Abstract
Quantifying the costs of mating is key for understanding life-history trade-offs. As a reflection of metabolic rate, body temperature is one metric for assaying these costs. However, conventional methods for measuring body temperature are invasive and unsuitable for the study of free-living populations of endangered species, including great apes. A promising proxy for body temperature is fecal temperature, the internal temperature of fecal deposits shortly following defecation. We validated this method with humans, finding that maximum fecal temperature is a reliable proxy for rectal temperature. We then applied this method to wild chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. We collected and analyzed 101 fecal temperature measurements from 43 adult chimpanzees (male: N = 28; female: N = 15). Chimpanzee fecal temperature ranged from 33.4 to 38.9 °C, with a mean of 35.8 °C. Although fecal temperature was not predicted by sex, age, or ambient temperature, male fecal temperature was 1.1 °C higher on days when sexually receptive females were present and was positively correlated with male dominance rank. Post hoc analyses showed that overall copulation rates, but not aggression rates, were positively correlated with fecal temperature, suggesting that sexual physiology and behavior best explain mating-related temperature variation. Together, these results indicate fecal temperature is a reliable proxy for core body temperature in large-bodied mammals, captures metabolic costs associated with male mating behavior, and represents a valuable noninvasive tool for biological field research.
Significance statement
Body temperature illuminates an animal’s physiological condition and energy expenditure, but it is difficult to measure in wild animals. Consequently, basic data on body temperature and its socioecological correlates in wild animals are scant, especially when noninvasive measures must be used. To address this problem, we demonstrated that the temperatures of fecal deposits reliably estimate body temperatures in a large bodied primate and are approximately as reliable as invasive, subcutaneous transponder methods used in other mammals. We then found that fecal temperature in chimpanzees varied by ecologically and reproductively relevant variables including time of year, the presence of sexually receptive females, and dominance rank. Sexual behavior was likely responsible for increased male fecal temperature, as overall copulation frequency, but not aggression, was correlated with fecal temperature. We therefore provide evidence that fecal temperature can be used to assay body temperature and address questions regarding physiological condition and metabolic expenditure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many mammalian species, males invest less in offspring care than do females (Bateman 1948; Trivers 1972) and invest instead in mating effort (Hämäläinen et al. 2017). Male mating effort has been well documented in monkeys and apes and includes aggressive contest competition (Bercovitch 1997; Muller 2002; MacCormick et al. 2012) and social dominance hierarchies, with high-ranking males more likely to mate with receptive females (Altmann 1962; Dewsbury 1982; Cowlishaw and Dunbar 1991) and sire offspring (Launhardt et al. 2001; Wroblewski et al. 2009; Feldblum et al. 2014; Georgiev et al. 2015). As contest competition requires considerable investment, male mating effort may impose a number of costs (reviewed in Emery Thompson and Georgiev 2014).
Efforts to identify and quantify costs associated with mating effort have been numerous. Notable methods include observation of feeding time (Georgiev et al. 2014), assessment of gastrointestinal parasite richness (Muehlenbein and Watts 2010), and social network analyses of viral exposure (Rushmore et al. 2013). The energetic and psychosocial costs of male mating effort have also been assessed using physiological biomarkers measured noninvasively in urine or feces (Behringer and Deschner 2017), including cortisol (Muller and Wrangham 2004b; Anestis et al. 2006; Muehlenbein and Watts 2010) and C-peptide of insulin (Emery Thompson et al. 2009; Surbeck et al. 2015).
Variation in body temperature, as a reflection of metabolic demands (Hayward et al. 1977; Berger and Phillips 1988; Westerterp-Plantenga et al. 2002; Abreu-Vieira et al. 2015), may offer another metric of mating effort. Although the majority of body temperature research emphasizes fever and innate immune responses (Palmes and Park 1965; Baracos et al. 1987), body temperature also has critical implications for animal growth (Verbeek 1988; Köhler et al. 2012), reproductive physiology (Royston 1982; Kusuda et al. 2011), body condition (Haftorn 1972), and even social behavior (Gestich et al. 2014). For instance, female vervet monkeys (Chlorocebus pygerythrus) with more social partners exhibited higher minimum body temperatures, which may confer fitness benefits by reducing the costs of homeothermy and increasing energy available for reproduction (McFarland et al. 2015). Critically, body temperature has repercussions for mate competition in both ectotherms and endotherms. Increased body temperature may promote physical traits that increase reproductive success. For instance, male Moor Frogs (Rana arvalis) with higher body temperatures exhibit bluer coloration, which, for small males, is positively correlated with mating success (Hettyey et al. 2009). Similarly, in lions (Panthera leo), females preferentially mate with males who have darker, longer manes; these preferred males also exhibit higher body temperatures (West and Packer 2002). Reproductive physiology and behavior also affect body temperature. Notably, sexual arousal corresponds to increased genital temperature in both men and women (Kukkonen et al. 2007). In Macleay’s marsupial mice (Antechinus stuartii), male body temperature increases during the mating season, due in part to male-male aggression (Körtner and Geiser 1995), and in female Nile grass rats (Arvicanthis niloticus), mating frequently occurs during the time of day when body temperature is highest (McElhinny et al. 1997).
Considering the various physiological and ecological applications of body temperature, there have been numerous efforts to measure temperature in wild mammals. Internal temperature probes provide highly accurate core temperature measurements and allow remote access of data (Lovegrove 2009; Torrao et al. 2011; Langer and Fietz 2014). However, such devices are highly invasive and therefore unsuitable for the study of free-ranging and protected species, including great apes. Indeed, the ethical considerations are not clearly defined for many species (Wilson and McMahon 2006). Furthermore, invasive methods can generate methodological complications. Surgical implants, for example, can be logistically and financially prohibitive, and if improperly implanted, harmful to the study subject (Horning et al. 2017). Similarly, methods entailing animal capture can detrimentally alter body temperature—for instance, via restraint-induced stress (Busnardo et al. 2010).
Although strides have been made in the use of noninvasive temperature measurements, these methods remain imperfect (McCafferty et al. 2015). For instance, infrared thermography provides high-precision surface temperatures and has been used to measure responsiveness to reproductive and social changes in wild chimpanzees (Dezecache et al. 2017a, b). However, infrared thermography is affected by climatic conditions, making absolute body temperatures difficult to ascertain (Cilulko et al. 2013; McCafferty et al. 2015). Indeed, in primates, measurements derived by infrared thermography predict ambient temperature better than they predict body temperature (Thompson et al. 2017). Another noninvasive method is therefore required for use when implants or collars are unethical, impractical, or cost prohibitive.
