Abstract
Group-living species are often organized into social dominance hierarchies, where high-ranking individuals have priority of access to resources, including estrous females. Traits associated with male dominance status should thus be correlated with reproductive success, but, with the exception of research on some primates, studies with both behavioral data to determine dominance hierarchies and a pedigree to identify male siring success are rare. For a wild population of sexually dimorphic eastern grey kangaroos Macropus giganteus, we characterized the social hierarchy over 6 non-consecutive years to investigate the relationship between male dominance and yearly reproductive success. Dominance hierarchies were steep, linear, and stable over time. Asymmetries in body mass and size between contestants were strong predictors of contest outcomes, and these morphological traits were positively correlated with dominance status. Males did not spatially avoid each other but tended to fight with individuals of similar size, suggesting that when asymmetries were large, contests were unlikely. Dominance status was under strong sexual selection, despite moderate monopolization of paternities by highly dominant males. Overall, these results suggest that body size and weapons are important determinants of dominance status and male reproductive success but that other traits play a considerable role. A clear-cut dominance hierarchy and strong selection on dominance status do not necessarily lead to monopolization of reproduction by the most dominant males in this strongly sexually dimorphic species.
Significance statement
It is generally assumed that high social rank in males is a very strong determinant of access to mates in polygamous species. Like other sexually dimorphic species, male kangaroos are thought to establish dominance hierarchies based on size. A few high-ranking males are then believed to monopolize matings. Our study confirms a strong correlation between body size and dominance status, and that males mostly engage in contests with males of similar size. However, we also found weak monopolization of reproduction, despite strong, positive sexual selection on dominance status. This result suggests that strong selection for high rank does not imply that subordinate males cannot sire offspring. A few highly dominant males had high reproductive success but did not monopolize matings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In gregarious species, individuals often compete for space, resources, and mates (West-Eberhard 1979). Competition for mates is central to sexual selection theory, which presumes that phenotypic traits assisting mate acquisition yield fitness gains in terms of reproductive success (Andersson 1994). This causal dependence of fitness on phenotype can strongly affect the distribution of siring success in some polygynous species, where a few males may monopolize matings (Emlen and Oring 1977; Clutton-Brock 1989). Animal societies are often organized into social dominance hierarchies, and social rank can greatly affect individual fitness (Dewsbury 1982; Ellis 1995). Extensive research has been conducted on traits signaling dominance status and fighting skills (McCullough et al. 2016; Rico-Guevara and Hurme 2019). Individual rank and fighting skills can also reliably advertise individual condition, which can be selected through mate choice (Qvarnström and Forsgren 1998). Dominant males of polygynous and polygynandrous species thus often experience high reproductive success (Dewsbury 1982; Ellis 1995; e.g., savannah baboons Papio cynocephalus, Alberts et al. 2006; southern elephant seals Mirounga leonina, Hoelzel et al. 1999; North American bison Bison bison, Wyman et al. 2021), but in some species, dominance is a weak determinant of male reproductive success (e.g., tammar wallabies Notamacropus eugenii, Hynes et al. 2005; horned dung beetles Onthophagus taurus, McCullough and Simmons 2016).
The concept of dominance is pivotal in studies of social behavior, although its definition varies among scholars. Typically, dominance implies a stable asymmetry in the outcome of contests between two individuals (a dyad), predictably in favor of one individual (dominance-subordination relationship; Drews 1993). Accordingly, individual dominance can be defined by an ordinal rank as the result of contest outcomes (Pelletier and Festa-Bianchet 2006). Dominance, however, can also be characterized as a score or rating (Neumann et al. 2011), which measures success of competing individuals during contests (Jennings et al. 2017, 2021). Finally, within a social environment, dominance can be viewed as a latent trait intrinsic to each individual, broadly representing its resource-holding potential (RHP; Parker, 1974). Age, relative body and/or weapon size, aggressiveness, or signals of fighting ability can affect the outcome of contests and thus reflect a male’s RHP. Regardless of how dominance is defined, if two individuals have similar RHP, contests may escalate to fights (Parker 1974; Jennings et al. 2006; Bergeron et al. 2010). Because escalated contests, such as fights, are costly and potentially lethal (Clutton-Brock et al. 1979; Crespi 1988), game theory predicts that they should be rare and individuals should be under strong selection to avoid escalation of interactions (Maynard Smith and Price 1973; Maynard Smith 1974). Males may thus engage in ritualized displays (sensu Maynard Smith, 1974), where asymmetries in RHP define relative dominance status. In a social group, these asymmetries can determine a dominance hierarchy, based on individual dominance rank (Dewsbury 1982).
Several indices have been developed to quantify dominance hierarchies, either through ranking systems (de Vries 1998; Douglas et al. 2017) or indices of success (David 1987; Albers and de Vries 2001). The reliability of a social hierarchy in predicting the outcome of dyadic interactions depends on its linearity. If the hierarchy is strictly linear, one individual consistently dominates certain other individuals, and triads are transitive, so that outcomes of dyadic encounters are predictable since the individual with the higher rank or dominance score is expected to win (McDonald and Shizuka 2013). Dominance hierarchies can be quantified from interaction matrices, in which dominant-subordinate relationships are deduced for each dyad. Sparse datasets, with a low proportion of observed dyads, increase uncertainty in rank assessment, so it is important to record interactions among as many dyads as possible to robustly quantify dominance hierarchies (Neumann et al. 2011; Sánchez-Tójar et al. 2018). Alternatively, sparse datasets can be tackled statistically (Foerster et al. 2016; Sánchez-Tójar et al. 2018) or by staging dyadic interactions. The latter solution is particularly useful to study determinants of the dominance-subordination relationship (Stuart-Fox et al. 2006; Wilson et al. 2011a). These approaches, however, ignore the possibility that unobserved contests may be biologically meaningful if some individuals actively avoid interacting with other, highly dominant individuals that could cause injury or death (Shizuka and McDonald 2012).
In polygamous, gregarious, and non-territorial species, encounters between individuals may create social conflict, especially at high population density (Schoepf and Schradin 2012; Piza-Roca et al. 2018). Depending on social status and previous interactions, individuals can thus adjust their home-range use to avoid other individuals (Kaufmann 1983). High-ranking individuals could force subordinates into areas of lower resource value (Murray et al. 2007), and subordinate males may avoid dominant males to escape harassment. High-ranking males could also avoid each other to reduce the risk of fighting injuries (Parker 1974). Spatial avoidance thus likely induces non-random association patterns among individuals according to dominance status (Shizuka and McDonald 2012; McDonald et al. 2013).
Here, we investigate which traits are associated with male dominance status, to understand the drivers of contests among males and to determine whether yearly reproductive success is facilitated by high dominance rating in a species where dominance status should favor access to mates. We use longitudinal data on behavior, space use, and siring success of eastern grey kangaroos (Macropus giganteus), a species known for intense male-male escalated contests (Russell 1974).
Macropus giganteus is a large, sexually dimorphic (Jarman 1989), gregarious (Kaufmann 1975) marsupial with fission–fusion social dynamics (Jarman and Coulson 1989), which inhabits temperate and subtropical open habitats, often at high density (Russell 1974). Males do not defend territories (Jarman and Southwell 1986), and home ranges overlap extensively (King et al. 2015b; Montana et al. 2020), creating opportunities for male-male contests. Kangaroos appear to mostly rely on body size to win contests (Jarman 1983), but dominance status may also depend upon aggressiveness since rank does not correlate perfectly with size (Grant 1973; Miller et al. 2010). Kangaroos grow throughout much of life (LM, WJK, GC, and MF-B unpubl. data), so older adult males should be dominant. Most male-male contests are ritualized displays (Grant 1973) but can lead to serious injuries or death (Miller et al. 2010; Toni 2018) if they escalate to violent fights (Coulson 1997). The outcome of contests can be used to define a dominance hierarchy, which has been described as linear in previous studies with captive populations and/or limited sample sizes (< 13 males/group) (Grant 1973; Miller et al. 2010).
