Abstract
Understanding how individual behavior shapes the structure and ecology of populations is key to species conservation and management. Like many elasmobranchs, manta rays are highly mobile and wide-ranging species threatened by anthropogenic impacts. In shallow water environments, these pelagic rays often form groups and perform several apparently socially mediated behaviors. Group structures may result from active choices of individual rays to interact or passive processes. Social behavior is known to affect spatial ecology in other elasmobranchs, but this is the first study providing quantitative evidence for structured social relationships in manta rays. To construct social networks, we collected data from more than 500 groups of reef manta rays (Mobula alfredi) over 5 years in the Raja Ampat Regency of West Papua. We used generalized affiliation indices to isolate social preferences from non-social associations, the first study on elasmobranchs to use this method. Longer lasting social preferences were detected mostly between female rays. We detected assortment of social relations by phenotype and variation in social strategies, with the overall social network divided into two main communities. Overall network structure was characteristic of a dynamic fission-fusion society, with differentiated relationships linked to strong fidelity to cleaning station sites. Our results suggest that fine-scale conservation measures will be useful in protecting social groups of M. alfredi in their natural habitats and that a more complete understanding of the social nature of manta rays will help predict population responses to anthropogenic pressures, such as increasing disturbance from dive tourism.
Significance statement
In social animals, relationships between individuals have important implications for species conservation. Like many other sharks and rays, manta rays are threatened species, and little is known about their natural behavior or how their populations are structured. This study provides evidence of social structure in a wild, free-ranging population of reef manta rays. We show for the first time that individual manta rays have preferred relationships with others that are maintained over time, and structured societies. This study extends our knowledge of elasmobranch ecology and population structuring. Results suggest that understanding social relationships in manta rays will be important in protecting populations from human impacts and developing sustainable, localized conservation and management initiatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of how individual behavior drives population structure and dynamics is required to predict the response of populations to human impacts (Sutherland 1998; Sih 2013). In group-living species, social interactions are a fundamental part of population ecology (Hinde 1976) important in enabling collective behaviors (Couzin et al. 2002; Couzin and Krause 2003; Sumpter 2006), such as cooperative foraging (Sih et al. 2009), predator avoidance (Ward et al. 2011), and social learning (Brown et al. 2011). Social interactions directly affect key ecological and evolutionary processes such as disease transmission, habitat use, and genetic exchange (Kurvers et al. 2014). Social animals are often able to modify their behavior depending on the status of their relationship with various social partners (Krause and Ruxton 2002). Social preferences between individuals may have profound effects on movement decisions that lead to the formation of structured social groups (Bode et al. 2011). Understanding this structure can aid conservation approaches by explaining individual behavior in the context of a population’s social environment (Berger-Tal et al. 2011; Krause et al. 2014; Snijders et al. 2017). Social heterogeneity tends to produce organization of animal societies into units that respond differently to environmental conditions, such as in their foraging success (Whitehead and Rendell 2004). This is likely to cause stratification in survival and reproductive success of group members, so it can be misleading to assess population dynamics without considering the impact of this structure (Lusseau et al. 2006). Social network analysis may be used to describe and quantify social structure (Croft et al. 2008) that may be particularly useful for populations in which the existence of social relationships between individuals is not immediately evident, such as in fission-fusion societies (Snijders et al. 2017).
Despite wide literature on social structuring in terrestrial vertebrates and marine mammals (e.g., Baird and Whitehead 2000; Gero et al. 2005; Lusseau et al. 2006; Wolf et al. 2007; Foster et al. 2012), there are few equivalent studies on marine fish. These are particularly lacking for elasmobranchs, despite their high potential for socially structured populations (Jacoby et al. 2010). Sharks and rays are often thought to be solitary creatures, but many species across elasmobranch phylogeny are found in groups or loose aggregations (for review see Jacoby et al. 2012). Where individuals vary in their movements and habitat preferences, some are likely to interact more than others by chance. Group formation via passive processes occurs in elasmobranchs during feeding aggregations (e.g., Heyman et al. 2001) and seasonal migrations (e.g., Heupel and Simpfendorfer 2005; Bass et al. 2017). Many elasmobranchs have developmental shifts in habitat and diet (Wetherbee et al. 2004) that may drive assortment in size- or sex-segregated groups (Wearmouth and Sims 2008). Recently, ex situ studies have shown that some species exhibit complex sociality, including social structure (Jacoby et al. 2010; Mourier et al. 2012), social learning (Guttridge et al. 2013; Thonhauser et al. 2013), and individual personalities (Jacoby et al. 2014; Byrnes et al. 2016). Due to the difficulty in observing multiple interactions between wild elasmobranchs, however, quantitative analysis of the importance of social relations to the structure of their populations is lacking (but see Guttridge et al. 2011; Mourier et al. 2012). It is usually a considerable challenge to disentangle passive aggregation driven by external forces from active social preferences.
Manta rays (Mobula spp.) are excellent candidates for studies on elasmobranch sociality, including social preferences. Individuals can often be easily observed and accurately identified in the wild. Mobulid rays have the largest brains relative to body size of all elasmobranchs (Lisney et al. 2008), with a highly developed central nucleus that has been linked to social intelligence and formation of hierarchical social structures (Ari 2011). Social recognition may be important in mate choice (Marshall and Bennett 2010). Manta rays perform group-based behaviors including collective foraging, following, breaching, copying, play, and curiosity towards humans (Marshall 2008; Deakos 2010; Gadig and Neto 2014; RJYP pers. obs.), which are associated with social functions and reminiscent of highly social marine mammals (Bradbury 1986).
Globally, both species of manta ray (M. alfredi and M. birostris) are considered vulnerable to extinction (Marshall et al. 2018a, b) due to evidence for recent, large-scale population declines in several regions (e.g., Rohner et al. 2017). Populations are extremely vulnerable to overfishing, among other threats such as ocean pollution, climate change and bycatch (Marshall et al. 2011; Lawson et al. 2017; Stewart et al. 2018), exacerbated by their extremely low reproductive output (Dulvy et al. 2014; Stevens 2016), and high mobility (Germanov and Marshall 2014; Jaine et al. 2014). Populations inhabit subtropical waters, typically those of developing nations where funding for conservation or policing initiatives is scarce, and are unlikely to receive adequate protection from small marine reserves. Indonesia is a globally significant area for both species, having some of the largest identified populations of manta rays in the world (Marshall and Holmberg 2019). Despite receiving protection throughout Indonesian waters in 2014 (Lawson et al. 2017), fishers continue to exploit mobulid rays with impunity, impacting local populations (Couturier et al. 2012; Lewis et al. 2015; Croll et al. 2016). Manta rays are an important attraction in dive tourism (O’Malley et al. 2013; Venables et al. 2016a), and unrestricted growth of this industry may cause disturbance at known aggregation sites (Anderson et al. 2011; Venables et al. 2016b). Understanding the nature of manta ray group and social structuring will aid the implementation of measures to mitigate any negative impacts of dive tourism in these areas.