The temperature of fecal deposits provides a compelling, and largely overlooked, noninvasive proxy for rectal temperature in free-ranging mammals [e.g., elephants (Benedict and Lee 1936; Kusuda et al. 2007)]. Rectal temperature reflects core temperature (Robinson et al. 1998; Mazerolle et al. 2011). Consequently, studies of captive chimpanzees have measured rectal temperatures (Fox 1923; Morrison 1962; Melis et al. 2012) or tympanic temperatures (Fowler et al. 1999), which are highly correlated with rectal temperatures (Stewart et al. 1998; Sehgal et al. 2002; Boere et al. 2003; Long et al. 2011). Jensen et al. (2009) estimated body temperature in wild chimpanzees (Pan troglodytes) by applying a sigmoid curve to fecal temperature decline. However, this method requires upwards of 10 min to obtain adequate points for the sigmoid curve, during which time air bubbles may open in the deposit that will adversely affect the estimate. Simplicity and speed are highly desirable methodological qualities when monitoring wild chimpanzees. Therefore, a modified, simpler version of Jensen et al. (2009)’s method, in which the maximum fecal temperature is used as a proxy for rectal body temperature, would be useful for the study of chimpanzees and other large-bodied mammals.
Here, we tested whether reproductively relevant behavioral and ecological variables predict differences in fecal temperature in adult chimpanzees at Ngogo, Kibale National Park, Uganda. We first validated a single measurement of maximum fecal temperature as a reliable proxy for rectal body temperature in humans. We then assessed variance in chimpanzee fecal temperature according to variables that may affect primate body temperature, including age (Lane et al. 1996; Obermeyer et al. 2017), sex (Thompson et al. 2014), ambient temperature (Aujard and Vasseur 2001), season (Takemoto 2004; van Ooijen et al. 2004), and time of day (Fuller and Sulzman 1982). Next, we assessed variance in fecal temperature with two measures of male chimpanzee mating effort: the presence of sexually receptive females and dominance rank. Male-male competition peaks in the presence of sexually receptive females (Muller 2002; Sobolewski et al. 2013). We predict that increases in body temperature correspond to such increased physical and social activity. We assessed the presence of sexually receptive females as a proxy for mating effort to assess the cumulative influence of behavioral and physiological changes that define male mating effort (from aggression to sexual arousal and copulation). This was also done because we did not observe mating behavior from the focal males when fecal temperatures were collected. Importantly, we only assess associations for sexually receptive females in the same party as the focal male, as increased male competition is contingent on spatial association with the receptive female. Similarly, high-ranking male chimpanzees engage in greater levels of physical competition, as reflected in elevated aggression rates (Muller 2002; Muller and Wrangham 2004a) and perhaps body size (Foster et al. 2009). We therefore expected that if status competition imposes notable metabolic costs through physical competition, dominance rank should also positively correlate with fecal temperature.
Methods
Study site and subjects
We conducted this study at Ngogo, Kibale National Park, Uganda. Ngogo is home to the largest community of wild chimpanzees yet studied (Wood et al. 2017). At the beginning of the study period, there were 204 individuals in this community, including 34 adult males. The chimpanzees range over approximately 35 km2 (Mitani et al. 2010). The unusually large size of this community provides a unique sample with which to assess the ecological and demographic correlates of noninvasively measured body temperature in wild chimpanzees. As we collected data from focal chimpanzees, we did not use blinded methods in this study.
Measuring fecal temperature
We collected all temperature measurements with a commercial digital data-logging thermometer (TMD-56, Amprobe, USA). The thermometer was inserted into the fecal deposit, and we recorded until the temperature started to decline. We used the peak temperature as a given deposit’s temperature. As Jensen et al. (2009) observed decreased quality in their estimates with longer lag times between defecation and measurement (beginning at approximately two minutes post-defecation), we only took fecal temperatures when we could begin measuring within 90 s of deposition. Jensen et al. (2009) did not observe an effect of fecal weight on temperature estimates; consequently, we did not control for fecal weight.
Human validation
We validated our method by collecting 18 paired measurements of fecal temperature and rectal body temperature from two adult human males, using the same thermometer used for fecal temperatures and at the same field site where chimpanzee data were collected. One individual provided 14 paired measurements from 17 November 2017 and 13 January 2018, almost always in the morning after having eaten a light breakfast. The second individual provided four paired measurements from 19 to 23 September 2017 between 7:30 and 13:15. Fecal deposits were excreted onto a piece of cardboard over the opening of a “long-drop” pit latrine (n = 10) or onto a piece of cardboard on the ground (n = 8). Deposits from one individual were relatively soft, but formed (n = 14), while samples from the other individual were firm and compact (n = 4). Small, diarrheal, and exceptionally dry deposits were not measured. To determine if defecation greatly biased rectal temperature measurements, we compared rectal temperatures taken before and after defecation for 15 paired measurements. We found a significant positive correlation (rm = 0.661, p = 0.010), indicating that pre- and post-defecation rectal measurements were comparable. Because we collected more measurements post-defecation, we limited comparisons of rectal and fecal temperature to post-defecation rectal measurements.
Chimpanzee fecal temperature measurements
Measurements of chimpanzee fecal temperature were collected by JDN or a Ngogo Chimpanzee Project field assistant between February 2016 and April 2017. To ensure that the fecal deposit was large enough, we limited our sample to adults. Furthermore, we only measured full deposits left when the defecator was seated on the ground to avoid the dissipation of heat that may occur when feces fall from great heights and disperse. As a result, we measured deposits after an individual had been engaging in stationary behavior (e.g., resting, grooming). Only soft deposits with ample fecal matrix were measured. Small or diarrheal deposits, exceptionally dry deposits, and deposits composed mostly of seeds were not measured. In this regard, chimpanzee fecal deposits were comparable to those of the humans. We collected 101 fecal temperatures (males: N = 80; females: N = 21) from 43 individuals (males: N = 28; females: N = 15). We measured 37 fecal temperatures from males when sexually receptive females were absent (i.e., were not in association with the sampled individuals) and 41 temperatures from males when sexually receptive females were present in the party. Females were considered sexually receptive if they exhibited full sexual swellings and were observed copulating with adult males on the day the measurement was taken, or on an adjacent day.
Observational data collection
To assess dominance rank and mating environment, observational data were collected between January 2016 and July 2017 as per routine Ngogo Chimpanzee Project protocol. During focal animal follows (Altmann 1974), observers recorded all chimpanzees present in the party (i.e., ≤ 50 m of the focal) at 15-min intervals. Observers also recorded the presence of sexually receptive females, as well as all copulations, pant-grunts—i.e., unidirectional vocalizations given by subordinant to dominant individuals (Goodall 1986)—and acts of aggression, noting both the aggressor(s) and recipient(s). Aggression included charges, chases, and physical attacks directed at a conspecific. We collected a total of 20,450 scans of party composition, 751 copulations, and 939 acts of aggression by adult males.