The mating system of M. giganteus is considered polygynous (Jarman 1983). Males court and follow estrous females; dominant males account for more than 75% of observed mountings (Jarman and Southwell 1986). Before and after copulating, dominant males guard estrous females from other males. They may also interrupt copulation by other males, similar to other promiscuous marsupials (reviewed in Russell 1984). Given that estruses rarely overlap in time (Russell 1984), a few dominant males could monopolize estrous females, resulting in high variance in male reproductive success. A study of two free-ranging M. giganteus populations, however, found low reproductive skew (Rioux-Paquette et al. 2015), possibly because males do not have equal access to all females (Montana et al. 2020), but also because females can mate with multiple males (Paplinska et al. 2010; Montana et al. 2020), indicating a polygynandrous mating system. The role of female mate choice in determining male reproductive success is unclear (Miller et al. 2010) and could partly explain the low reproductive skew found recently (Rioux-Paquette et al. 2015). Rioux-Paquette et al.’s (2015) result for M. giganteus challenges the long-standing hypothesis that high dominance status leads to monopolization of estrous females, a hypothesis that also may not be supported in other polygynous and polygynandrous systems (Wroblewski et al. 2009).
We first evaluated how morphological traits influenced dominance status, defined by estimating male dominance ratings. Consequently, dominance status was quantified as the rate of success (or failure) of males during contests. We evaluated the role of physical traits on dominance status with two complementary analyses. First, we estimated yearly dominance hierarchies and their associated uncertainties (Sánchez-Tójar et al. 2018) to determine whether physical traits correlated with individual dominance ratings. Given the consistent pattern of larger, heavier individuals being dominant in many species (Alpine ibex Capra ibex, Bergeron et al. 2010; bighorn sheep Ovis canadensis, Pelletier and Festa-Bianchet 2006; New Zealand sheet-web spider Cambridgea foliata, Walker and Holwell 2018; eastern gorilla Gorilla beringei, Wright et al. 2019), we expected large, heavy males to have high dominance ratings. We then tested whether asymmetry in body mass and limb length between contestants predicted winning probability within a dyad in unescalated contests (such as displays, displacements, and play-fights) and in escalated contests (henceforth, fights). We thus use “male-male contests” as a general term for both unescalated contests and fights. We expected trait asymmetry to be an important determinant of contest outcome. The asymmetry analysis is complementary to the size/mass analysis and provides statistical refinement. Moreover, individual success in contests could represent a phenotypic measure of RHP. Kangaroo males can differ widely in size (Montana et al. 2020); therefore, we tested whether male-male contests were size-assortative, expecting small males to avoid large ones, especially for fights. We also investigated whether (i) subordinate males spatially avoided dominant males, which should prefer areas of high female density, and (ii) high-ranking males avoided each other, potentially generating a subdivision of mating opportunities that could explain, in addition to the possible influence of female mate choice, the low reproductive skew found of this kangaroo population (Rioux-Paquette et al. 2015). Finally, we investigated the relationship between dominance ratings and yearly male reproductive success. If dominance rating positively influences access to mates, individuals with high ratings should obtain greater reproductive success than males that lose more dyadic contests (Andersson 1994). Miller et al. (2010) found a positive relationship between dominance and reproductive success in a small captive population. Examining the dynamics of male-male contests, the determinants of dominance status, and the relationship between dominance status and male reproductive success in a polygynandrous species is essential for a comprehensive understanding of the association between social competition and reproductive success, which is the foundation of sexual selection theory (Andersson 1994).
Methods
Study site and population
We studied eastern grey kangaroos in Wilsons Promontory National Park (38°57′S, 146°17′E), Victoria, Australia. We monitored a 1.1-km2 grassland around an emergency landing strip, where kangaroos are habituated to observers and easily identified. Monitoring started in 2008, and during our 9-year study (breeding seasons of 2010–2018), density varied from 2 to 7 kangaroos/ha (Plaisir et al. 2022). Similarly to other populations (Coulson et al. 2014), most females are philopatric and sedentary, with smaller home ranges than adult males, on average (King et al. 2015b). The study site is the main breeding area within the park due to a large number of resident adult females. Many males move into the site during the peak of the breeding season and decrease their attendance during autumn and winter (Montana et al. 2020). Despite the small size of the study site, about 2.5% of male dyads showed no spatial overlap (LM unpubl. data).
Adult kangaroos were marked with unique combinations of Allflex-colored eartags and visual plastic collars after being immobilized by injection of Zoletil (King et al. 2011). While kangaroos were immobilized, we measured hind leg (hereafter “leg length”) and forearm length (“arm length”) to the nearest mm as proxies of skeletal size and body mass to the closest 250 g using a spring scale. Leg length and body mass were used to estimate individual body condition with the relative condition index (Kn; Peig and Green 2010), which is uncorrelated to skeletal size or date in kangaroo males (Montana et al. 2020). Kn was estimated independently for each year and ranged from 0.903 to 1.085 (SD = 0.024). Only males measured between 1 July and 15 February were included in analyses.
Molecular analyses and paternity assignment
Laboratory analyses and paternity assignment are detailed in Montana et al. (2020). In brief, ear tissue samples were collected at first capture either using a 2-mm biopsy punch or by collecting tissue displaced by eartags. Samples were stored in 95% ethanol and refrigerated at 4 °C. We extracted DNA from these samples using a modified version of the salting-out method (Chambers and Garant 2010) and genotyped samples at nine microsatellite loci. Mother–offspring pairs were established by field observations and confirmed by genetic analyses since about 3% of offspring are adopted (King et al. 2015a). We assigned paternities using the likelihood-based approach of Cervus v 3.0 (Kalinowski et al. 2007). Only individuals with at least eight loci genotyped were retained, and only paternities assigned with 95% confidence were used for analyses. We assigned sires to 88% of 573 young.
Behavioral observations
Each year, kangaroos were observed with binoculars from 15 to 50 m while walking around the study area approximately 3 h after dawn and before dusk between early August and late March. Ten male-male contests were also recorded in April through July in 2010 and 2011. Contests were recorded ad libitum (Altmann 1974). Because kangaroos were marked with unique combinations of tags and collars, it was not possible to record data blind. Observers recorded time, global positioning system location, male identities, behaviors performed by each male, and the outcome of contests. Contests included displays, such as staring, stiff-legged walking, grass-pulling, grass-rubbing, high-standing (Kaufmann 1975; Coulson 1997), displacements (“supplanting” in Gansloßer 1989), play-fights, and fights (Gansloßer 1989). The dominance outcome was clear for about 96% of male-male contests, because one opponent performed at least one subordinate behavior: coughing, grooming, or avoiding (Coulson 1997). Besides play-fights, we never observed subordinate behavior by both individual of a dyad during a single contest, and reversal of dominance-subordination relationships within a dyad within the same year was rare (< 2%). Consequently, observing one subordinate behavior was enough to ascertain the dominance-subordination relationship within a dyad for all contests other than play-fights. Small males often engaged in play-fighting, and sometimes individuals performed both dominant and submissive behaviors; the outcome of these play-fights was regarded as a tie. Play-fights involved pawing, cuffing, and kicking at low intensity, often interspersed with bouts of grooming and grazing by both males (“ritualized fights” in Gansloßer 1989). Fights involved wrestling, pushing, and kicking that can lead to serious injury (“non-ritualized fights” in Gansloßer 1989) and lasted until one of the contestants showed signs of submission: retreating (avoiding), coughing, or both, likely followed by grooming. Compared to play-fights, fights are much more violent and are not interspersed by grooming and grazing interludes. We did not record any reversal of dominance among the observed fights. Ties and unresolved male-male unescalated contests were excluded from all analyses.