Research on manta rays to date has focused mainly on broad population demographic and ecological studies (e.g., Marshall and Bennett 2010; Deakos et al. 2011; Kashiwagi et al. 2011; Marshall et al. 2011; Jaine et al. 2012; Couturier et al. 2014), as well as individual-based movement tracking and behavioral studies (e.g., Dewar et al. 2008; Jaine et al. 2014; Stewart et al. 2016a; Ari and D’Agostino 2016). While these provide comprehensive baseline data for management of manta rays, considering social structure will aid a more nuanced approach, where the behavior of individuals is linked to group- or population-level responses to the environment (Bowler and Benton 2005). Recent studies have shown that individuals within shark populations exhibit large differences in movements, feeding behavior and personality (Jacoby et al. 2014; Matich and Heithaus 2015; Finger et al. 2016, 2017), suggesting that network analyses may be vital to provide reliable data for population ecology and conservation. Though several studies have provided anecdotal evidence of social behavior in manta rays (Deakos 2010; Stevens 2016; Stewart et al. 2016b, 2018), this is the first study to provide a quantitative description of their social organization. Our aims were to describe the temporal and spatial structure of social relations and determine whether manta rays had genuine social preferences (caused by active choice of individuals to interact) by controlling for non-social structural factors, including location, time, phenotype, and individual gregariousness. We expected to find heterogeneity in social relations, as in most social species (Foster et al. 2012). Based on previous knowledge of manta ray ecology and habitat use, we predicted that location fidelity would be an important driver of association and that individuals would have differentiated social strategies. We expected that assortment by phenotype, including sex, maturity, color morph, and reproductive status would be important in structuring the society, potentially enabling the division of the population into distinct social communities.
Methods
Sampling procedure
Data on reef manta ray group compositions were collected from November 2013 to May 2018 in the Dampier Strait region of Raja Ampat, West Papua, by trained researchers diving using SCUBA equipment, or freediving, depending on the position of rays in the water column. Where exact times and locations could be verified, some records (approx. 10% of all data) were obtained by photographic uploads to “MantaMatcher.org,” an online citizen-science based catalog (Marshall and Holmberg 2019). It was not possible to record data blind because our study involved observing animals in the field. Sightings of reef manta rays at five sites, including three cleaning stations: Manta Sandy (MS), Manta Ridge (MR) and Rob's Secret Bommie (RSB), and two feeding areas: East Study Area (ESA) and West Study Area (WSA) within a 20-km2 area were recorded to allow analysis of fine-scale social structure, with data collected from an additional 5km2 area: Pulau Wai (PW) that was used by manta rays for both feeding and cleaning behaviors. Sampling occasions were dives or snorkels of approx. one hour, at one of these sites, restricted to one sampling occasion at each site per day. The total area covered during a single dive or snorkel was approximately 0.5–1 km2. We alternated sampling effort by site and time to minimize environmental bias, using variables expected to influence manta ray behavior (location, tidal phase, tidal range, time, and lunar phase) (Jaine et al. 2012). See Appendix Section 1 for details of study area (Appendix Fig. 9) and sampling effort (Appendix Table 4).
Recording individual encounters
Individual reef manta rays were identified by standard photo-ID methods (see Fig. 1), using unique, lifelong spot patterns on the ventral surface (Pierce et al. 2018). Rays were sexed by the presence/absence of claspers, and maturity and reproductive status/sexual activity were estimated as in Marshall and Bennett (2010) using evidence from female pregnancies and mating scars, and male clasper size/calcification. Disc width (DW) was estimated by visual comparison of manta rays to coral structures of known size. Based on 55 individual females of known maturity, size-at-maturity in the population was estimated to be 3–3.5 m DW, similar to populations in Hawaii and Australia (Deakos 2012; Couturier et al. 2014). Where maturity could not be determined using morphological features, females with estimated DW ≥ 3.5 m were considered mature and estimated DW ≤ 3 m immature. Photographic records of each distinct encounter (sighting of an individual) were stored in an online database (www.MantaMatcher.org). For each individual, an “encounter rate” (ER = no. sightings of individual at site, divided by no. sampling occasions at site) was calculated and ranked by site to define individual site preferences. Sex ratios were compared at each study site using exact binomial tests. We constructed logistic mixed effects models using the glmer function of the lme4 package (Bates et al. 2014) in R version 3.4.4 (R Core Team 2018) to compare the probability of encounter of different phenotypes (sex, maturity, color morph) at cleaning stations/feeding sites, and at individual sites, using presence/absence of individuals during a sampling occasion as the dependent variable, site and phenotype as fixed effects, and individual ID as a random effect. We used deviation coding to compare probability of encounter to a grand mean over all sites (see Appendix Section 3, Table 5).
Identification of reef manta rays: (1a) female typical morph with distinct ventral spot pattern; (1b) mating scars on female indicating maturity; (2a) male typical morph, with claspers; (2b) juvenile male typical morph, with undeveloped claspers; (3) melanistic morph with distinct white patches between gills; (4) pregnant female
Defining associations
Associations between individuals were defined using the “Gambit of the Group” (GoG) (Whitehead and Dufault 1999), which assumes all individuals observed together are associated, without necessarily interacting socially. This is appropriate where individuals move between groups (Franks et al. 2010), and where direct interactions are difficult to observe regularly, but groups can easily be defined and have meaningful structure (Farine and Whitehead 2015). Each dive was considered an independent sampling occasion (Whitehead 2008a), and all individuals observed during a dive were considered as part of the same group if a gap of < 10 min between encounters occurred (this addressed difficulty in observing a highly mobile species with restricted visibility underwater). In practice, we were confident that observed associations gave an accurate representation of true structure, because groups were spatio-temporally well-defined, and it was usually possible to record the identity of all individuals seen.
Data were recorded in a group by individual binary matrix with rows representing each sampling occasion and columns representing individuals. Network analyses were performed in R, using the asnipe (Farine 2017a), igraph (Csardi and Nepusz 2006), and tnet (Opsahl et al. 2010) packages. Network diagrams were drawn in Gephi 0.9.2 (Bastian et al. 2009). We calculated simple-ratio indices (SRIs) (Cairns and Schwager 1987) to measure strength of association between all pairs. The SRI is the recommended association index (AI) where calibration data are unavailable (Hoppitt and Farine 2018). SRIs were calculated within 45 sampling periods (SPs) of length 15 days. This length was chosen according to results from lagged association rate (LAR) analysis (see “Stability of identifications and associations over time”), and prior knowledge of the species’ movements (e.g., Marshall 2008; Deakos 2012), to be short enough that individuals were likely to remain in the area, but long enough to allow sufficient opportunity for swaps between groups required for independence of observations. We identified 112 individuals ≥ 10 times. All individuals observed < 10 times were removed from subsequent network analyses, because various studies suggest that prioritizing edge accuracy is preferable to including a large proportion of the population (Whitehead 2008b; Franks et al. 2010). We calculated social differentiation (S): the variability of the “true” AIs estimated using maximum likelihood approximation (Whitehead 2008a). Values of S close to 0 indicate homogenous relationships within the population, while values near or greater than 1 indicate highly varied relationships. To determine the accuracy of AIs and their power in testing for social relationships, we calculated the correlation coefficient r, between S and the observed (measured) AIs (Whitehead 2009) as follows:\( r=\frac{S}{\mathrm{CV}\left({\mathrm{SRI}}_{ab}\right)} \). Sufficient statistical power to test for preferred or avoided associations was accepted when S2 × H > 5, (where H is the mean no. identifications per individual) (Whitehead 2008b). Standard errors for S and r were estimated using 100 bootstrap replicates of the observed data.