Calculation of male dominance rank
During the study period, males organized into two subsets or “neighborhoods” (i.e., Central and West) that rarely interacted. Therefore, a dominance hierarchy was calculated for each neighborhood. Individuals were assigned to only one neighborhood. Ordinal dominance ranks were calculated in SOCPROG (Whitehead 2009) using pant grunts (N = 580) and decided dyadic agonistic encounters (N = 354) in which one individual submitted to the aggression of another. We first determined if each dominance hierarchy was significantly linear by comparing Landau’s index of linearity (H′) generated from observed dominance interactions to an index calculated from 10,000 random matrix permutations. Both the Central and West hierarchies were significantly linear (Central: p < 0.001; West: p = 0.035). We then applied the I & SI method of linear ordering (de Vries 1998), which minimizes inconsistencies in a linear sequence when data for every dyadic relationship are not available. For the central neighborhood, two equally likely dominance hierarchies were generated in which the dominance scores of three low-ranking individuals differed; these individuals were assigned new dominance scores based on the averages of their values from the two calculated hierarchies. To adjust for differences in the size of the two dominance hierarchies, dominance scores were normalized by subtracting the ordinal rank from the total number of adult males and dividing this value by one less than the total number of adult males; thus, the highest ranking male had a value of one, and the lowest had a value of zero (Muller et al. 2006).
Statistical analyses
All statistical analyses were completed in R version 3.5.1 (R Core Team 2013) using RStudio version 1.1.463 (RStudio Team 2015). To validate our method in paired human rectal and fecal measurements, we calculated repeated-measures correlation coefficients using the “rmcorr” function in package rmcorr version 0.3.0 (Bakdash and Marusich 2017). To assess predictors of chimpanzee fecal temperature, we constructed linear mixed models (LMMs) with Gaussian error structures and fitted with restricted maximum likelihood using the “lmer” function in package lme4 version 1.1.20 (Bates et al. 2015). We considered a Gaussian error structure appropriate, as we assessed normality of residuals with Shapiro-Wilk tests (Shapiro and Wilk 1965) using the “shapiro.test” function, Jarque-Bera tests of skewness and kurtosis (Jarque and Bera 1980, 1987) using the “jarque.bera.test” function in package tseries version 0.10.46 (Trapletti and Hornik 2015), and inspection of Q-Q plots (Wilk and Gnanadesikan 1968). We observed no significant deviance from normality.
In model 1, we analyzed samples from both males and females; we included age, sex, time of day, and ambient temperature as fixed effects. In model 2, we assessed social predictors of fecal temperature in males; we included the presence of sexually receptive females in the party, dominance rank, and time of day as fixed effects. To improve model accuracy, we also included the significant predictors from model 1 as additional fixed effects. In both models, to control for large-scale temporal variation in thermoregulation (Aujard and Vasseur 2001; Wessling et al. 2018a), we included the sine and cosine of the Julian date (divided by 365.25 and multiplied by 2π) as additional fixed effects per Stolwijk et al. (1999). To control for multiple sampling of individuals, we included the identity of individual chimpanzees as a random effect, with time of day included as a random slope to keep the probability of type I error at the nominal 5% (Barr et al. 2013). Age and ambient temperature were originally included as random slopes in the first LMM; however, they explained relatively little variance (age = 0.006253; ambient temperature < 0.000001) and thus prevented model convergence. They were therefore not included as random slopes in the final model. Similarly, in the second LMM, the mating variable prevented model convergence and was therefore not included as a random slope in the final model.
To produce comparable estimates for each fixed effect, we Z-transformed continuous fixed effects (except Julian date) to a mean of 0 and a standard deviation of 1. We used Satterthwaite approximations in package lmerTest version 3.0.1 (Kuznetsova et al. 2017) to estimate degrees of freedom and probability values, as this method produces low type I error rates (Luke 2017). We also examined collinearity by checking variance inflation factors (VIFs), using the “vif” function in the package car version 3.0.2 (Fox and Weisberg 2011). Multicollinearity in relatively small datasets is often acknowledged when VIFs are ≥ 2.5 (Johnston et al. 2018); all VIFs in our models were < 2.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Results
Human validation
Human rectal temperature (mean ± SD 36.46 ± 0.43) and fecal temperature (mean ± SD 36.38 ± 0.36) were significantly correlated (rm = 0.751, p < 0.001) (Fig. 1). The mean difference between paired rectal and fecal temperatures was 0.21 °C (SD ± 0.22 °C). In 12 of 18 measurements, the fecal measurement differed from the rectal measurement by ≤ 0.20 °C.
Chimpanzee fecal temperature
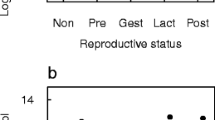
The mean fecal temperature of chimpanzees was 35.8 ± 1.0 °C (range 33.4 °C to 38.9 °C). Results of the LMM assessing demographic and ambient predictors of fecal temperature (n = 87 measurements) are listed in Table 1. Males and females exhibited comparable temperatures (Fig. 2a), and adults did not vary in temperature based on age (Fig. 2b). In addition, temperature of fecal deposits did not vary with ambient temperature (Fig. 2c) but significantly increased with time of day (Fig. 2d). Furthermore, fecal temperature varied with time of year (Table 1).
To assess which environmental variables may drive temporal variation, we conducted a post hoc analysis on a reduced subset of the male samples (n = 76) for which corresponding data were available for daily rainfall, minimum temperature, and maximum temperature. We found positive effects of rainfall (estimate = 0.389, SE = 0.106, df = 2.974, p = 0.036) and minimum temperature (estimate = 0.527, SE = 0.118, df = 17.300, p < 0.001), but not maximum temperature (estimate = − 0.067, SE = 0.010, df = 55.273, p = 0.505). A second post hoc analysis with the full dataset, in which we included month as a fixed effect (using January as the reference month), indicated that samples were hotter when collected in May (estimate = 1.913, SE = 0.453, df = 64.414, p < 0.001) and November (estimate = 1.328, SE = 0.583, df = 48.242, p = 0.027) and colder when collected in September (estimate = − 1.356, SE = 0.424, df = 61.469, p = 0.002) and October (estimate = − 1.188, SE = 0.492, df = 43.176, p = 0.020).