Dominance ratings and associated properties
Due to small samples of observed interactions in some years, we could not estimate dominance ratings in 2012, 2013, and 2014 (N < 100 male-male contests/year). We estimated yearly dominance ratings for marked and a few unmarked (N = 13 male-years, or 6%) males, which were recognizable from morphological features such as spots on pelage, unusual coat color, and ripped ears, using the randomized Elo-rating algorithm (Sánchez-Tójar et al. 2018). Because of the opportunistic nature of observations, 13.3% of males (or 40 males) were observed interacting only once/year. Both the number of male-male contests and the steepness of the hierarchy (the probability that a dominant male would defeat a subordinate based on their rating difference) affect the performance of algorithms used to estimate Elo-ratings (Sánchez-Tójar et al. 2018). To examine how the hierarchy was influenced by individuals that were seldom observed interacting, we compared Elo-ratings after removing males that interacted fewer than 2, 3, 4, and 5 times/year (details in Supplementary Materials – SM – S.1.1.). We also calculated multiple indices to assess the reliability of the estimated ratings and the associated uncertainty (see SM S.1.2.). We estimated linearity for yearly interaction datasets using the triangle transitivity index (Shizuka and McDonald 2012). We used Pearson correlations to assess the stability of standardized individual Elo-ratings between consecutive years. All estimations were computed in R 4.1.4 (R Core Team 2021). Triangle transitivity was estimated with EloRating 0.46.11 (Neumann and Kulik 2020); yearly randomized Elo-ratings and uncertainty indices were estimated with aniDom 0.1.4 (Farine and Sánchez-Tójar 2019).
Home-range size and overlap
Individual locations (N = 11,426 locations for 183 male-years) between 10 July and 31 January were used to estimate yearly home ranges (median = 63 locations/male/year, range = 15–128). We estimated both 95% and 50% home ranges (N = 189) and their utilization distributions (UDs; Van Winkle 1975) using fixed-kernel density estimators (KDEs; Worton 1989). UD uses a density function to estimate how areas inside the home range are used. We estimated UDs using grid = 500 and extent parameter = 0.250. The individual smoothing parameters (h) were determined by least-squared cross-validation, and ad hoc multipliers were constrained between 0.5 and 1.5. KDE assumes that location data are independent. If this assumption is violated, KDE underestimates home-range size (Noonan et al. 2019). To avoid biases in individual UD and home-range size caused by temporal autocorrelation, we retained only one randomly selected location per individual per observation session (morning or afternoon). We restricted spatial analyses to individuals with at least 15 locations/year (Montana et al. 2020). We used the utilization distribution overlap index (UDOI; Fieberg and Kochanny 2005) to estimate overlap between either 95 or 50% UDs (total home range and core area, respectively) of all adult male dyads (yearly average = 646 dyads; range = 406–903; N global = 5,812). We estimated home ranges, UDs, and overlap using the adehabitatHR 0.4.19 package (Calenge 2006) in R (R Core Team 2021).
Statistical analyses
All statistical analyses were performed in R (R Core Team 2021). All (generalized) linear mixed models ((G)LMMs) were fitted using the glmmTMB function of the package glmmTMB 1.1.3 (Brooks et al. 2017). Model fit was quantified via type-II Wald χ2 tests using the car 3.0.12 package (Fox and Weisberg 2019). Model assumptions were verified using the DHARMa 0.4.5 package (Hartig 2022).
Determinants of dominance
Males weighing less than 30 kg and those not measured in a given year were excluded from analyses. We used 30 kg as the lower mass threshold because active spermatogenesis in M. giganteus occurs by 48 months (Poole and Catling 1974), and mean mass of known-aged 4-year-old males was about 32 kg. The lightest male assigned a paternity weighed 31 kg (Montana 2021). We fitted LMMs to investigate the influence of body mass, leg length, and arm length on male yearly Elo-ratings. Because mean and dispersion in Elo-ratings varied among years (mean range = − 23.19–26.88, SD range = 227.58–329.13), we standardized Elo-ratings within each year. Using yearly standardized Elo-ratings as the response variable, we fit LMMs with Gaussian error structure. Initially, we included male identity, year, and yearly number of contests recorded as random effects to control for repeated measures of males, shared environmental conditions, and possible biases caused by different numbers of contests recorded. The number of contests recorded was not included in final models because its variance was very low. First, to correctly estimate parameter coefficients, standard errors (SEs) and P-values for body mass, leg length, and arm length, we fitted separate LMMs because these traits were strongly correlated (Spearman r mass-leg = 0.843, P < 0.001; r mass-arm = 0.905, P < 0.001; Pearson rarm-leg = 0.913 [95% CI: 0.887, 0.934], P < 0.001) and produced moderate variance inflation factors (VIFs; average VIF ≃ 7) when included in the same model. To test linearity of the relationship between a trait and Elo-ratings, the linear and quadratic terms of the trait were included in the model. In models of the effect of leg and arm length, we included the quadratic effect of body condition, as well as interactions between either the linear or quadratic body size trait and linear or quadratic body condition (Table 1). We then fitted a model including all three size measures, despite their multicollinearity (Morrissey and Ruxton 2018), to understand how much variance was left unexplained and could thus be attributable to other traits. Coefficients of determination for each model were estimated using pseudo-R2 (henceforth, R2; Nakagawa et al. 2017) within the performance 0.8.0 package (Lüdecke et al. 2021). All morphological traits were standardized (mean = 0, SD = 1).
Outcome of unescalated contests and fights
To evaluate individual probability of winning unescalated contests and fights, each dyadic contest was treated as the primary datum. One male per contest was randomly selected as the focal male, and the other was considered the opponent (Wilson et al. 2011b; Walker and Holwell 2018; Couchoux et al. 2021). Consequently, the outcome was binary (0 = lose, 1 = win), trait asymmetry was relative to the focal male (positive if the focal male was larger or heavier), and pseudoreplication was avoided. We fitted GLMMs (logit link and binomial error structure) to estimate the probability of winning for each focal male. We also included both males’ identities and year as random effects. Asymmetry in mass, leg, and arm length between contestants were again modeled separately. All variables were standardized. Fitting separate models for unescalated contests vs fights allowed us to compare the slope of the effects of each trait asymmetry between unescalated contests and fights using Z-tests and their associated P-values. Z-tests were also used to compare if any one trait asymmetry best predicted the outcome of unescalated contests or fights. We estimated the coefficient of determination for models evaluating the males’ winning probability in unescalated contests (but not for fights, where the variance of random effects was low) using R2 (Nakagawa et al. 2017).
Are male-male contests size-assortative?
To assess whether male-male contests were size-assortative, we generated random unescalated contests and fights by resampling dyads from the list of males seen interacting at least 5 times in a year. The number of random contests/year was equal to the number observed each year. We simulated 50 yearly datasets each for fights (N = 51 males/dataset) and unescalated contests (N = 2,059 males/dataset). To estimate trait asymmetries for all four datasets (observed and simulated fights, observed and simulated unescalated contests), we calculated the absolute (unsigned) trait asymmetry between contestants. We then compared the variance of asymmetries in mass, leg length, and arm length between dyads using Levene’s test (Hosken et al. 2018). We compared variances of (i) simulated and observed unescalated contests, (ii) simulated and observed fights, and (iii) observed unescalated contests and observed fights. To test whether larger males fought more often than small ones, we compared average absolute body mass, leg length, and arm length of focal males involved in unescalated contests vs the average traits of focal males that fought using Mann–Whitney U tests.
Spatial avoidance
To test whether males of similar ratings avoided each other, we first determined if ratings and home-range size were correlated using the Pearson correlation coefficient (r). If dominant males had larger home ranges, they would have higher odds of meeting than subordinate males and thus would more often encounter other dominant than subordinate males. We then explored if males shared space by fitting two gamma (log link) GLMMs with rescaled versions of 95% and 50% UDOIs as response variables. When overlap is nearly non-existent, UDOI can be approximated to 0. To fit gamma GLMMs, we thus transformed the pairwise overlap of males with UDOI = 0 to very small values (1e-10). We included year and male identity of both males as distinct random effects. The fixed structure of each model included yearly standardized Elo-ratings and 95% or 50% home-range sizes of both males. We used natural cubic splines with 3 degrees of freedom to control for the likely non-linear influence of home-range size on spatial overlap. Elo-ratings as well as home-range sizes of the two males were included as interactions in the model to test whether (i) high-ranking males avoided each other, (ii) low-ranking males avoided high-ranking males, and (iii) there was no rank-related spatial structure. Because model assumptions were violated, we repeated the analyses using two additional link and error structures to verify whether results were consistent (see SM S.1.4. for additional details).