Stability of identifications and associations over time
We calculated lagged identification rates (LIRs) (see Appendix Section 3) and LARs (Fig. 5) to describe changes in the presence of individuals in the study area and their relationships over time (Whitehead 1995). For these analyses, we used sampling periods of 1 day. We used LAR rather than standardized LAR because we were confident of identifying most individuals within groups. We calculated three LARs: for all individuals, between females only, and between males only. Due to large time gaps between study seasons, a maximum time lag of 180 days was used to restrict LARs to within a single study season. We used a moving average (A) over the possible no. of associations (p), multiplied by 0.25 (Ap0.25), to smooth the line (Ap0.25 (all individuals) = 3630, Ap0.25 (females) = 1208, Ap0.25 (males) = 431). We fit models describing different potential aspects of relationships within animal societies (see Appendix Section 4) and compared LAR to a null association rate (NAR—the expected rate if associations in the population were randomly distributed). Standard errors were obtained using jackknife resampling (Whitehead 1995). All LIR and LAR analyses were run using SOCPROG 2.7 (Whitehead 2009). The most parsimonious LAR model was selected using the quasi-Akaike Information Criterion (QAICc) (Burnham and Anderson 2004; Whitehead 2007).
Quantifying social preferences
Social networks derived using AIs may be the result of many inter-related factors, including joint locational preferences or overlap in time (passive grouping with unknown others), individual gregariousness (active choice to form groups with unknown others), as well as social preferences (active choice to form groups with known others). Manta rays in this study had high location fidelity and phenotypic variation in site preferences (see “Site use and encounter rates” and “Structure of associations”). We needed to disentangle non-social factors from the social preferences that we were interested in. Researchers often use location-constrained permutations for this purpose, but these only change P values and do not control for bias in effect sizes, which can lead to spurious conclusions. We therefore used generalized affiliation indices (GAIs) that control for various non-social factors when constructing network weights (Whitehead and James 2015). GAIs in our study were deviance residuals (divided by the denominator of the corresponding SRI value) from a generalized linear model with a binomial error structure and log link function, with SRIs as the dependent variable, and corresponding matrix elements of predictors of pairwise association as independent variables. High positive values for GAIs indicate affiliation (dyads are more associated than expected given the structural predictor variables), and negative values indicate avoidance. GAIs may therefore be considered an estimate of the strength of social preference between pairs, with variation due to non-social factors statistically removed. Predictor variables used in calculation of GAIs were the following: site use similarity—the Euclidean distance between the encounter rate (see “Recording individual encounters”) of each pair at each study site, temporal overlap (custom SRI calculated on whether pairs were observed in the study area within 14 days of each other, within sampling periods of 60 days), gregariousness (based on Godde et al. 2013, joint pairwise gregariousness was calculated as follows: Gab = log(ΣSRIaΣSRIb) where ΣSRIa and ΣSRIb are the sums of all the SRIs for individuals a and b, respectively), sex class (male/female, 1 if same sex, 0 if not), maturity class (adult/juvenile, 1 if same maturity class, 0 if not), and color morph class (typical or melanistic, 1 if same color morph, 0 if not). Multiple regression quadratic assignment procedure (MRQAP) tests (Dekker et al. 2007) were used to identify the relative influences of each predictor variable on associations (see Appendix Section 6, Table 8).
Permutation tests
We tested various hypotheses regarding preferred associations, social preferences, assortment by phenotype, and community structure by comparing observed statistics against equivalent statistical distributions produced by data stream permutations of the observed group by individual matrix (Bejder et al. 1998; Croft et al. 2011). All tests used 1000 permutations of the data, with 100 flips per permutation. P values were calculated by the number of times the randomized statistic was higher than the observed statistic. In all cases, permutations were sufficient for P values to stabilize. Permutation tests for SRIs were conducted on all individuals and for GAIs were conducted on subnetworks of individuals divided by the sex and maturity of individuals, as follows: (1) overall network (all ties between all individuals); (2) female:female—female ties with other females; (3) male:male—male ties with other males; (4) female:male—female ties with males; (5) adult:adult—adult ties with other adults; (6) juvenile:juvenile—juvenile ties with other juveniles; (7) adult:juvenile—adult ties with juveniles. This allowed us to retain variation associated with sex or age differences within GAIs while interpreting differences in social relations between sex and age classes. For each network, we tested the hypothesis that there were more preferred and avoided relationships than expected by chance. Short-term preferred relationships were indicated by a significantly lower than expected mean of all tie weights, long-term preferred relationships indicated by a significantly higher than expected SD of all tie weights, and overall preferred relationships indicated by a significantly higher CV of all tie weights (vice versa for avoided relationships), following Whitehead (2009). We used the same permutation method to find dyadic values that were significantly higher than expected within each network. These were used to build a network of estimated social preferences (Fig. 7).
Assortment by phenotype
We tested for assortment in the reef manta ray population by sex, maturity, and color morph, with the null hypothesis that assortment would be no stronger than expected if relationships were random. To test for assortment while controlling for the structure of the dataset, we compared assortativity coefficients (ACs) calculated on observed SRIs (to check if rays assorted non-socially) and GAI values (to check if social preferences were assorted) to equivalent coefficients calculated from data stream permutations (see “Permutation tests”). ACs were positive if vertices of similar phenotype tended to positively connect, or if vertices of different phenotype tended to negatively connect. ACs were negative if vertices of different phenotype tended to positively connect, or if vertices of similar phenotype tended to negatively connect. GAIs had both negative values (indicating avoidance) and positive values (indicating social preference). Due to the difference in meaning of positive/negative values here, it did not make sense to calculate ACs for all GAI values combined. We therefore tested for assortment among positive and negative GAI values separately.
Community structure
We used the leading.eigenvector.community algorithm in igraph to identify community structure within the overall networks of SRIs/GAIs. This method divided networks successively into clusters, with the most parsimonious network division being that maximized the modularity coefficient, Q (Newman 2006). Data stream permutations (see “Permutation tests”) were used to evaluate whether this value was meaningful. We obtained confidence intervals for Q using the method of Lusseau et al. (2008). We assessed robustness of community assignment using a coefficient of assortativity (Rcom), which directly assessed the degree to which empirical community assignments of nodes agreed with assignments from bootstrap replicates (Shizuka and Farine 2016). We then calculated within-community social differentiation (“Defining associations”) to measure social complexity in the population.
