biol 80:538–554
Johnson NS, Yun S-S, Thompson HT, Brant CO, Li W (2009) A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc Natl Acad Sci USA 106:1021–1026
LeMaster MP, Mason RT (2002) Variation in a female sexual attractiveness pheromone controls male mate choice in garter snakes. J Chem Ecol 28:1269–1285
Li K, Wang H, Brant CO, Ahn S, Li W (2011) Multiplex quantification of lamprey specific bile acid derivatives in environmental water using UHPLC–MS/MS. J Chromatogr B 879:3879–3886
Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun S-S, Gage DA (2002) Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 296:138–141
López P, Amo L, Martín J (2006) Reliable signaling by chemical cues of male traits and health state in male lizards, Lacerta monticola. J Chem Ecol 32:473–488
Mager JN, Walcott C, Piper WH (2007) Male common loons, Gavia immer, communicate body mass and condition through dominant frequencies of territorial yodels. Anim Behav 73:683–690
Morbey YE, Ydenberg RC (2001) Protandrous arrival timing to breeding areas: a review. Ecol Lett 4:663–673
Ng SH, Shankar S, Shikichi Y, Akasaka K, Mori K, Yew JY (2014) Pheromone evolution and sexual behavior in Drosophila are shaped by male sensory exploitation of other males. Proc Natl Acad Sci USA 111:3056–3061
Pisanski K, Oleszkiewicz A, Sorokowska A (2016) Can blind persons accurately assess body size from the voice? Biol Lett 12:20160063
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Raihani G, Székely T, Serrano-Meneses MA, Pitra C, Goriup P (2006) The influence of sexual selection and male agility on sexual size dimorphism in bustards (Otididae). Anim Behav 71:833–838
Scott AP, Ellis T (2007) Measurement of fish steroids in water—a review. Gen Comp Endocrinol 153:392–400
Siefkes MJ, Scott AP, Zielinski B, Yun S-S, Li W (2003) Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biol Reprod 69:125–132
Siefkes MJ, Winterstein SR, Li W (2005) Evidence that 3-keto petromyzonol sulphate specifically attracts ovulating female sea lamprey, Petromyzon marinus. Anim Behav 70:1037–1045
Smith SJ, Marsden JE (2009) Factors affecting sea lamprey egg survival. N Am J Fish Manag 29:859–868
Symonds MR, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228
Wiklund C, Fagerström T (1977) Why do males emerge before females? Oecologia 31:153–158
Zonneveld C (1996) Being big or emerging early? Polyandry and the trade-off between size and emergence in male butterflies. Am Nat 147:946–965
Acknowledgements
Tyler Bruning, Scott Couture, Brooklyn Idalski, Michelle VanCompernolle, Julia Krohn, Mike Siemiantkowski, and Joseph Reidy assisted with experiments. The U.S. Fish and Wildlife Service Marquette Biological Station provided experimental animals. Heather Eisthen, Jenny Boughman, Andrea Pilastro and two anonymous reviewers provided valuable suggestions that improved the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was funded by the Great Lakes Fishery Commission. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and all procedures were approved by Michigan State University’s Animal Use and Care Committee (Approval no. 02/13-040-00).
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability statement
The data collected during the current study are provided in supplemental files.
Additional information
Communicated by A. Pilastro
Electronic supplementary material
Supplemental Table 1
(XLSX 21 kb)
Supplemental Table 2
(XLSX 18 kb)
Rights and permissions
About this article
Cite this article
Buchinger, T.J., Bussy, U., Buchinger, E.G. et al. Increased pheromone signaling by small male sea lamprey has distinct effects on female mate search and courtship. Behav Ecol Sociobiol 71, 155 (2017). https://doi.org/10.1007/s00265-017-2384-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2384-3