Results of a linear mixed model investigating the effects of male mating effort on fecal temperature (n = 76 measurements) are listed in Table 2. Average fecal temperature in male chimpanzees was approximately 1.1 °C higher in the presence of sexually receptive females (Fig. 3). Furthermore, we found a significant positive correlation between dominance rank and fecal temperature. In this model, time of day was not correlated with fecal temperature.
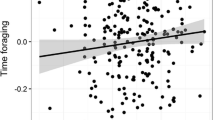
To further explore dominance and mating-related temperature variation, we performed post hoc analyses of copulation and aggression rates as predictors of male fecal temperature. Because fecal temperatures were mainly collected from individuals ad libitum rather than from individuals during focal follows, we lacked the data necessary to test the relationship between variation in fecal temperature and variation in aggression/copulation at or around the time of fecal sample collection. Instead, we examined the relationship between fecal temperature and long-term individual aggression and copulation rates, as calculated over the entire 1-year duration of the study period. Individual copulation rates were calculated as the number of observed copulations divided by the number of party scans in which the male appeared with a female displaying a full sexual swelling. Similarly, individual aggression rates were calculated as the number of observed aggression acts divided by the number of party scans in which that individual appeared during the study period. Although dominance rank was positively correlated with both overall copulation rate (rs = 0.597, n = 34, p = 0.001) and aggression rate (rs = 0.671, n = 34, p < 0.001), overall rates of copulation and aggression were not significantly correlated (rs = 0.301, n = 34, p = 0.136). A LMM including dominance rank as well as overall copulation and aggression rates indicated potential multicollinearity (VIF for dominance rank = 2.706); we ran the model again after excluding dominance rank. Fecal temperature was higher in the presence of sexually receptive females (estimate = 0.686, SE = 0.207, df = 59.992, p = 0.002) and increased with individual rate of copulation (estimate = 0.316, SE = 0.109, df = 18.322, p = 0.010), but not aggression (estimate = −0.015, SE = 0.114, df = 14.039, p = 0.896) (Fig. 4).
Male chimpanzee fecal temperature as a function of a overall copulation rate and b overall aggression rate. Rates were calculated as the number of scan samples in which a both the sampled individual and a sexually receptive female were present and b the sampled individual was present. Shading around regression lines represents 95% confidence intervals. Each point represents an individual’s mean fecal temperature; note that correlation estimates and significance values were calculated from linear mixed models controlling for the presence of sexually receptive females, collection time, and collection date
Discussion
Results of our validation using two humans indicate that our simplification of Jensen et al. (2009)‘s method was effective. The mean difference between paired fecal and rectal temperature measurements observed in this study (0.21 °C) was approximately as small as the mean difference between paired subcutaneous transponder and rectal temperature measurements reported in previous studies of other mammalian species (e.g., 0.20 °C, Torrao et al. 2011; 0.90 °C, Wacker et al. 2012). Therefore, our results indicate that maximum fecal temperature is an effective proxy for rectal body temperature in humans and is an appropriate proxy in other large-bodied mammals, including adult chimpanzees. To confirm this method’s reliability and validity, we recommend our study be replicated with a larger sample size.
Demographic and ecological predictors of chimpanzee fecal temperature
In apparently healthy chimpanzees at Ngogo, fecal temperatures ranged from 33.4 to 38.9 °C, which are comparable to measurements reported in previous studies of chimpanzees (Table 3). Our mean temperature was 1.4 °C lower than fecal temperatures reported for chimpanzees at Taï (Jensen et al. 2009) and rectal temperatures at the Philadelphia Zoological Garden (Fox 1923; Morrison 1962). Notably, we derived nearly equivalent average values from the tympanic temperature of captive chimpanzees at the Yerkes Primate Center (Fowler et al. 1999) and rectal temperatures from the Animal Advocacy and Protection Sanctuary (Melis et al. 2012), derived from studies with substantial sample sizes (Table 3). The results of some previous studies in captive populations, however, are potentially confounded by experimental pathogenic infection (Morrison 1962) or the use of anesthetics that may artificially reduce body temperature (Melis et al. 2012). Consequently, we cannot draw definitive conclusions about normal chimpanzee body temperatures given the relative paucity of comparative data from unimpaired great apes, whether free-living or captive. More data are needed (1) from apparently healthy study populations, and (2) that are collected in ways that do not artificially modify results.
Chimpanzee fecal temperatures in our study exhibited a somewhat wider range than that of normal rectal temperature in humans, which is 34.4 to 37.8 °C (Sund-Levander et al. 2002). However, some primates are known to have wide ranges in body temperature. For instance, chacma baboons (Papio ursinus) living in the Namib desert reach maximum core temperatures of more than 42 °C (Brain and Mitchell 1999). Similarly, Thompson et al. (2014) report that subcutaneous body temperatures of howler monkeys (Alouatta palliata) in Belize range from 30.97 to 42.64 °C. Therefore, compared with humans, the normal body temperatures of nonhuman primates may be more variable.
Fecal temperature did not vary by age or sex, but we found a positive correlation with time of day. This relationship accords with previously observed circadian patterns in humans (Mackowiak et al. 1992) and captive chimpanzees (Fowler et al. 1999), such that body temperature peaks in the late afternoon or evening. However, in the males-only model, in which we included mating environment and dominance rank, there was no longer a significant correlation with time of day. Therefore, the relationship we observed between time of day and temperature may reflect a complex interaction of physiological and behavioral rhythms. Further data are necessary to disentangle the relative contributions of these factors to chimpanzee body temperature.
Fecal temperature also varied by time of year. Post hoc analyses indicated that daily values for rainfall and minimum temperature were positively correlated with fecal temperature. Furthermore, samples collected in May and November were hotter, and samples collected in September and October were colder than average. September and October constitute approximately the middle of the second rainy season in Kibale, while May and November represent the start of the two dry seasons (Hartter et al. 2012). However, ambient temperature at time of sample collection did not appear to influence fecal temperature. This is somewhat surprising. Positive correlations between body temperature and environmental temperature have been observed in other species, for instance, in bush rats (Rattus fuscipes) (Glanville and Seebacher 2010) and round-tailed ground squirrels (Spermophilus tereticaudus) (Wooden and Walsberg 2002). Yet, short-term (e.g., hourly) ambient conditions may be poor predictors of fecal temperature in wild chimpanzees. Furthermore, monthly variation may reflect variation in locomotor activity, which in llamas (Lama glama), for instance, is correlated with body temperature (Riek et al. 2017).
Although ambient temperature and precipitation fluctuate substantially with season, so too do other critical variables such as food availability, which may affect metabolic rate and body temperature. For instance, in golden spiny mice (Acomys russatus), dietary supplementation increases body temperature (Levy et al. 2011), while caloric restriction reduces body temperature in a variety of mammals (Duffy et al. 1990; Lane et al. 1996; Rikke and Johnson 2004), including humans (Soare et al. 2011). In addition, differences in dietary composition may alter gut microfloral activity (Rowland et al. 1985) and thereby increase the temperature of feces. These dynamics require further attention.