Individual network positions
To investigate social strategies and classify overall network structure, we calculated network metrics for individuals within the overall network of GAIs. This allowed us to test hypotheses that individuals of different phenotypic class (sex, maturity status, color morph) or reproductive status (females observed as pregnant at least once/females never observed as pregnant, and females observed as sexually active/never observed as sexually active) had different average network positions. We used the tnet package (Opsahl et al. 2010) in R to calculate the following metrics: weighted degree (summed weight of all connections for each individual), weighted betweenness centrality (measure of how often an individual is located on the shortest path between two others), and local clustering coefficient (measure of how complete the neighborhood of each individual is). For this analysis, all negative GAI values were treated as zeros, because we were primarily interested in the effect of direct social preferences (positive values), rather than avoidance (negative values). Our measure of weighted betweenness favored shorter paths composed of weaker ties over longer paths with stronger ties (α tuning parameter = 0.5). We compared metrics between phenotypes by calculating the coefficient of the slope of linear models for different levels of each phenotype (Farine 2017b). Empirical slope values were compared to equivalent random values produced via data stream permutations, which provided a null model; P values were obtained as in “Permutation tests.”
Results
Individual identification and group structures
A total of 3411 encounters of 594 M. alfredi individuals were recorded over 512 sampling occasions. The highest number of sightings of a single individual was 57, with 112 individuals observed ≥ 10 times, including 70 females (43 mature, 13 immature, 14 unknown maturity) and 42 males (32 mature, 9 immature, 1 unknown maturity). A declining discovery curve (see Appendix Fig. 10) indicated that most of the total population was recorded at least once. Observed group sizes ranged from 1 to 67 individuals, with most smaller than 20 (mean 6.66 over all sites, 95% CI = 6.03–7.30), median 4 over all sites, 95% CI = 4–5). Feeding sites typically had larger group sizes, but large groups were also observed at cleaning stations (see Appendix Section 2, Fig. 11 for group size and sightings frequency data).
Site use and encounter rates
For individuals observed ≥ 10 times, especially females, encounters were much more likely at that individual’s 1st preference site than any other (Fig. 2). Many individuals were observed multiple times at a single cleaning station, but infrequently or not at all at others (see Appendix Fig. 12), indicating strong site preferences. In general, female rays were more likely to be encountered at cleaning stations than males, while mature males were more likely to be encountered at feeding areas (see Appendix Section 3, Table 5). However, encounter rates at individual cleaning stations MS, MR, and RSB varied considerably between sexes, with different results for each site (Fig. 3). Site MS had a strongly female-biased sex ratio (exact binomial tests Nenc = 1198, 77% female, 95% CIs 74.6–80.1% female, P < 0.001), site MR had no difference from parity (Nenc = 1052, (53% female, CI = 48.9–56.8% female, P = 0.163), and site RSB (Nenc = 321, 40% female, CI 34.2–46.3% female, P = 0.002) had a male-biased sex ratio.
Encounter rates over all study sites by sex (F females, M males), ranked by site preference. The thick black lines represent the medians, the notches represent the 95% confidence interval of the medians, the boxes encompass the interquartile ranges, the whiskers extend to the most extreme data points within 1.5× the interquartile range outside the box, and the circles show data points beyond the whiskers. Note the much higher average values at first preference sites than other sites, indicating strong site fidelity, particularly for females. There was considerable variability in the level of site fidelity between individuals, but not between sexes
Encounter rates at each of the six study sites by sex (F females, M males). The thick black lines represent the medians, the notches represent the 95% confidence interval of the medians, the boxes encompass the interquartile ranges, the whiskers extend to the most extreme data points within 1.5× the interquartile range outside the box, and the circles show data points beyond the whiskers. Note the difference between attendance of males and females at the three cleaning stations (MS, MR and RSB), showing marked differences in site preferences. There were a greater number of zero values at the three feeding areas (WSA, ESA and LDS) due to lower sampling effort there
Structure of associations
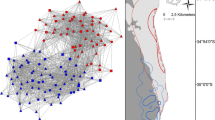
The population had moderate social differentiation (S = 0.574, SE = 0.067), and estimated AIs were a useful representation of the true AIs (r = 0.450, SE = 0.048). We had sufficient power to test the hypothesis that reef manta rays had no preferred or avoided relationships (S2 × H = 5.59). Most pairs that had associated at least once were not strongly associated (70% of recorded associations had an SRI value of ≤ 0.1, and only 3% had a value ≥ 0.2, median non-zero SRI value 0.071). The highest SRI value between any pair of individuals was 0.357. Figure 4 shows the network of associations between individuals in the context of their site preferences, which appear to be an important factor structuring associations. The network was highly connected (56.4% of possible connections realized), but connections among individuals with similar site preferences were more common and typically stronger. Manta rays with preference for site “MS” appeared partly segregated from the rest of the population.
Network of simple ratio indices. Node colors indicate individual site preferences (green: MS, purple: MR, bottle green: RSB, red = ESA, orange = WSA, blue: LDS). Node size scaled by the SD of encounter rates of an individual at each site, indicating level of overall site fidelity. Edge widths represent weight of SRIs (min = 0.118, max = 0.444). Individuals with 8 or more encounters included as nodes (n=163). Only the 30% highest SRI values were included as edges to show strongest associations. ForceAtlas algorithm was used to construct network
Stability of identifications and associations over time
LIRs fell steeply over the first few days but remained stable thereafter for at least a year (see Appendix Section 4, Fig. 13, Table 6), and individuals were much more likely to be re-sighted at the same site than a different site over the full study period (1603 days). The re-identification rate at a different site to initial sighting was low, remaining constant throughout the study period. Identifications at the same location were best described using a model that indicated the occurrence of emigration (including permanent emigration from the study area) while re-identifications at a different location were best described by a model indicating a closed population (Appendix Table 6). LARs (Fig. 5) showed that time was an important influence on group structures. Among all individuals, the LAR declined slowly but gradually over several months. Re-associations between females occurred more frequently than those between males, with overall and female LAR remaining higher than equivalent null rates over several months, whereas male LAR approached the null rate after ~ 55 days. Models of exponential decay fit to the LAR data are shown in Table 7 (Appendix). The best-fit model based on QAICc suggested that preferred relationships were important in structuring relationships between females (and among all individuals), while casual acquaintances were important in structuring relationships between males.
Lagged association rates (LAR) compared to null association rate (NAR) between all individuals, between females and between males. Bars indicate approximate standard errors generated by jackknife resampling. Females dissociated gradually, and LAR did not approach the null rate, whereas males dissociated more rapidly, and LAR approached null rate more frequently. Figure created in SOCPROG
Tests for preferred associations and social preferences
Results of tests for association preferences (co-occurrence in time and space) and social preferences (active decisions to interact) are given in Table 1. Associations are measured by simple ratio indices (SRIs), whereas social preferences are measured by generalized affiliation indices (GAIs). The CV of SRIs was significantly higher (observed mean 1.14, mean of random CVs 1.10, P = 0.001) than expected, indicating that reef manta rays had preferred associations. These preferences were not evenly distributed throughout the full network. Results were similar for associations between females (F:F network), mixed sex (F:M), and mixed maturity (A:J) associations, indicating preferred associations within these networks. Associations between adult rays (A:A) and between juvenile rays (J:J) had CV values that were not significantly higher than expected. Associations between males (M:M), however, had a lower than expected CV, indicating that males did not have preferred associations with other males, and may tend to avoid each other.