Dominance and reproductive success
We measured reproductive success as the number of offspring genetically assigned to each male each year (range 0–13). We first assessed how dominance was related to male reproductive success by estimating a standardized selection differential (Lande and Arnold 1983). Randomized Elo-ratings (see 9 above) were standardized yearly (mean = 0, SD = 1). The most dominant males had high ratings and positive yearly Z-values. Relative yearly reproductive success was estimated by dividing yearly reproductive success (min = 0, max range = 4–13 offspring/year) by mean reproductive success for that year (mean range = 1.1–2.9 offspring/year). To assess statistical significance of the linear selection differential, we fitted a GLMM with negative binomial error structure and log link. Yearly absolute male reproductive success was the response variable. We included male identity and year as random effects.
Results
Dominance hierarchy and determinants of rank
Yearly dominance hierarchies were linear, as triads were strongly transitive (mean transitivity = 0.987, range = 0.978–1.000, P < 0.001 for all years). Elo-ratings were estimated for 211 male-years (116 males) observed interacting at least five times in any 1 year (N males: 2010 = 44; 2011 = 40; 2015 = 27; 2016 = 41; 2017 = 32; 2018 = 27). The correlation between Elo-ratings of the same individual in consecutive years was high (Pearson r = 0.827 [0.747, 0.883], P < 0.001), indicating that ratings were relatively stable across years. Elo-rating repeatability, together with the orderliness showed by triangle transitivity and the correlation of ratings in consecutive years, suggested that dominance hierarchies were steep and stable within and between years, and uncertainty was acceptably low (SM S1.2, Table S1).
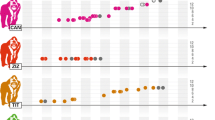
Body mass, leg length, and arm length correlated positively with Elo-ratings (Table 1, Fig. 1). The relationship with Elo-ratings (standardized yearly) was linear for body mass but quadratic for leg length, arm length, and body condition (Table 1, Fig. 1). The models highlighted an interactive effect of body condition and leg length, whereas the effect was additive when body condition was modeled with arm length. These results indicated that males with longer legs benefitted more than those with shorter ones from body condition, whereas males of all arm lengths benefitted similarly from good condition (Table 1, Fig. 1B, C). No trait was a superior predictor of Elo-ratings (Zmass-leg = 0.949, P = 0.342; Zmass-arm = 1.021, P = 0.307; Zleg-arm = 0.038, P = 0.969), and the variance explained by each model was similar (marginal R2 range = 0.729–0.734; conditional R2 range = 0.858–0.892). The model including all three physical traits had a marginal R2 = 0.753 and conditional R2 = 0.862.
Effects of body mass (A), leg length (B), and arm length (C) on yearly standardized randomized Elo-ratings of 198 eastern grey kangaroo male-years at Wilsons Promontory National Park (Australia), 2010–2011 and 2015–2018. Body mass was used as the sole predictor of estimated Elo-ratings in A. Models for leg (B) and arm (C) length included an interaction with body condition, as well as its additive effect. Body condition was a continuous variable, but for illustration purposes, males in poor condition (Kn < 0; N = 110) are represented by empty triangles and broken lines and males in good condition (Kn > 0; N = 88) by dots and continuous lines. Lines and associated shaded areas are model predictions ± 95% CIs
Outcomes of unescalated contests and fights
Of 2,110 male-male contests observed in 2010–2018, only 51 (2.4%) escalated into fights, one of which was fatal (Toni 2018). Males that were heavier, larger, or had longer arms than their opponents were more likely to win unescalated contests (Table 2, Fig. 2A–C). According to Z-tests, there was no difference between the effects of mass and arm asymmetry, nor between leg and arm asymmetry, on probability of winning unescalated contests (Zmass-arm = 1.109, P = 0.268; Zarm-leg = 1.085, P = 0.277). Mass asymmetry, however, had a stronger effect than leg asymmetry on the probability of winning unescalated contests (Zmass-leg = 2.110, P = 0.035). Asymmetry in both body mass and arm length explained more variance (marginal R2: mass asymmetry = 0.753, arm length asymmetry = 0.713; conditional R2: mass asymmetry = 0.933, arm length asymmetry = 0.921) than asymmetry in leg length (marginal R2 = 0.523, conditional R2 = 0.928) for unescalated contests. Similar trends were found for the probability of winning fights: mass, leg, and arm asymmetries all increased winning probability (Table 2, Fig. 2D–F). We found no difference when comparing the effects of the three traits on fight outcome using Z-tests (Zmass-leg = 1.112, P = 0.266; Zmass-arm = 0.517, P = 0.605; Zarm-leg = 0.542, P = 0.588). Asymmetry in body mass, leg length, and arm length had stronger effects on outcome of unescalated contests than of fights (Zmass = 5.151, Zleg = 5.778, Zarm = 5.135, P < 0.001). For example, a positive difference of 3 kg increased a male’s probability to win an unescalated contest by 18% compared to his probability of winning a fight.
Probability of an eastern grey kangaroo male winning unescalated contests (upper row, N = 2,059; displays, displacements, and play-fights) or fights (lower row, N = 51) depending on asymmetry in body mass (A, D), leg length (B, E), and arm length (C, F) compared to its opponent at Wilsons Promontory National Park (Australia), 2010–2018. Solid lines and associated grey-shaded areas represent logistic regressions ± 95% CIs from binomial models. Dots are raw data points for probability of winning
Are male-male contests size-assortative?
Levene’s tests revealed that for all body measures, the variance in trait asymmetries differed between males that fought (N = 51), those that were involved in unescalated contests (N = 2,059), and random samples (Nrandom fights = 2,550; Nrandom unescalated = 102,950) (Fig. 3). Males of similar size were involved in contests more often than males randomly chosen from the population (two-way Levene’s tests; unescalated contests: Fmass asymmetry = 100.52; Fleg asymmetry = 263.75; Farm asymmetry = 205.03, P < 0.001 for all asymmetries; escalated contests: Fmass asymmetry = 20.71; Fleg asymmetry = 8.08; Farm asymmetry = 12.48, P < 0.005 for all asymmetries). Fights appeared more size-assortative than unescalated contests for mass and arm length (Fmass asymmetry = 10.94, P < 0.001; Farm asymmetry = 9.75, P = 0.001), but the variance in leg length asymmetry in unescalated contests compared to fights was similar (Fleg asymmetry = 2.82, P = 0.093) (Fig. 3B). Average absolute mass and limb length did not differ between males that fought and those involved in unescalated contests (Mann–Whitney U tests: medianmass unescalated = 52.3 kg, medianmass fight = 49.8 kg, Umass = 58,320, P = 0.176; medianleg unescalated = 633 mm, medianleg fight = 636 mm, Uleg length = 50,528, P = 0.646, medianarm unescalated = 324 mm, medianarm fight = 323 mm, Uarm length = 57,628, P = 0.233).
Absolute body-mass and limb-length asymmetries of eastern grey kangaroo male pairs that were involved in fights and unescalated contests at Wilsons Promontory National Park (Australia) in 2010–2018, compared to randomly selected male pairs. Boxplots show medians, first, and third quartiles (center, lower, and upper borders, respectively). Whiskers represent the values within 1.5 times the interquartile range below and above the 25th and 75th percent percentile, respectively. Median, first, and third quartiles and whiskers of simulated fights and unescalated contests are the mean values obtained from the 50 resampling datasets
Spatial avoidance
The mean 95% home-range size of males (0.386 km2) comprised more than a third of the study area, generating overlap between most male dyads (mean 95% UDOI = 0.353; range of 95% UDOI = 0.000–1.298) and thus multiple opportunities for male-male contests. There was weak evidence that yearly standardized dominance ratings were positively associated with home-range size (Pearson r = 0.125 [0.004, 1.000], df = 187, P = 0.044), but not with core-area size (50% kernel; r = 0.086 [-0.034, 1.000], df = 187, P = 0.119). We found no evidence of rating-based spatial avoidance or attraction at 95% or 50% UDOIs (Table S3).