The fact that ambient temperature did not predict variation in chimpanzee fecal temperature at Ngogo may indicate that chimpanzees are thermoregulating efficiently. Although ambient temperature varied, it was not at the extremes found in other habitats. For instance, chimpanzees living in hot, arid savannas at Fongoli, Senegal, exhibit signs of heat stress (Wessling et al. 2018b), including higher cortisol levels than chimpanzees in the rainforests of Taï National Park (Wessling et al. 2018a). Cave use, which is exceedingly rare in wild chimpanzees, has been documented at Fongoli, presumably as a way to combat heat stress (Pruetz 2007). Measurements of body temperature in savanna chimpanzees will help assess the effectiveness of their behavioral thermoregulation and how they cope with such extreme heat.
Male mating effort and fecal temperature
Importantly, our method captured variation in chimpanzee body temperature pertaining to mating effort. Male chimpanzees exhibited higher fecal temperatures in the presence of reproductively receptive females, and high-ranking males exhibited higher fecal temperatures than low-ranking males. In our study, fecal temperature measurements could not be matched to corresponding short-term copulation or aggression rates. Nevertheless, post hoc analyses indicated that male chimpanzees with higher long-term copulation rates, but not those with higher long-term aggression rates, exhibited higher fecal temperature. In chimpanzees, sexual arousability—calculated from penile erection time and mean erection length—is correlated with copulation frequency (Nadler and Bartlett 1997). Therefore, the elevated temperature exhibited by males in the presence of receptive females may be due to both copulatory behavior specifically and sexual arousal more generally. Although male aggression rates are higher in the presence of preferred female mating partners (Muller and Wrangham 2004a; Sobolewski et al. 2013), increased physical activity is likely inadequate to explain increased fecal temperature, given that we did not observe a correlation with overall aggression rates. Furthermore, as overall copulation rates were correlated with dominance rank, we suggest the relationship observed between dominance rank and fecal temperature was due to sexual behavior rather than physical aggression.
Although temperature changes throughout the body are well-recognized components of sexual physiology (Kukkonen et al. 2007; Merla and Romani 2007; Kiyatkin 2010), the detectible increase in male chimpanzee fecal temperature in the presence of sexually receptive females and the correlation with overall copulation rates suggest that sexual behavior imposes non-trivial metabolic costs. Similar patterns have been observed in rams (Ovis aries): Ungerfeld and Fila (2012) reported an increase in rectal temperature with the onset of sexual behavior, while Godfrey et al. (1998) observed that the number of mounts was positively correlated with rectal temperature. Studies of skin temperature and oxygen consumption similarly indicate that sexual behavior is energetically costly. For example, in healthy heterosexual men, sexual activity yields an energy expenditure approximating 10 min of moderate endurance running (Frappier et al. 2013), and in male millipedes (Alloporus uncinatus), copulations raise energy expenditure by 30% of basal levels (Telford and Webb 1998). We therefore suggest the energetic expenses of sexual physiology and behavior require more consideration in the study of mating strategies and life history trade-offs.
However, we cannot discount other behavioral factors. Overall increases in activity during periods of mating activity may contribute to increased body temperature, as observed, for instance, in Macleay’s marsupial mice (Körtner and Geiser 1995). Non-sexual social stimuli may also cause mating-based differences in male chimpanzee fecal temperature. In captivity, chimpanzees shown videos of severe aggression exhibited increased right tympanic membrane temperature (Parr and Hopkins 2000). Similarly, wild chimpanzees in the Budongo Forest Reserve, Uganda, exhibited increased ear temperatures after hearing aggressive vocalizations and decreased ear temperatures after hearing non-aggressive vocalizations (e.g., whimpers) from conspecifics (Dezecache et al. 2017b). Further research is necessary to distinguish the effects of social and sexual arousal from those of mating behavior itself.
While the higher temperature of high-ranking males likely reflects sexual behavior, it may be due to other factors such as body size and mass. In various mammalian species, body size and mass predict dominance rank (Haley et al. 1994; McElligott et al. 2001; Huang et al. 2011; Chelliah and Sukumar 2013). Muscle mass, in particular, predicts physical competitiveness (Mitani et al. 1996; Lassek and Gaulin 2009). While overall body mass predicts body temperature in humans (Bastardot et al. 2019), muscle tissue is especially thermogenic (Rowland et al. 2015; Payne et al. 2018). Therefore, variation in fecal temperature in adult male chimpanzees may also be due, in part, to variation in lean muscle mass. Yet, we did not find a significant difference in male and female fecal temperatures. Given that males likely have greater muscle mass on average than females, as has been reported in humans (Janssen et al. 2000) and bonobos, P. paniscus (Zihlman and Bolter 2015), the absence of a sex difference in our study suggests the thermogenic effects of lean muscle are mitigated by other thermoregulatory processes. Regardless, inter-individual variation in wild chimpanzee muscle mass has yet to be explored (Watts 2018) and may have informed our results.
In conclusion, we found that fecal temperature is an easy and effective method for inferring body temperature in adult chimpanzees and could be applied to other species when invasive methods are inappropriate or cost prohibitive. Although we found the method accurate for a human-sized fecal deposit, caution should be exercised when measuring fecal temperature in smaller bodied primates, as heat retention—and the subsequent correlation between fecal and rectal temperatures—may be lower for smaller fecal deposits. Further study is required to determine if fecal temperature is similarly reliable in smaller animals with correspondingly smaller fecal deposits. Given that body temperature is a key variable in many aspects of reproductive ecology, energetics, and health, we consider fecal temperature an important method in animal field studies.