Associations between individuals in our study may be highly influenced by non-social factors (see Fig. 4 main text, Appendix Table 8). Our use of generalized affiliation indices (GAIs) controlled for this. GAIs gave similar results to SRIs in some cases, but not all. Generally, we found that social preferences were more common than preferred associations (see Appendix Fig. 14). For all networks, the mean of GAI values was negative, indicating that avoidance between pairs was common, particularly between males and between juveniles (the M:M and J:J GAI networks had the lowest means). The CV of all observed GAIs was significantly higher, and the mean of observed GAI values significantly were lower than expected, indicating that social preferences occurred between all individuals, particularly over short (< 15 days) time periods. All statistics for female:female GAIs (network 2) were significantly (or nearly significantly) different to random expectation, indicating the presence of short and long-term social preferences between female rays. In contrast, for male:male GAIs, only short-term social preferences were significantly stronger than random expectations. There were also a lower percentage of preferred dyadic values between males (4.9%) than between females (8.1%). The highest percentage of preferred dyadic values was between individuals of different sexes (12.6%) (Table 1C), though these appeared to be mainly short-term preferences. Social preferences were not common between adult rays (A:A network). The CV and mean for the J:J and A:J networks indicated that short-term social preferences were stronger than expected between juveniles and between juveniles and mature adults. The percentage of social preferences was similar for all three networks separated by maturity (7.3–9.0%).
Assortment by phenotype
Results for assortment by phenotype are reported in Table 2. Assortativity coefficients (ACs) for SRI values were significantly higher than expected when grouping individuals by sex and maturity, indicating that associations were positively assorted by these phenotypic attributes. There was no evidence for assortment of associations by color morph. For GAI values, the AC was significantly higher than expected (considering only positive GAI values) and significantly lower than expected (considering only negative GAI values) when grouping by sex. This indicated that same-sex pairs tended to have social preferences and did not avoid each other. There was limited or no evidence for assortment of GAIs by maturity or color morph. Figure 7 shows the network of social preferences by sex and maturity. While all individuals are highly connected, there was partial segregation between the sexes.
Community structuring
We found support for subdivision of the observed manta ray society into communities of individuals with stronger in-group relationships. The most parsimonious division of the association (SRI) network (Fig. 4) was into two communities with a Qmax value of 0.168 (95% CIs 0.162-0.257). This indicates that the population had only a weak modular structure, but there was significantly more structure than expected if associations were random (mean of random Qmax values = 0.106, P = 0). Robustness of community assignment (Rcom) for SRIs was 0.580, which is considered reliable evidence for the empirical structure (Shizuka and Farine 2016) (see Fig. 6). Within-community social differentiation was quite different for the two communities. Community 1 (S = 0.393, observed CV = 0.926, correlation = 0.427) had a moderately differentiated social structure, while community 2 (S = 0.093, observed CV = 0.919, correlation = 0.100) had a strongly homogeneous social structure.
Network of community assortativity assignments (based on SRIs) showing how often (represented by edge widths) empirical community assignment of each pair agreed with bootstrap replicate networks. Edges < 0.25 removed. Node sizes indicate maturity status: large = adult, small = juvenile, medium = unknown). Community 1 (white nodes) contained an approximately equal no. females (24) and males (34), but community 2 (black nodes) had a strong female bias (46 females, 8 males). ForceAtlas2 algorithm used to construct network
Network of social preferences (Nedges = 480). Node colors indicate sex (red = female, blue = male). Node size indicates the centrality of the individual (measured by weighted betweenness). Edge widths represent weights of GAI values. Edge colors represent relations between females (red), between males (blue), and mixed sex relations (purple). While all individuals are highly connected, there is clear partitioning of the network by sex. ForceAtlas algorithm used to construct network
Significant differences in network metrics by phenotype, including a weighted degree for adult (A) and juveniles (J), b weighted betweenness for adult (A) and juveniles (J), c weighted betweenness for females observed pregnant (Y) and never observed pregnant (N), d clustering coefficient for females seen (Y) pregnant and never seen (N) pregnant. The thick black lines represent the medians, the notches represent the 95% confidence interval of the medians, the boxes encompass the interquartile ranges, the whiskers extend to the most extreme data points within 1.5× the interquartile range outside the box, and the circles show data points beyond the whiskers
Variability in network positions
Results comparing network metrics of GAIs between phenotypes are presented in Table 3 and Fig. 8. They suggest some variation in social strategies between phenotypic groups and according to reproductive status. Juveniles had significantly higher weighted degree and weighted betweenness than mature adults and were therefore more central in the overall network of GAIs. Females observed to be pregnant at least once during the study had significantly lower weighted betweenness and significantly lower clustering coefficients than females with no observed pregnancies. Mature females may therefore be more segregated from the overall network than other individuals. No other metrics were significant, with similar values for degree, betweenness, and clustering between individuals of different sex, color morph, and for mated and non-mated females.
Discussion
Reef manta rays in the Dampier Strait region of Raja Ampat, West Papua formed a complex and heterogeneously structured society, with non-random associations between individuals that divided the population into two distinct communities. Associations were the result of more than just similarities in habitat use, gregariousness, or overlaps in time, indicating that individuals actively chose to group with preferred social partners. As such, this is the first study to provide quantitative evidence for structured social relationships in manta rays. Such relationships may provide survival benefits across a range of contexts (Frère et al. 2010; Ellis et al. 2017; Kalbitzer et al. 2017). Familiarity and kin recognition over extended time periods (Griffiths and Ward 2011) have been shown to enhance the benefits of group living in fishes through antipredator effects (Chivers et al. 1995), increased foraging efficiency (Swaney et al. 2001), reduction in competition (Frostman and Sherman 2004), release of time budget constraints (Griffiths et al. 2004), and improved social learning (Lachlan et al. 1998). However, it is not yet clear to what extent sharks and rays recognize familiar individuals, including their capability for long-term social recognition (LTSR) of multiple partners and long-term memory of relationship histories.
Our results show that stable, differentiated social relationships lasting over several weeks or months are an important driver of group structures in reef manta rays, which suggests that both familiarity and LTSR are important in structuring their societies. In complex social systems, such capabilities can be essential to identify partners in reciprocal altruism, maintain social hierarchies, and avoid inbreeding (Trivers 1971; Axelrod and Hamilton 1981; Bruck 2013). Simultaneous relationships with multiple partners may be required for social behaviors in manta rays, such as in initiation of mating trains and during collective feeding events. Social preferences were detected mostly between female rays, in mixed sex relations, and between juveniles, with only weak evidence for short-term preferences between males. Time-based analyses suggested that associations between manta rays dissociated gradually over time, but often remained stable over weeks or months (particularly among females). Associations and social preferences were assorted by sex and maturity, and network metrics showed that social relationships were highly differentiated and indicative of varied social strategies. The overall network of observed associations was weakly modular, with two main communities that had quite different structures, one having a mixed sex ratio with differentiated social relations and the other having a highly biased female sex ratio, with homogeneous social structure. Female reef manta rays therefore appear to choose to associate mostly with other females (in more stable groups) or with males (in more dynamic groups). This decision may depend on factors such as age/maturity and reproductive status, as discussed further below. Reef manta rays did not form tight-knit social groups, such as those observed in many dolphin and larger toothed whale populations (Baird and Whitehead 2000; Cantor et al. 2012), although in several aspects our findings were comparable to social network studies on bottlenose dolphins (Tursiops sp.) including a recent study using GAIs (Zanardo et al. 2018). Bottlenose dolphins typically live in open and fluid hierarchical societies with fission-fusion dynamics, LTSR, and a high number of potential affiliates (Lusseau et al. 2003; Gero et al. 2005; Bruck 2013). Social structure in these dolphins is flexible depending on environmental conditions (Lusseau et al. 2003; Karczmarski et al. 2005), enabling efficient flow of information required in foraging and predator avoidance (King and Janik 2015). It is possible that social relationships in reef manta rays have similar structure and functions.