Dominance and reproductive success
Among 203 male-years with estimated dominance ratings, 61% sired at least 1 offspring. On average, males sired 1.4 ± 0.1 (mean ± SE) young/year. Overall, males with the top 10% ratings sired 72 out of 291 sampled young (or 25.4%), accounting for 18.9–36.4% offspring each year (Fig. 4B). The standardized linear selection differential (i) for yearly randomized Elo-ratings on yearly relative reproductive success was 0.582 (SE = 0.081; Fig. 4A). Paternities were broadly distributed among males of all ranks (Fig. 4B).
Relationship between estimated dominance ratings and reproductive success for 203 eastern grey kangaroo male-years at Wilsons Promontory National Park (Australia) in 2010–2011 and 2015–2018. Randomized Elo-ratings were standardized within each year. A) Standardized linear selection differential for yearly standardized randomized Elo-ratings on yearly relative reproductive success. The line represents the strength of selection (i = 0.582). B) Relationship between estimated randomized ratings and yearly reproductive success. The line and associated shaded areas show predicted reproductive success ± 95% CI. Dots are raw data points. Note that the scales on the Y-axes for panels A and B are different
Discussion
We aimed to investigate which traits were associated with male dominance status, to understand the drivers of male-male contests, and to determine whether high dominance rank was sexually selected in eastern grey kangaroos. We confirmed that males formed yearly linear social hierarchies, based on size (Grant 1973; Miller et al. 2010). Asymmetry in morphological traits between contestants determined the outcome of contests; asymmetry for all three traits better predicted the outcome of unescalated contests than fights. Males engaged in both types of contests mostly with individuals of similar size. Unlike a study of semi-captive kangaroos (Miller et al. 2010), our study did not show that alpha males monopolize access to females. Although dominance status experienced strong sexual selection, reproductive skew was low (this study; Rioux-Paquette et al. 2015), with many males of high ratings siring no offspring, while several males of low ratings sired some young. The high proportion of low-ranking males able to sire offspring suggests that dominance was not the sole predictor of access to females.
As expected, male kangaroos formed steep, linear, and stable dominance hierarchies each year. Individuals engaged in unescalated contests and occasional fights to establish and maintain dominant status. Heavy males with long legs and arms ranked at the top of yearly hierarchies. Body size and mass commonly affect social status in sexually dimorphic species (Haley et al. 1994; Pelletier and Festa-Bianchet 2006; Bergeron et al. 2010). Dominance status increased linearly with mass, and since M. giganteus grow through much of their life, male dominance status should tend to increase with age. However, we did not know the exact age of marked males because most were first captured as adults. Body condition also increased dominance status and increased the positive effect of limb length on ratings. Interestingly, large males gained relatively more from better condition than small males, albeit the difference was weak, whereas we observed no difference in the effect of condition at different arm lengths. Perhaps large males are better than small males at exploiting their body condition when competing, as the former are likely older and more experienced (Briffa and Lane 2017). Physical traits clearly had a strong effect on dominance status in M. giganteus, but other unmeasured phenotypic traits, such as aggressiveness, endurance, or experience (Briffa and Lane 2017), could also influence a male’s yearly rating.
Given that body mass and limb length predicted male rank, it was not surprising that asymmetry in body size between contestants strongly influenced the outcome of male-male contests. Large size is associated with physical strength and often ensures access to resources (Wilson et al. 2011b; Couchoux et al. 2021). For unescalated contests, the winning probability was between 85 and 90% when the focal male was 10% heavier or larger (3.7 kg or 13.5 mm), or had 10% (12 mm) longer arms than its opponent, based on maximum trait asymmetry. Estimated coefficients of determination indicated that other phenotypic traits could be part of males’ RHP. None of the three physical traits appeared to be a superior predictor of the outcome of fights. Interestingly, the relationship between winning probability and asymmetry in body mass, leg length, and arm length between contestants was not as steep as for unescalated contests. Since fighting males wrestle and use their arms and claws to attack each other’s heads (Gansloßer 1989; Croft and Snaith 1991; Coulson 1997), we expected that arm length would be a stronger predictor of victory in fights than unescalated contests. Because fights require physical strength and fighting males appear to try to keep their opponent at a distance, heavier males and males with longer arms should theoretically be advantaged. As in most animals that rely on body size during male-male contests, body size is highlighted in kangaroo displays, such as standing tall on the tip of their tail or stiff-legged walking on the toes with the shoulders held high (Kaufmann 1975; Gansloßer 1989; Croft and Snaith 1991; Coulson 1997). Asymmetry in body size can be a cue for assessing potential opponents (Maynard Smith and Price 1973; Maynard Smith 1974), and the smaller male often signals subordinance to avoid dangerous fights (Maynard Smith and Price 1973; Maynard Smith 1974). In our study system, it appeared that the smaller male of a dyad conceded less easily during fights than during unescalated contests, possibly because success in fights may depend more on aggressiveness (Briffa et al. 2015) or previous fighting experience (Briffa and Lane 2017).
Fights accounted for only 2.5% of all contests, suggesting that males are under strong selection to avoid the potential costs of fights (Maynard Smith and Price 1973; Maynard Smith 1974). Fights occurred among males that were more similar in size than males involved in unescalated contests, in accordance with Parker’s (1974) prediction that fights should involve only closely matched combatants to reduce risks of injury. Similar results have been reported in ungulates such as Alpine ibex (Bergeron et al. 2010), fallow deer Dama dama (Jennings et al. 2006), and red deer Cervus elaphus (Clutton-Brock et al. 1979). Fights between kangaroos with mismatched size can have lethal consequences (Miller et al. 2010; Toni 2018). Closely matched males of all sizes fought: we found no difference in average size between males involved in fights or unescalated contests. Overall, these results suggested that we did not observe escalated contests between males of very different sizes due to mutual assessment strategies from a distance. Males thus avoided contests with opponents much larger than themselves.
We found no evidence of differential space use by males according to social status, supporting Kaufmann’s (1975) observations of high tolerance among kangaroos of all ages and sexes. This finding is meaningful since spatial structure can influence association patterns (Strickland et al. 2017), with downstream effects on social competition and sexual selection (McDonald et al. 2013). Therefore, our conclusion that males interacted with opponents similar in body size, and thus ratings, was not biased by variability of spatial overlap between males with high and low ratings. We cannot exclude the possibility, however, that males temporally avoided other males (Minta 1992).
Earlier observational studies of kangaroos suggested that most females mate with the highest-ranking male (Grant 1973; Russell 1984; Jarman and Southwell 1986). Instead, our results suggested that most dominant males enjoy a substantial advantage in reproduction: the selection differential for Elo-ratings of 0.582 ± 0.081 was almost three times higher than the average (0.198) estimated by studies on selection over traits enhancing reproductive success (Kingsolver et al. 2012). Yet, high-rating males were unable to monopolize reproductions (Rioux-Paquette et al. 2015), which is surprising given that the ability to monopolize mates is often considered a fundamental determinant of the strength of sexual selection (Emlen and Oring 1977; Clutton-Brock 1989; Klug et al. 2010). Our results, however, align with those of multiple recent studies which have used genetic analyses to assess paternities (see references in 1ntroduction) and emphasized the benefits of body size and weaponry on reproductive success of males (Clutton-Brock 2017). The advantage over reproduction for dominant males in strongly sexually dimorphic species like kangaroos was expected. Notable examples of strongly sexually dimorphic species that show strong reproductive advantages for dominant males are fallow deer and southern elephant seals (Hoelzel et al. 1999; McElligott et al. 2001). Like our results for kangaroos, dominance hierarchies in fallow deer and elephant seals are steep and linear, and body size is a strong predictor of social status. Compared to fallow deer and elephant seals, however, highly dominant kangaroo males obtained a lower proportion of matings/paternities. While alpha males monopolize more than 50% of matings in some years in fallow deer and elephant seals (Apollonio et al. 1989; Hoelzel et al. 1999), the most dominant eastern grey kangaroo males obtained fewer than 10% of paternities each year. Paternities were spread among males of differing rating, resulting in low reproductive skew, and > 60% of males, including one weighing only 31 kg, sired at least one offspring. About 15% of offspring were sired by males in the bottom quartile of mass (Montana 2021), which mostly achieved low ratings. This result is surprising considering the strong dominance hierarchy and sexual selection on dominance ratings we report here. Based on their competitive advantage, dominant males should be able to monopolize matings, since the long breeding season occurs in an open environments with asynchronous estruses (Russell 1984) and the most dominant males appear to effectively defend estrous females from other males (Jarman and Southwell 1986). Instead, our results match those of several recent studies of various taxa that found modest yearly reproductive skew despite males forming clear dominance hierarchies (Hynes et al. 2005; Lidgard et al. 2005; McCullough and Simmons 2016).