References
Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML (2015) Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 4:461–470
Altmann SA (1962) A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci 102:338–435
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–266
Anestis SF, Bribiescas RG, Hasselschwert DL (2006) Age, rank, and personality effects on the cortisol sedation stress response in young chimpanzees. Physiol Behav 89:287–294
Aujard F, Vasseur F (2001) Effect of ambient temperature on the body temperature rhythm of male gray mouse lemurs (Microcebus murinus). Int J Primatol 22:43–56
Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:456
Baracos VE, Whitmore WT, Gale R (1987) The metabolic cost of fever. Can J Physiol Pharmacol 65:1248–1254
Barr DJ, Levy R, Scheepers C, Tily HJ (2013) Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68:255–278
Bastardot F, Marques-Vidal P, Vollenweider P (2019) Association of body temperature with obesity. The CoLaus study. Int J Obes 43:1026–1033
Bateman AJ (1948) Intra-sexual selection in Drosophila. Heredity 2:349–368
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Behringer V, Deschner T (2017) Non-invasive monitoring of physiological markers in primates. Horm Behav 91:3–18
Benedict FG, Lee RC (1936) Studies on the body temperature of elephants. P Natl Acad Sci USA 22:405–408
Bercovitch FB (1997) Reproductive strategies of rhesus macaques. Primates 38:247–263
Berger RJ, Phillips NH (1988) Regulation of energy metabolism and body temperature during sleep and circadian torpor. In: Lydic R, Biebuyck JF (eds) Clinical physiology of sleep. Springer New York, New York, pp 171–189
Boere V, Silva I, Canale G, Ferreira Pianta T, Tomaz C (2003) Correlation between tympanic and rectal temperature in marmosets (Callithrix penicillata) under acute stress. Braz J Vet Res Anim Sci 40:90–95
Brain C, Mitchell D (1999) Body temperature changes in free-ranging baboons (Papio hamadryas ursinus) in the Namib Desert, Namibia. Int J Primatol 20:585–598
Busnardo C, Tavares RF, Resstel LBM, Elias LLK, Correa FMA (2010) Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Auton Neurosci 158:51–57
Chelliah K, Sukumar R (2013) The role of tusks, musth and body size in male–male competition among Asian elephants, Elephas maximus. Anim Behav 86:1207–1214
Cilulko J, Janiszewski P, Bogdaszewski M, Szczygielska E (2013) Infrared thermal imaging in studies of wild animals. Eur J Wildl Res 59:17–23
Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Cowlishaw G, Dunbar RIM (1991) Dominance rank and mating success in male primates. Anim Behav 41:1045–1056
de Vries HAN (1998) Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Anim Behav 55:827-843
Dewsbury DA (1982) Dominance rank, copulatory behavior, and differential reproduction. Q Rev Biol 57:135–159
Dezecache G, Wilke C, Richi N, Neumann C, Zuberbühler K (2017a) Skin temperature and reproductive condition in wild female chimpanzees. PeerJ 5:e4116
Dezecache G, Zuberbühler K, Davila-Ross M, Dahl CD (2017b) Skin temperature changes in wild chimpanzees upon hearing vocalizations of conspecifics. R Soc Open Sci 4:160816
Duffy PH, Feuers RJ, Hart RW (1990) Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int 7:291–303
Emery Thompson M, Georgiev AV (2014) The high price of success: costs of mating effort in male primates. Int J Primatol 35:609–627
Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB (2009) Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm Behav 55:299–305
Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC (2014) Sexually coercive male chimpanzees sire more offspring. Curr Biol 24:2855–2860
Foster MW, Gilby IC, Murray CM, Johnson A, Wroblewski EE, Pusey AE (2009) Alpha male chimpanzee grooming patterns: implications for dominance “style”. Am J Primatol 71:136–144
Fowler LA, Hopkins WD, Albers HE, Morris RD, Hyatt CW (1999) Establishing the presence of a body temperature rhythm in chimpanzees (Pan troglodytes) using a tympanic membrane thermometer. Primates 40:499–508
Fox H (1923) c. In: Fox H, Penrose CB (eds) Diseases in captive wild mammals and birds. Incidence, description, comparison. Lippincott, Philadelphia, pp 520–525
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Frappier J, Toupin I, Levy JJ, Aubertin-Leheudre M, Karelis AD (2013) Energy expenditure during sexual activity in young healthy couples. PLoS One 8:e79342
Fuller CA, Sulzman FM (1982) Circadian control of body temperature in primates. In: Aschoff J, Daan S, Groos GA (eds) Vertebrate circadian systems: structure and physiology. Springer, Berlin, pp 224–236
Georgiev AV, Russell AF, Emery Thompson M, Otali E, Muller MN, Wrangham RW (2014) The foraging costs of mating effort in male chimpanzees (Pan troglodytes schweinfurthii). Int J Primatol 35:725–745
Georgiev AV, Muehlenbein MP, Prall SP, Emery Thompson M, Maestripieri D (2015) Male quality, dominance rank, and mating success in free-ranging rhesus macaques. Behav Ecol 26:763–772
Gestich CC, Caselli CB, Setz EZF (2014) Behavioural thermoregulation in a small neotropical primate. Ethology 120:331–339
Glanville EJ, Seebacher F (2010) Advantage to lower body temperatures for a small mammal (Rattus fuscipes) experiencing chronic cold. J Mammal 91:1197–1204
Godfrey RW, Collins JR, Gray ML (1998) Evaluation of sexual behavior of hair sheep rams in a tropical environment. J Anim Sci 76:714–717
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Belknap Press, Cambridge
Haftorn S (1972) Hypothermia of tits in the Arctic winter. Ornis Scand 3:153–166
Haley MP, Deutsch CJ, Le Boeuf BJ (1994) Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Anim Behav 48:1249–1260
Hämäläinen A, McAdam AG, Dantzer B, Lane JE, Haines JA, Humphries MM, Boutin S (2017) Fitness consequences of peak reproductive effort in a resource pulse system. Sci Rep 7:9335
Hartter J, Stampone MD, Ryan SJ, Kirner K, Chapman CA, Goldman A (2012) Patterns and perceptions of climate change in a biodiversity conservation hotspot. PLoS One 7:e32408
Hayward JS, Eckerson JD, Collis ML (1977) Thermoregulatory heat production in man: prediction equation based on skin and core temperatures. J Appl Physiol 42:377–384
Hettyey A, Crochet P-A, Merilä J, Herczeg G, Laurila A (2009) Body temperature, size, nuptial colouration and mating success in male moor frogs (Rana arvalis). Amphibia-Reptilia 30:37–43
Horning M, Haulena M, Tuomi PA et al (2017) Best practice recommendations for the use of fully implanted telemetry devices in pinnipeds. Anim Biotelem 5:13
Huang B, Wey TW, Blumstein DT (2011) Correlates and consequences of dominance in a social rodent. Ethology 117:573–585
Janssen I, Heymsfield SB, Wang Z, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89:81–88
Jarque CM, Bera AK (1980) Efficient tests for normality, homoscedasticity and serial independence of regression residuals. Econ Lett 6:255–259