In addition to preferred social relationships, we found that passive aggregation and assortment of individuals with similar phenotypic attributes were important non-social factors influencing network structure. Many rays had strong philopatry to individual cleaning stations, resulting in marked differences in site sex ratios. This was surprising given the close proximity of all sites (Appendix Fig. 9c) and known wide-ranging movements of the species. Fidelity to areas of coastal reef has been described previously in M. alfredi in various locations (Deakos et al. 2011; Marshall et al. 2011; Jaine et al. 2014), including in Raja Ampat (Setyawan et al. 2018), but our study is novel in that it demonstrates that this can occur variably at multiple sites in close proximity (at a finer scale than the daily movements of the species). This result suggests that broad processes such as food availability or habitat quality may not be as important as individually distinct environmental or social preferences in driving manta ray movements and habitat use at fine scales. Associations were closely correlated with individuals’ site preferences. Site fidelity is often a prerequisite for sociality in gregarious animals, creating an environment for social relationships to develop (Wolf et al. 2007) and controlling the emergence of social preferences (Mourier et al. 2012). Time was also an important influence on social organization. Being present in the study at the same time was a strong predictor of association between pairs. Re-sightings were increasingly unlikely only a few days after initial sighting but were much more likely to occur at a previously visited site over long time periods. Rather than having broad area residency (where isolation by distance might explain location fidelity), this suggests that individuals typically stayed in a certain location for hours or days and made frequent movements in and out of the study area, returning to visit preferred sites (i.e., philopatry) over several years. It is likely that many individuals ranged widely throughout a larger area than we could cover in the scope of this study. LAR results suggested that casual acquaintances between rays were as important (or more) than preferred companionships to network structure. M. alfredi are known to travel up to 95 km per day (van Duinkerken 2010; Jaine et al. 2014) and move to deeper waters during the night (Braun et al. 2014). In Raja Ampat (Setyawan et al. 2018) and other locations (Marshall 2008; Dewar et al. 2008), visits to cleaning station sites occur mainly during daylight hours. Social structure in reef manta rays may therefore depend on daily fission-fusion dynamics. A limitation of our study is that associations between rays were only recorded at a few specific locations for short time periods during daylight hours. Preliminary observations via remotely piloted aircraft show that manta rays often follow each other when leaving cleaning stations or feeding areas (RJYP unpublished) and suggest that group structures formed in these areas are maintained outside them. Therefore, the network of associations that we recorded may underestimate true social relationships.
Sex, age, and size-based assortment are common in shark aggregations (Heupel and Simpfendorfer 2005; Wearmouth and Sims 2008; Guttridge et al. 2011), so it was not surprising to detect phenotypic structuring here. Sex ratios at manta ray aggregation sites are often female dominated (Marshall et al. 2011), though here we document a male-dominated site. Assortment may occur without any individual recognition capability, for example, if individuals differ in behavior or motivation, they may spontaneously form closer associations to similar individuals, known as “self-sorting” (Couzin 2006). Social preferences are, however, often important in creating assortative structures in dynamic systems (Croft et al. 2015), and assortative interactions suggestive of active partner preference are reported in a wild elasmobranch (Guttridge et al. 2011). Here we detected sex and maturity-based assortment of GAIs, suggesting that social preferences were a driver of assortative structuring. This could be linked to reef manta rays’ reproductive strategy, which is not yet well described, but appears to be promiscuous (Stevens 2016). In several M. alfredi populations, most non-juvenile male and female manta rays display evidence of reproductive activity, males initiate courtship with multiple females at different times, while females may take part in mating chains with multiple males (Marshall and Bennett 2010; Deakos 2012; Stevens et al. 2018; RJYP unpublished data). A single female manta ray has been observed to mate with two males in close succession (Yano et al. 1999). Sexual conflict in promiscuous systems is common (Parker 2006), and social factors are known to be drivers of sexual segregation in elasmobranchs (Wearmouth et al. 2012). Fish are also known to avoid mating with familiar conspecifics in promiscuous systems (e.g., Simcox et al. 2005), and the use of familiarity is often varied between sexes (e.g., Griffiths and Magurran 1997; Croft et al. 2003). While both sexes may have equal ability to recognize familiar individuals, they may not have equal motivation—for example, males may only behave differently towards familiar individuals in the context of mate choice (Griffiths and Ward 2011). Differences in motivation to be social in manta rays could explain why social preferences were rare between males and why pregnant females were significantly less central and less connected to the overall population than non-pregnant females. Mature females often appeared to dominate cleaning stations and were rarely observed performing cleaning behaviors with mature males. When females (including many pregnant individuals) were alone, they were often pursued by males (RJYP, pers. obs.). Enabling social behavior may be a primary cause of manta ray visitations to cleaning stations, that act as “social gathering points” (Stevens 2016). Hierarchical social organization in these locations could allow mature females to group with preferred social partners and simultaneously avoid unwanted mating attempts by mature males. Familiarity has been shown to reduce aggression among sharks within recently established social hierarchies (Brena et al. 2018). Social gathering points could also facilitate exchange of information (e.g., regarding the distribution of ephemeral food patches) in species which appear to lack the ability to communicate over medium-long distances, for example, breaching may be used as a social signal of food availability (Stevens 2016). Some elasmobranchs use body positioning and fin movements in gestural communication (Martin 2007; Sperone et al. 2012), and this may occur in reef manta rays (Stewart et al. 2016b; RJYP unpublished). Research into the communicative capabilities of manta rays is warranted.