Our long-term analyses, based on a substantial sample size, revealed strong estimated selection on dominance status despite low monopolization of reproductions by highly dominant males. Female choice could partly determine which males gain paternities: females could find dominance status attractive (Qvarnström and Forsgren 1998), but other traits could also play a role in mate selection. Possibly, male-male competition and female choice select toward different optima (Qvarnström and Forsgren 1998). Additionally, a large number of competitors likely reduce the ability of high-rating males to monopolize paternities (Montana et al. 2020), suggesting that the level of male-male competition should be accounted for, particularly for polygynandrous species.
Conclusions
For more than half a century, dominance has been viewed as a major determinant of individual fitness because high rank should confer priority of access to limiting resources (Altmann 1962). Success in male-male contests should thus be of paramount importance for males in species where direct competition defines dominance status (Dewsbury 1982). The study of social behavior and its association to individual fitness has benefitted from major technical advances over the past five decades in the field, laboratory, and statistical analyses (Martin and Bateson 2007; Neumann et al. 2011; Sánchez-Tójar et al. 2018), and our understanding of the determinants of reproductive success of both sexes has improved through an increasing number of long-term studies of wild species (Ellis 1995; Pelletier and Festa-Bianchet 2006; Wyman et al. 2021). Extensive research on traits signaling dominance status and fighting skills reveal that body and weapon size are important determinants of a male’s RHP (Haley et al. 1994; Bergeron et al. 2010; Wright et al. 2019), but do not fully explain it. Other phenotypic traits such as aggressiveness and experience (Briffa and Lane 2017), often depending on social environment (Spong et al. 2008), can also affect RHP. A promising research avenue is the cost of phenotypic traits that contribute to RHP. If dominance rating and its determinants are honest signals, they should be costly to produce and maintain (Zahavi 1975). We still know little about the costs of high dominance rank and whether or not males adopt different strategies, likely condition-dependent, by spending more or less time pursuing higher dominance status. Another key issue is the possibility that not all unobserved dyadic interactions are weaknesses of a dataset, due to sparse sampling. Instead, it seems likely that dyads of mismatched opponents are not often observed interacting because the weaker individual tends to avoid these confrontations (Shizuka and McDonald 2012). Finally, new theory may be required to explain the co-occurrence of strong sexual selection and low monopolization of reproduction by high-rating males. The idea that the ability of males to monopolize mates determines the strength of sexual selection (Emlen and Oring 1977) is deeply rooted in sexual selection research. More studies quantitatively comparing the strength of sexual selection to indices of monopolization are required.
Supplementary information
Data availability
Data are attached as Supplementary Information Files.
Code availability
R functions are attached as Supplementary Information Files.
References
Albers PCH, de Vries H (2001) Elo-rating as a tool in the sequential estimation of dominance strengths. Anim Behav 61:489–495. https://doi.org/10.1006/anbe.2000.1571
Alberts SC, Buchan JC, Altmann J (2006) Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav 72:1177–1196. https://doi.org/10.1016/j.anbehav.2006.05.001
Altmann SA (1962) A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann NY Acad Sci 102:338–435. https://doi.org/10.1111/j.1749-6632.1962.tb13650.x
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267. https://doi.org/10.1163/156853974X00534
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Apollonio M, Festa-Bianchet M, Mari F (1989) Correlates of copulatory success in a fallow deer lek. Behav Ecol Sociobiol 25:89–97. https://doi.org/10.1007/BF00302925
Bergeron P, Grignolio S, Apollonio M, Shipley B, Festa-Bianchet M (2010) Secondary sexual characters signal fighting ability and determine social rank in Alpine ibex (Capra ibex). Behav Ecol Sociobiol 64:1299–1307. https://doi.org/10.1007/s00265-010-0944-x
Briffa M, Lane SM (2017) The role of skill in animal contests: a neglected component of fighting ability. Proc R Soc B 284:20171596. https://doi.org/10.1098/rspb.2017.1596
Briffa M, Sneddon LU, Wilson AJ (2015) Animal personality as a cause and consequence of contest behaviour. Biol Lett 11:20141007. https://doi.org/10.1098/rsbl.2014.1007
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Modell 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Chambers JL, Garant D (2010) Determinants of population genetic structure in eastern chipmunks (Tamias striatus): the role of landscape barriers and sex-biased dispersal. J Hered 101:413–422. https://doi.org/10.1093/jhered/esq029
Clutton-Brock TH (1989) Mammalian mating systems. Proc R Soc Lond B 236:339–372. https://doi.org/10.1098/rspb.1989.0027
Clutton-Brock TH (2017) Reproductive competition and sexual selection. Phil Trans R Soc B 372:20160310. https://doi.org/10.1098/rstb.2016.0310
Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE (1979) The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim Behav 27:211–225. https://doi.org/10.1016/0003-3472(79)90141-6
Couchoux C, Garant D, Aubert M, Clermont J, Réale D (2021) Behavioral variation in natural contests: integrating plasticity and personality. Behav Ecol 32:277–285. https://doi.org/10.1093/beheco/araa127
Coulson G (1997) Repertoires of social behaviour in captive and free-ranging grey kangaroos, Macropus giganteus and Macropus fuliginosus (Marsupialia: Macropodidae). J Zool 242:119–130. https://doi.org/10.1111/j.1469-7998.1997.tb02933.x
Coulson G, Cripps J, Wilson M (2014) Hopping down the main street: eastern grey kangaroos at home in an urban matrix. Animals 4:272–291. https://doi.org/10.3390/ani4020272
Crespi BJ (1988) Risks and benefits of lethal male fighting in the colonial, polygynous thrips Hoplothrips karnyi (Insecta: Thysanoptera). Behav Ecol Sociobiol 22:293–301. https://doi.org/10.1007/BF00299845
Croft DB, Snaith F (1991) Boxing in red kangaroos, Macropus rufus: aggression or play? Int J Comp Psychol 4:221–236
David HA (1987) Ranking from unbalanced paired-comparison data. Biometrika 74:432–436. https://doi.org/10.1093/biomet/74.2.432
de Vries H (1998) Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Anim Behav 55:827–843. https://doi.org/10.1006/anbe.1997.0708
Dewsbury DA (1982) Dominance rank, copulatory behavior, and differential reproduction. Q Rev Biol 57:135–159. https://doi.org/10.1086/412672
Douglas PH, Ngonga Ngomo A-C, Hohmann G (2017) A novel approach for dominance assessment in gregarious species: ADAGIO. Anim Behav 123:21–32. https://doi.org/10.1016/j.anbehav.2016.10.014
Drews C (1993) The concept and definition of dominance in animal behaviour. Behaviour 125:283–313
Ellis L (1995) Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol 16:257–333. https://doi.org/10.1016/0162-3095(95)00050-U
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223. https://doi.org/10.1126/science.327542
Farine DR, Sánchez-Tójar A (2019) aniDom: inferring dominance hierarchies and estimating uncertainty. R package version 0.1.4, https://CRAN.R-project.org/package=aniDom
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildlife Manage 69:1346–1359. https://doi.org/10.2193/0022-541X(2005)69[1346:QHOTIO]2.0.CO;2
Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE (2016) Chimpanzee females queue but males compete for social status. Sci Rep 6:35404. https://doi.org/10.1038/srep35404
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks, CA
Gansloßer U (1989) Agonistic behaviour in macropodids - a review. In: Grigg G, Jarman PJ, Hume I (eds) Kangaroos, Wallabies and Rat-Kangaroos, vol 2. Surrey Beatty & Sons. Chipping Norton, NSW, Australia, pp 475–503
Grant TR (1973) Dominance and association among members of a captive and a free-ranging group of grey kangaroos (Macropus giganteus). Anim Behav 21:449–456. https://doi.org/10.1016/S0003-3472(73)80004-1
Haley MP, Deutsch CJ, Le Boeuf BJ (1994) Size, dominance and copulatory success in northern elephant seals. Anim Behav 48:1249–1260. https://doi.org/10.1006/anbe.1994.1361
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.5, https://CRAN.R-project.org/package=DHARMa
Hoelzel AR, Le Boeuf BJ, Reiter J, Campagna C (1999) Alpha-male paternity in elephant seals. Behav Ecol Sociobiol 46:298–306. https://doi.org/10.1007/s002650050623
Hosken DJ, Buss DM, Hodgson DJ (2018) Beware the F test (or, how to compare variances). Anim Behav 136:119–126. https://doi.org/10.1016/j.anbehav.2017.12.014
Hynes EF, Rudd CD, Temple-Smith PD, Sofronidis G, Paris D, Shaw G, Renfree MB (2005) Mating sequence, dominance and paternity success in captive male tammar wallabies. Reproduction 130:123–130. https://doi.org/10.1530/rep.1.00624
Jarman PJ (1983) Mating system and sexual dimorphism in large, terrestrial, mammalian herbivores. Biol Rev 58:485–520
Jarman PJ (1989) Sexual dimorphism in Macropodoidea. In: Macropods: The Biology of Kangaroos, Wallabies, and Rat-kangaroos, vol. 2. Surrey Beatty & Sons, Chipping Norton, NSW, Australia, pp 433–447
Jarman PJ, Coulson G (1989) Dynamics and adaptiveness of grouping in macropods. In: Grigg G, Jarman PJ, Hume I (eds) Kangaroos, Wallabies and Rat-kangaroos, vol 2. Surrey Beatty & Sons. Chipping Norton, NSW, Australia, pp 527–547
Jarman PJ, Southwell CJ (1986) Grouping, associations, and reproductive strategies in eastern grey kangaroos. In: Rubenstein DI, Wrangham RW (eds) Ecological aspects of social evolution. Princeton University Press, Princeton, NJ, pp 399–428
Jennings DJ, Gammell MP, Carlin CM, Hayden TJ (2006) Is difference in body weight, antler length, age or dominance rank related to the number of fights between fallow deer (Dama dama)? Ethology 112:258–269. https://doi.org/10.1111/j.1439-0310.2006.01154.x
Jennings DJ, Boys RJ, Gammell MP (2017) Weapon damage is associated with contest dynamics but not mating success in fallow deer (Dama dama). Biol Lett 13:20170565. https://doi.org/10.1098/rsbl.2017.0565
Jennings DJ, Amin B, Gammell MP (2021) Third-party assessment of contestants during fallow deer fights increases with resource abundance and dominance rank. Anim Behav 177:81–89. https://doi.org/10.1016/j.anbehav.2021.04.020
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106. https://doi.org/10.1111/j.1365-294X.2007.03089.x
Kaufmann JH (1975) Field observations of the social behaviour of the eastern grey kangaroo, Macropus giganteus. Anim Behav 23:214–221. https://doi.org/10.1016/0003-3472(75)90066-4
Kaufmann JH (1983) On the definitions and functions of dominance and territoriality. Biol Rev 58:1–20. https://doi.org/10.1111/j.1469-185X.1983.tb00379.x
King WJ, Wilson ME, Allen T, Festa-Bianchet M, Coulson G (2011) A capture technique for free-ranging eastern grey kangaroos (Macropus giganteus) habituated to humans. Aust Mammal 33:47–51. https://doi.org/10.1071/AM10029
King WJ, Forsyth DM, Coulson G, Festa-Bianchet M (2015a) Adoption in eastern grey kangaroos: a consequence of misdirected care? PLoS ONE 10:e0125182. https://doi.org/10.1371/journal.pone.0125182
King WJ, Garant D, Festa-Bianchet M (2015b) Mother-offspring distances reflect sex differences in fine-scale genetic structure of eastern grey kangaroos. Ecol Evol 5:2084–2094. https://doi.org/10.1002/ece3.1498
Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM (2012) Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol Ecol 26:1101–1118. https://doi.org/10.1007/s10682-012-9563-5
Klug H, Heuschele J, Jennions MD, Kokko H (2010) The mismeasurement of sexual selection. J Evol Biol 23:447–462. https://doi.org/10.1111/j.1420-9101.2009.01921.x
Lande R, Arnold SJ (1983) The measurement of selection and correlated characters. Evolution 37:1210–1226
Lidgard DC, Boness DJ, Bowen WD, McMillan JI (2005) State-dependent male mating tactics in the grey seal: the importance of body size. Behav Ecol 16:541–549. https://doi.org/10.1093/beheco/ari023
Lüdecke D, Ben-Shachar M, Patil I, Waggoner P, Makowski D (2021) performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Martin P, Bateson P (2007) Measuring behaviour: an introductory guide, 3rd edn. Cambridge University Press, Cambridge
Maynard Smith J (1974) The theory of games and the evolution of animal conflicts. J Theor Biol 47:209–221. https://doi.org/10.1016/0022-5193(74)90110-6
Maynard Smith J, Price GR (1973) The logic of animal conflict. Nature 246:15–18
McCullough EL, Simmons LW (2016) Selection on male physical performance during male-male competition and female choice. Behav Ecol 27:1288–1295. https://doi.org/10.1093/beheco/arw033
McCullough EL, Miller CW, Emlen DJ (2016) Why sexually selected weapons are not ornaments. Trends Ecol Evol 31:742–751. https://doi.org/10.1016/j.tree.2016.07.004
McDonald DB, Shizuka D (2013) Comparative transitive and temporal orderliness in dominance networks. Behav Ecol 24:511–520. https://doi.org/10.1093/beheco/ars192
McDonald GC, James R, Krause J, Pizzari T (2013) Sexual networks: measuring sexual selection in structured, polyandrous populations. Phil Trans R Soc B 368:20120356–20120356. https://doi.org/10.1098/rstb.2012.0356
McElligott AG, Gammell MP, Harty HC et al (2001) Sexual size dimorphism in fallow deer (Dama dama): do larger, heavier males gain greater mating success? Behav Ecol Sociobiol 49:266–272. https://doi.org/10.1007/s002650000293
Miller EJ, Eldridge MDB, Cooper DW, Herbert CA (2010) Dominance, body size and internal relatedness influence male reproductive success in eastern grey kangaroos (Macropus giganteus). Reprod Fertil Dev 22:539–549. https://doi.org/10.1071/RD09061
Minta SC (1992) Tests of spatial and temporal interaction among animals. Ecol Appl 2:178–188
Montana L (2021) Sexual selection in a sexually dimorphic species: Effect of body size on reproductive success and offspring sex ratio in kangaroos. PhD thesis, Université de Sherbrooke
Montana L, Rousseu F, Garant D, Festa-Bianchet M (2020) Siring success in kangaroos: size matters for those in the right place at the right time. Behav Ecol 31:750–760. https://doi.org/10.1093/beheco/araa020
Morrissey MB, Ruxton GD (2018) Multiple regression is not multiple regressions: the meaning of multiple regression and the non-problem of collinearity. Philos Theor Pract Biol 10:3. https://doi.org/10.3998/ptpbio.16039257.0010.003
Murray CM, Mane SV, Pusey AE (2007) Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Anim Behav 74:1795–1804. https://doi.org/10.1016/j.anbehav.2007.03.024
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:20170213. https://doi.org/10.1098/rsif.2017.0213
Neumann C, Kulik L (2020) EloRating: animal dominance hierarchies by Elo rating. R package version 0.46.11, https://CRAN.R-project.org/package=EloRating
Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A (2011) Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim Behav 82:911–921. https://doi.org/10.1016/j.anbehav.2011.07.016