Jarque CM, Bera AK (1987) A test for normality of observations and regression residuals. Int Stat Rev 55:163–172
Jensen SA, Mundry R, Nunn CL, Boesch C, Leendertz FH (2009) Non-invasive body temperature measurement of wild chimpanzees using fecal temperature decline. J Wildl Dis 45:542–546
Johnston R, Jones K, Manley D (2018) Confounding and collinearity in regression analysis: a cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual Quant 52:1957–1976
Kiyatkin EA (2010) Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci 15:73–92
Köhler M, Marín-Moratalla N, Jordana X, Aanes R (2012) Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487:358–361
Körtner G, Geiser F (1995) Body temperature rhythms and activity in reproductive Antechinus (Marsupialia). Physiol Behav 58:31–36
Kukkonen TM, Binik YM, Amsel R, Carrier S (2007) Thermography as a physiological measure of sexual arousal in both men and women. J Sex Med 4:93–105
Kusuda S, Wakimoto T, Sato T, Nishimura K, Kawakami S, Okuda K, Saito E, Shimada T, Sakamoto H, Yanagimoto H, Wada S, Nishio K, Fuji H, Suzuki T, Hashikawa H, Kusunoki H, Doi O (2007) Relationship between body temperature and ovarian cycle in Asian and African elephants. J Reprod Dev 53:1099–1105
Kusuda S, Kakizoe Y, Kanda K, Sengoku T, Fukumoto Y, Adachi I, Watanabe Y, Doi O (2011) Ovarian cycle approach by rectal temperature and fecal progesterone in a female killer whale, Orcinus orca. Zoo Biol 30:285–295
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 1:1–26
Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS (1996) Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. P Natl Acad Sci USA 93:4159–4164
Langer F, Fietz J (2014) Ways to measure body temperature in the field. J Therm Biol 42:46–51
Lassek WD, Gaulin SJC (2009) Costs and benefits of fat-free muscle mass in men: relationship to mating success, dietary requirements, and native immunity. Evol Hum Behav 30:322–328
Launhardt K, Borries C, Hardt C, Epplen JT, Winkler P (2001) Paternity analysis of alternative male reproductive routes among the langurs (Semnopithecus entellus) of Ramnagar. Anim Behav 61:53–64
Levy O, Dayan T, Kronfeld-Schor N (2011) Adaptive thermoregulation in golden spiny mice: the influence of season and food availability on body temperature. Physiol Biochem Zool 84:175–184
Long CT, Pacharinsak C, Jampachaisri K, McKeon GP, Howard AM, Albertelli MA, Felt SA (2011) Comparison of rectal and tympanic core body temperature measurement in adult Guyanese squirrel monkeys (Saimiri sciureus sciureus). J Med Primatol 40:135–141
Lovegrove BG (2009) Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J Comp Physiol B 179:451–458
Luke SG (2017) Evaluating significance in linear mixed-effects models in R. Behav Res Methods 49:1494–1502
MacCormick HA, MacNulty DR, Bosacker AL, Lehman C, Bailey A, Anthony Collins D, Packer C (2012) Male and female aggression: lessons from sex, rank, age, and injury in olive baboons. Behav Ecol 23:684–691
Mackowiak PA, Wasserman SS, Levine MM (1992) A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA 268:1578–1580
Mazerolle SM, Ganio MS, Casa DJ, Vingren J, Klau J (2011) Is oral temperature an accurate measurement of deep body temperature? A systematic review. J Athl Train 46:566–573
McCafferty DJ, Gallon S, Nord A (2015) Challenges of measuring body temperatures of free-ranging birds and mammals. Anim Biotelem 3:33
McElhinny TL, Smale L, Holekamp KE (1997) Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav 62:91–96
McElligott AG, Gammell MP, Harty HC, Paini DR, Murphy DT, Walsh JT, Hayden TJ (2001) Sexual size dimorphism in fallow deer (Dama dama): do larger, heavier males gain greater mating success? Behav Ecol Sociobiol 49:266–272
McFarland R, Fuller A, Hetem RS, Mitchell D, Maloney SK, Henzi SP, Barrett L (2015) Social integration confers thermal benefits in a gregarious primate. J Anim Ecol 84:871–878
Melis S, Schauvliege S, van Bolhuis H, Hoyer M, Gasthuys F (2012) Chemical immobilization of chimpanzees (Pan troglodytes) using a combination of detomidine and ketamine. Vet Anaesth Analg 39:520–528
Merla A, Romani GL (2007) Thermal signatures of emotional arousal: a functional infrared imaging study. Conf Proc IEEE Eng Med Biol Soc 2007:247–249
Mitani JC, Gros-Louis J, Richards AF (1996) Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am Nat 147:966–980
Mitani JC, Watts DP, Amsler SJ (2010) Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol 20:R507–R508
Morrison P (1962) An analysis of body temperature in the chimpanzee. J Mammal 43:166–171
Muehlenbein MP, Watts DP (2010) The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc Med 4:21
Muller MN (2002) Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant L (eds) Behavioral diversity in chimpanzees and bonobos. Cambridge University Press, Cambridge, pp 112–124
Muller MN, Wrangham RW (2004a) Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Anim Behav 67:113–123
Muller MN, Wrangham RW (2004b) Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55:332–340
Muller MN, Emery Thompson M, Wrangham RW (2006) Male chimpanzees prefer mating with old females. Curr Biol 16:2234–2238
Nadler RD, Bartlett ES (1997) Penile erection: a reflection of sexual arousal and arousability in male chimpanzees. Physiol Behav 61:425–432
Obermeyer Z, Samra JK, Mullainathan S (2017) Individual differences in normal body temperature: longitudinal big data analysis of patient records. BMJ 359:j5468
Palmes ED, Park CR (1965) The regulation of body temperature during fever. Arch Environ Health 11:749–759
Parr LA, Hopkins WD (2000) Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiol Behav 71:363–371
Payne S, Macintosh A, Stock J (2018) Body size and body composition effects on heat loss from the hands during severe cold exposure. Am J Phys Anthropol 166:313–322
Pruetz JD (2007) Evidence of cave use by savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal: implications for thermoregulatory behavior. Primates 48:316–319
Riek A, Brinkmann L, Gauly M, Perica J, Ruf T, Arnold W, Hambly C, Speakman JR, Gerken M (2017) Seasonal changes in energy expenditure, body temperature and activity patterns in llamas (Lama glama). Sci Rep 7:7600
Rikke BA, Johnson TE (2004) Lower body temperature as a potential mechanism of life extension in homeotherms. Exp Gerontol 39:927–930
Robinson JL, Seal RF, Spady DW, Joffres MR (1998) Comparison of esophageal, rectal, axillary, bladder, tympanic, and pulmonary artery temperatures in children. J Pediatr 133:553–556
Rowland IR, Mallett AK, Wise A (1985) The effect of diet on the mammalian gut flora and its metabolic activities. Crit Rev Toxicol 16:31–103
Rowland LA, Bal NC, Periasamy M (2015) The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev 90:1279–1297
Royston JP (1982) Basal body temperature, ovulation and the risk of conception, with special reference to the lifetimes of sperm and egg. Biometrics 38:397–406