Our study provides the first evidence for structured social relationships in manta rays and suggests that detailed information on their social organization (including structure, dynamics, and social preferences) will help to understand their natural behaviors and response to human and environmental impacts. Social preferences may lead to formation of distinct social units that are differentially at risk of disturbance (Jacoby et al. 2012). Social structures may be adapted to current selective environments, so rapid environmental changes may have severe consequences in disrupting demographically important social processes, influencing population genetic and demographic structure. Species that occur in small, isolated populations, with a low rate of reproduction, and a high reliance on social interactions are likely to be vulnerable to sudden population crashes due to changes in social structure (Snijders et al. 2017). We recommend long-term monitoring of manta rays in the Raja Ampat marine park to understand the effects of dive tourism, including increases in boating and SCUBA diving activities, which may cause displacement from certain locations and changes to social and reproductive behaviors. Knowledge on social interactions and fine-scale site fidelity in manta rays may be used to prioritize the protection of key sites and develop guidelines for sustainable ecotourism. It is important, however, to stress that fine-scale monitoring and protection within small MPAs are not likely to protect these highly mobile species from target fisheries, bycatch, environmental change, or ocean pollution, which are the major global dangers that manta rays face (Marshall et al. 2018a, b). In the light of these more nefarious threats, network-based studies that link movements and behavior to population ecology are required. These might combine social information with animal tracking technology (Wilson et al. 2015; Jacoby et al. 2016) or information on genetic relatedness (Frère et al. 2010); use temporal networks to investigate social stability and assortativity in the context of a changing environment (Blonder et al. 2012); determine network resilience to removal of individuals (Williams and Lusseau 2006; Mourier et al. 2017); link habitat connectivity to social connectivity (Snijders et al. 2017); or model disease, information, and gene flow using a network approach (Hamede et al. 2009). Such studies will improve our understanding of the ecology and evolution of mobulid rays and other elasmobranchs and help to provide a more holistic approach to their conservation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request. Photographs of each encounter are available in the MantaMatcher online repository www.mantamatcher.org
References
Anderson RC, Shiham AM, Kitchen-Wheeler A-M, Stevens G (2011) Extent and economic value of manta ray watching in Maldives. Tour Mar Environ 7:15–27. https://doi.org/10.3727/154427310X12826772784793
Ari C (2011) Encephalization and brain organization of mobulid rays (Myliobatiformes, Elasmobranchii) with ecological perspectives. Open Anat J 3:1–13. https://doi.org/10.2174/1877609401103010001
Ari C, D’Agostino DP (2016) Contingency checking and self-directed behaviors in giant manta rays: Do elasmobranchs have self-awareness? J Eth 34 (2):167-174. https://doi.org/10.1007/s10164-016-0462-z
Axelrod R, Hamilton WD (1981) The evolution of cooperation. Science 211:1390–1396. https://doi.org/10.1126/science.7466396
Baird RW, Whitehead H (2000) Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can J Zool 78:2096–2105. https://doi.org/10.1139/z00-155
Bass NC, Mourier J, Knott NA, Day J, Guttridge T, Brown C (2017) Long-term migration patterns and bisexual philopatry in a benthic shark species. Mar Freshw Res 68:1414–1421. https://doi.org/10.1071/MF16122
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. Accessed October 18, 2018, from https://gephi.org/
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bejder L, Fletcher D, Bräger S (1998) A method for testing association patterns of social animals. Anim Behav 56:719–725. https://doi.org/10.1006/anbe.1998.0802
Berger-Tal O, Polak T, Oron A, Lubin Y, Kotler BP, Saltz D (2011) Integrating animal behavior and conservation biology: a conceptual framework. Behav Ecol 22:236–239. https://doi.org/10.1093/beheco/arq224
Blonder B, Wey TW, Dornhaus A, James R, Sih A (2012) Temporal dynamics and network analysis. Methods Ecol Evol 3:958–972. https://doi.org/10.1111/j.2041-210X.2012.00236.x
Bode NWF, Wood AJ, Franks DW (2011) The impact of social networks on animal collective motion. Anim Behav 82:29–38. https://doi.org/10.1016/j.anbehav.2011.04.011
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225. https://doi.org/10.1017/S1464793104006645
Bradbury JW (1986) Social complexity and cooperation behavior in delphinids. In: Schusterman RJ, Thomas JA, Wood FG (eds) Dolphin cognition and behavior: a comparative approach. Erlbaum, Hilsdale, pp 361–372
Braun CD, Skomal GB, Thorrold SR, Berumen ML (2014) Diving behavior of the reef manta ray links coral reefs with adjacent deep pelagic habitats. PLoS One 9:e88170. https://doi.org/10.1371/journal.pone.0088170
Brena PF, Mourier J, Planes S, Clua EE (2018) Concede or clash? Solitary sharks competing for food assess rivals to decide. Proc R Soc B 285:2018006. https://doi.org/10.1098/rspb.2018.0006
Brown C, Laland K, Krause J (eds) (2011) Fish cognition and behavior. Wiley, Chichester. https://doi.org/10.1002/9781444342536
Bruck JN (2013) Decades-long social memory in bottlenose dolphins. Proc R Soc B 280:20131726. https://doi.org/10.1098/rspb.2013.1726
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Socio Meth Res 33(2), 261-304. https://doi.org/10.1177/0049124104268644
Byrnes EE, Pouca CV, Chambers SL, Brown C (2016) Into the wild: developing field tests to examine the link between elasmobranch personality and laterality. Behaviour 153:1777–1793. https://doi.org/10.1163/1568539X-00003373
Cairns SJ, Schwager SJ (1987) A comparison of association indices. Anim Behav 35:1454–1469. https://doi.org/10.1016/S0003-3472(87)80018-0
Cantor M, Wedekin LL, Guimaraes PR, Daura-Jorge FG, Rossi-Santos MR, Simoes-Lopes PC (2012) Disentangling social networks from spatiotemporal dynamics: the temporal structure of a dolphin society. Anim Behav 84:641–651. https://doi.org/10.1016/j.anbehav.2012.06.019
Chivers DP, Brown GE, Smith RJF (1995) Familiarity and shoal cohesion in fathead minnows (Pimephales promelas): implications for antipredator behaviour. Can J Zool 73:955–960. https://doi.org/10.1139/z95-111
Couturier LI, Marshall AD, Jaine FRA, Kashiwagi T, Pierce SJ, Townsend KA, Richardson AJ (2012) Biology, ecology and conservation of the Mobulidae. J Fish Biol 80:1075–1119. https://doi.org/10.1111/j.1095-8649.2012.03264.x
Couturier LI, Dudgeon CL, Pollock KH, Jaine FRA, Bennett MB, Townsend KA, Richardson AJ (2014) Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs 33:329–342. https://doi.org/10.1007/s00338-014-1126-5
Couzin ID (2006) Behavioral ecology: social organization in fission–fusion societies. Curr Biol 16:169–171. https://doi.org/10.1016/j.cub.2006.02.042