Noonan MJ, Tucker MA, Fleming CH et al (2019) A comprehensive analysis of autocorrelation and bias in home range estimation. Ecol Monogr 89:e01344. https://doi.org/10.1002/ecm.1344
Paplinska J, Bencini R, Fisher DO et al (2010) A review of evidence concerning sperm competition in the superfamily Macropodoidea. In: Coulson G, Eldridge MDB (eds) Macropods: The Biology of Kangaroos, Wallabies, and Rat-kangaroos. CSIRO Publishing, Collingwood, Victoria, pp 65–76
Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243. https://doi.org/10.1016/0022-5193(74)90111-8
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332. https://doi.org/10.1111/j.1365-2435.2010.01751.x
Pelletier F, Festa-Bianchet M (2006) Sexual selection and social rank in bighorn rams. Anim Behav 71:649–655. https://doi.org/10.1016/j.anbehav.2005.07.008
Piza-Roca C, Strickland K, Schoeman D, Frere CH (2018) Eastern water dragons modify their social tactics with respect to the location within their home range. Anim Behav 144:27–36. https://doi.org/10.1016/j.anbehav.2018.08.001
Plaisir C-A, King WJ, Forsyth DM, Festa-Bianchet M (2022) Effects of rainfall forage biomass and population density on survival and growth of juvenile kangaroos. J Mammal. https://doi.org/10.1093/jmammal/gyab132
Poole WE, Catling PC (1974) Reproduction in the two species of grey kangaroos, Macropus giganteus Shaw and M. foliginosus (Desmarest). I. Sexual maturity and oestrus. Aust J Zool 22:277–302
Qvarnström A, Forsgren E (1998) Should females prefer dominant males? Trends Ecol Evol 13:498–501. https://doi.org/10.1016/S0169-5347(98)01513-4
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/
Rico-Guevara A, Hurme KJ (2019) Intrasexually selected weapons. Biol Rev 94:60–101. https://doi.org/10.1111/brv.12436
Rioux-Paquette E, Garant D, Martin AM, Coulson G, Festa-Bianchet M (2015) Paternity in eastern grey kangaroos: moderate skew despite strong sexual dimorphism. Behav Ecol 26:1147–1155. https://doi.org/10.1093/beheco/arv052
Russell EM (1974) The biology of kangaroos (Marsupialia-Macropodidae). Mamm Rev 4:1–59
Russell EM (1984) Social behaviour and social organization of marsupials. Mamm Rev 14:101–154. https://doi.org/10.1111/j.1365-2907.1984.tb00343.x
Sánchez-Tójar A, Schroeder J, Farine DR (2018) A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J Anim Ecol 87:594–608. https://doi.org/10.1111/1365-2656.12776
Schoepf I, Schradin C (2012) Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J Anim Ecol 81:649–656. https://doi.org/10.1111/j.1365-2656.2011.01939.x
Shizuka D, McDonald DB (2012) A social network perspective on measurements of dominance hierarchies. Anim Behav 83:925–934. https://doi.org/10.1016/j.anbehav.2012.01.011
Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH (2008) Factors affecting the reproductive success of dominant male meerkats. Mol Ecol 17:2287–2299. https://doi.org/10.1111/j.1365-294X.2008.03734.x
Strickland K, Levengood A, Foroughirad V, Mann J, Krzyszczyk E, Frère CH (2017) A framework for the identification of long-term social avoidance in longitudinal datasets. R Soc Open Sci 4:170641. https://doi.org/10.1098/rsos.170641
Stuart-Fox DM, Firth D, Moussalli A, Whiting MJ (2006) Multiple signals in chameleon contests: designing and analysing animal contests as a tournament. Anim Behav 71:1263–1271. https://doi.org/10.1016/j.anbehav.2005.07.028
Toni P (2018) Combat leads to intraspecific killing in eastern grey kangaroos. Aust Mammal 40:109–111. https://doi.org/10.1071/AM16057
Van Winkle W (1975) Comparison of several probabilistic home-range models. J Wildlife Manage 39:118–123
Walker LA, Holwell GI (2018) The role of exaggerated male chelicerae in male–male contests in New Zealand sheet-web spiders. Anim Behav 139:29–36. https://doi.org/10.1016/j.anbehav.2018.02.020
West-Eberhard MJ (1979) Sexual selection, social competition, and evolution. Proc Am Philos Soc 123:222–234
Wilson AJ, de Boer M, Arnott G, Grimmer A (2011a) Integrating personality research and animal contest theory: aggressiveness in the green swordtail Xiphophorus helleri. PLoS ONE 6:e28024. https://doi.org/10.1371/journal.pone.0028024
Wilson AJ, Morrissey MB, Adams MJ, Walling CA, Guinness FE, Pemberton JM, Clutton-Brock TH, Kruuk LEB (2011b) Indirect genetics effects and evolutionary constraint: an analysis of social dominance in red deer, Cervus elaphus. J Evol Biol 24:772–783. https://doi.org/10.1111/j.1420-9101.2010.02212.x
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Wright E, Galbany J, McFarlin SC, Ndayishimiye E, Stoinski TS, Robbins MM (2019) Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim Behav 151:87–102. https://doi.org/10.1016/j.anbehav.2019.03.011
Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE (2009) Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav 77:873–885. https://doi.org/10.1016/j.anbehav.2008.12.014
Wyman MT, Pinter-Wollman N, Mooring MS (2021) Trade-offs between fighting and breeding: a social network analysis of bison male interactions. J Mammal 102:504–519. https://doi.org/10.1093/jmammal/gyaa172
Zahavi A (1975) Mate selection - a selection for a handicap. J Theor Biol 53:205–214. https://doi.org/10.1016/0022-5193(75)90111-3
Acknowledgements
We thank all students and assistants who helped with fieldwork and recorded behavioral observations from 2010 to 2018. Jennifer Chambers, Elise Rioux-Paquette, and Hélène Presseault-Gauvin performed genetic analyses before 2015. We are grateful to Patrick Bergeron, Marc Bélisle, Catherine Geoffroy, and members of the Festa-Bianchet research group for fruitful discussions, as well as to François Rousseu for statistical advice. We thank Alastair Wilson and an anonymous reviewer for constructive comments on an earlier version of the manuscript. We appreciate the logistical support of Parks Victoria, the University of Melbourne, and the Australian National University.
Funding
Funding was received from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to MF-B; Postgraduate Scholarship to WJK), Université de Sherbrooke (Doctoral Scholarship to LM), University of Queensland (International Research Tuition Award to WJK), Bishop’s University (Senate Research Committee grant to WJK), and the Holsworth Wildlife Research Endowment (research grant to WJK).
Author information
Authors and Affiliations
Contributions
LM conceived the idea with inputs from all co-authors. LM, WJK, GC, and MF-B collected field data. LM and WJK performed genetic labwork. LM performed statistical analyses and wrote the first draft. All authors contributed critically to the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and institutional guidelines for the use of animals were followed. Animal handling was approved by the Animal Ethics Committees of the University of Melbourne (approval 1312902.1) and the Australian National University (approval A2018/02), and by the Animal Care Committee of the Université de Sherbrooke (protocol MFB 2016–01). The Victorian Department of Environment, Land, Water and Planning authorized field research with permit 10008630.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J. Mann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection Measuring individual reproductive success in the wild—Guest Editors: Janet Mann and Marco Festa-Bianchet.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montana, L., King, W.J., Coulson, G. et al. Large eastern grey kangaroo males are dominant but do not monopolize matings. Behav Ecol Sociobiol 76, 78 (2022). https://doi.org/10.1007/s00265-022-03185-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03185-7