RStudio Team (2015) RStudio: integrated development for R. R Studio Inc., Boston
Rushmore J, Caillaud D, Matamba L, Stumpf RM, Borgatti SP, Altizer S (2013) Social network analysis of wild chimpanzees provides insights for predicting infectious disease risk. J Anim Ecol 82:976–986
Sehgal A, Dubey NK, Jyothi MC, Jain S (2002) Comparison of tympanic and rectal temperature in febrile patients. Indian J Pediatr 69:305–308
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L (2011) Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging 3:374–379
Sobolewski ME, Brown JL, Mitani JC (2013) Female parity, male aggression, and the challenge hypothesis in wild chimpanzees. Primates 54:81–88
Stewart JJ, Brown RD, Rowell JP, Wilson JT (1998) Correlation of continuously recorded rectal and axillary temperatures with tympanic membrane temperature in children. Pediatr Res 43:120 (abstract)
Stolwijk AM, Straatman H, Zielhuis GA (1999) Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health 53:235–238
Sund-Levander M, Forsberg C, Wahren LK (2002) Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci 16:122–128
Surbeck M, Deschner T, Behringer V, Hohmann G (2015) Urinary C-peptide levels in male bonobos (Pan paniscus) are related to party size and rank but not to mate competition. Horm Behav 71:22–30
Takemoto H (2004) Seasonal change in terrestriality of chimpanzees in relation to microclimate in the tropical forest. Am J Phys Anthropol 124:81–92
Telford S, Webb P (1998) The energetic cost of copulation in a polygynandrous millipede. J Exp Biol 201:1847–1849
Thompson CL, Williams SH, Glander KE, Teaford MF, Vinyard CJ (2014) Body temperature and thermal environment in a generalized arboreal anthropoid, wild mantled howling monkeys (Alouatta palliata). Am J Phys Anthropol 154:1–10
Thompson CL, Scheidel C, Glander KE, Williams SH, Vinyard CJ (2017) An assessment of skin temperature gradients in a tropical primate using infrared thermography and subcutaneous implants. J Therm Biol 63:49–57
Torrao NA, Hetem RS, Meyer LCR, Fick LG (2011) Assessment of the use of temperature-sensitive microchips to determine core body temperature in goats. Vet Rec 168:328
Trapletti A, Hornik K (2015) Tseries: time series analysis and computational finance. R package version 0.10–34, https://cran.r-project.org/web/packages/tseries/
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine-Atherton, Chicago, pp 1871–1971
Ungerfeld R, Fila D (2012) Testicular fluid content and scrotal surface temperature increase with rams’ sexual activity. Reprod Domest Anim 47:e56–e58
van Ooijen AMJ, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR (2004) Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav 82:545–553
Verbeek NAM (1988) Development of a stable body temperature and growth rates in nestlings of three ground nesting passerines in alpine tundra. J Ornithol 129:449–456
Wacker CB, Daniella Rojas A, Geiser F (2012) The use of small subcutaneous transponders for quantifying thermal biology and torpor in small mammals. J Therm Biol 37:250–254
Watts DP (2018) Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 155:969–1009
Wessling EG, Deschner T, Mundry R, Pruetz JD, Wittig RM, Kühl HS (2018a) Seasonal variation in physiology challenges the notion of chimpanzees (Pan troglodytes verus) as a forest-adapted species. Front Ecol Evol 6:60
Wessling EG, Kühl HS, Mundry R, Deschner T, Pruetz JD (2018b) The costs of living at the edge: seasonal stress in wild savanna-dwelling chimpanzees. J Hum Evol 121:1–11
West PM, Packer C (2002) Sexual selection, temperature, and the lion’s mane. Science 297:1339–1343
Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P (2002) Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr 56:288–296
Whitehead H (2009) SOCPROG programs: analysing animal social structures. Behav Ecol Sociobiol 63:765–778
Wilk MB, Gnanadesikan R (1968) Probability plotting methods for the analysis of data. Biometrika 55:1–17
Wilson RP, McMahon CR (2006) Measuring devices on wild animals: what constitutes acceptable practice? Front Ecol Environ 4:147–154
Wood BM, Watts DP, Mitani JC, Langergraber KE (2017) Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J Hum Evol 105:41–56
Wooden KM, Walsberg GE (2002) Effect of environmental temperature on body temperature and metabolic heat production in a heterothermic rodent, Spermophilus tereticaudus. J Exp Biol 205:2099–2105
Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE (2009) Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim Behav 77:873–885
Zihlman AL, Bolter DR (2015) Body composition in Pan paniscus compared with Homo sapiens has implications for changes during human evolution. P Natl Acad Sci USA 112:7466–7471
Acknowledgments
We thank the Uganda Wildlife Authority, Uganda National Council for Science and Technology, and Makerere University Biological Field Station for permission to work in Kibale National Park. Thanks to David Watts and John Mitani for establishing and maintaining long-term chimpanzee research at Ngogo. We are indebted to Chris Aliganyira, Natasha Bartolotta, Charles Birungi, Rebecca Davenport, Brian Kamugyisha, Godfrey Mbabazi, Lawrence Ndangizi, Alfred Tumusiime, Ambrose Twineomujuni, and David Watts for help with data collection. For logistical and/or analytical support, we thank Sam Angedakin, Charles Businge, Cheryl Knott, Roger Mundry, Rachna Reddy, and Carol Rowney. We also thank two anonymous reviewers and David Watts for constructive comments on the manuscript.
Funding
JDN was supported by the National Science Foundation (Award no. 1613393), National Geographic Society (Award no. 9824-15), Nacey Maggioncalda Foundation, and Boston University. AAS was supported by the National Science Foundation (Award no. 1540259), University of Michigan, and Arizona State University. KEL was supported by the National Institutes of Health Award 5R01AG049395 through the National Institute on Aging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the use of animals were followed. Noninvasive data collection from chimpanzees received a review exemption from Boston University’s Institutional Animal Care and Use Committee. Similarly, this study did not require approval from Boston University’s Institutional Review Board.
Informed consent
Although this study was not considered human subjects research by Boston University’s Institutional Review Board, human participants provided written consent.
Additional information
Communicated by D. P. Watts
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Negrey, J.D., Sandel, A.A. & Langergraber, K.E. Dominance rank and the presence of sexually receptive females predict feces-measured body temperature in male chimpanzees. Behav Ecol Sociobiol 74, 5 (2020). https://doi.org/10.1007/s00265-019-2788-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2788-3