Couzin ID, Krause J (2003) Self-organization and collective behavior in vertebrates. Adv Study Behav 32:1–75. https://doi.org/10.1016/S0065-3454(03)01001-5
Couzin ID, Krause J, James R, Ruxton GD, Franks NR (2002) Collective memory and spatial sorting in animal groups. J Theor Biol 218:1–11. https://doi.org/10.1006/jtbi.2002.3065
Croft DP, Arrowsmith BJ, Bielby J, Skinner K, White E, Couzin ID, Magurran AE, Ramnarine I, Krause J (2003) Mechanisms underlying shoal composition in the Trinidadian guppy, Poecilia reticulata. Oikos 100:429–438
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press, Princeton. https://doi.org/10.1515/9781400837762
Croft DP, Madden JR, Franks DW, James R (2011) Hypothesis testing in animal social networks. Trends Ecol Evol 26:502–507. https://doi.org/10.1016/j.tree.2011.05.012
Croft DP, Edenbrow M, Darden SK (2015) Assortment in social networks and the evolution of cooperation. In: Krause J, James AJ, Franks D, Croft DP (eds) Animal social networks. Oxford University Press, Oxford, pp 13–23. https://doi.org/10.1093/acprof:oso/9780199679041.003.0003
Croll DA, Dewar H, Dulvy NK, Fernando D, Francis MP, Galván-Magaña F, Hall M, Heinrichs S, Marshall A, Mccauley D, Newton KM, Notarbartolo-di-Sciara G, O'Malley M, O'Sullivan J, Poortvliet M, Roman M, Stevens G, Tershy BR, White WT (2016) Vulnerabilities and fisheries impacts: the uncertain future of manta and devil rays. Aquat Conserv Mar Freshwat Ecosyst 26:562–575. https://doi.org/10.1002/aqc.2591
Csardi G, Nepusz T (2006) The igraph software package for complex network research. Int J Complex Syst 1695:1–9
Deakos MH (2010) Ecology and social behavior of a resident manta ray (Manta alfredi) population off Maui, Hawai'i. PhD thesis, University of Hawai’i at Mānoa, http://www.hamerinhawaii.org/wp-content/uploads/DeakosDissertation2010.pdf
Deakos MH (2012) The reproductive ecology of resident manta rays (Manta alfredi) off Maui, Hawaii, with an emphasis on body size. Environ Biol Fish 94:443–456. https://doi.org/10.1007/s10641-011-9953-5
Deakos MH, Baker JD, Bejder L (2011) Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Mar Ecol Prog Ser 429:245–260. https://doi.org/10.3354/meps09085
Dekker D, Krackhardt D, Snijders TA (2007) Sensitivity of MRQAP tests to collinearity and autocorrelation conditions. Psychometrika 72:563–581. https://doi.org/10.1007/s11336-007-9016-1
Dewar H, Mous P, Domeier M, Muljadi A, Pet J, Whitty J (2008) Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar Biol 155:121–133. https://doi.org/10.1007/s00227-008-0988-x
Duinkerken DI (2010) Movements and site fidelity of the reef manta ray, Manta alfredi, along the coast of southern Mozambique. MSc thesis, Utrecht University, The Netherlands
Dulvy NK, Pardo SA, Simpfendorfer CA, Carlson JK (2014) Diagnosing the dangerous demography of manta rays using life history theory. PeerJ 2:e400. https://doi.org/10.7717/peerj.400
Ellis S, Franks DW, Nattrass S, Cant MA, Weiss MN, Giles D, Balcomb KC, Croft DP (2017) Mortality risk and social network position in resident killer whales: sex differences and the importance of resource abundance. Proc R Soc B 284:20171313. https://doi.org/10.1098/rspb.2017.1313
Farine DR (2017a) asnipe: animal social network inference and permutations for ecologists. R package version 1.1.4, https://CRAN.R-project.org/package=asnipe
Farine DR (2017b) A guide to null models for animal social network analysis. Methods Ecol Evol 8:1309–1320. https://doi.org/10.1111/2041-210X.12772
Farine DR, Whitehead H (2015) Constructing, conducting and interpreting animal social network analysis. J Anim Ecol 84:1144–1163. https://doi.org/10.1111/1365-2656.12418
Finger JS, Dhellemmes F, Guttridge TL, Kurvers RH, Gruber SH, Krause J (2016) Rate of movement of juvenile lemon sharks in a novel open field, are we measuring activity or reaction to novelty? Anim Behav 116:75–82. https://doi.org/10.1016/j.anbehav.2016.03.032
Finger JS, Dhellemmes F, Guttridge TL (2017) Personality in elasmobranchs with a focus on sharks: early evidence, challenges, and future directions. In: Vonk J, Weiss A, Kuczaj SA (eds) Personality in nonhuman animals. Springer, Cham, pp 129–152. https://doi.org/10.1007/978-3-319-59300-5_7
Foster EA, Franks DW, Morrell LJ, Balcomb KC, Parsons KM, Van Ginneken A, Croft DP (2012) Social network correlates of food availability in an endangered population of killer whales (Orcinus orca). Anim Behav 83:731–736. https://doi.org/10.1016/j.anbehav.2011.12.021
Franks DW, Ruxton GD, James R (2010) Sampling animal association networks with the gambit of the group. Behav Ecol Sociobiol 64:493–503. https://doi.org/10.1007/s00265-009-0865-8
Frère CH, Krützen M, Mann J, Connor RC, Bejder L, Sherwin WB (2010) Social and genetic interactions drive fitness variation in a free-living dolphin population. Proc Natl Acad Sci U S A 107:19949–19954. https://doi.org/10.1073/pnas.1007997107
Frostman P, Sherman PT (2004) Behavioral response to familiar and unfamiliar neighbors in a territorial cichlid, Neolamprologus pulcher. Ichthyol Res 51:283–285. https://doi.org/10.1007/s10228-004-0223-9
Gadig OBF, Neto DG (2014) Notes on the feeding behaviour and swimming pattern of Manta alfredi (Chondrichthyes, Mobulidae) in the Red Sea. Acta Ethol 17:119–122. https://doi.org/10.1007/s10211-013-0165-1
Germanov ES, Marshall AD (2014) Running the gauntlet: regional movement patterns of Manta alfredi through a complex of parks and fisheries. PLoS One 9:e110071. https://doi.org/10.1371/journal.pone.0110071
Gero S, Bejder L, Whitehead H, Mann J, Connor RC (2005) Behaviourally specific preferred associations in bottlenose dolphins, Tursiops spp. Can J Zool 83:1566–1573. https://doi.org/10.1139/z05-155
Godde S, Humbert L, Côté SD, Réale D, Whitehead H (2013) Correcting for the impact of gregariousness in social network analyses. Anim Behav 85:553–558. https://doi.org/10.1016/j.anbehav.2012.12.010
Griffiths SW, Magurran AE (1997) Familiarity in schooling fish: how long does it take to acquire? Anim Behav 53:945–949. https://doi.org/10.1006/anbe.1996.0315
Griffiths SW, Ward A (2011) Social recognition of conspecifics. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior, 2nd edn. Wiley-Blackwell, Ames, pp 186–216. https://doi.org/10.1002/9781444342536.ch9
Griffiths SW, Brockmark S, Höjesjö J, Johnsson JI (2004) Coping with divided attention: the advantage of familiarity. Proc R Soc Lond B 271:695–699. https://doi.org/10.1098/rspb.2003.2648
Guttridge TL, Gruber SH, DiBattista JD, Feldheim KA, Croft DP, Krause S, Krause J (2011) Assortative interactions and leadership in a free-ranging population of juvenile lemon shark Negaprion brevirostris. Mar Ecol Prog Ser 423:235–245. https://doi.org/10.3354/meps08929
Guttridge TL, van Dijk S, Stamhuis EJ, Krause J, Gruber SH, Brown C (2013) Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim Cogn 16:55–64. https://doi.org/10.1007/s10071-012-0550-6
Hamede RK, Bashford J, McCallum H, Jones M (2009) Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol Lett 12:1147–1157. https://doi.org/10.1111/j.1461-0248.2009.01370.x
Heupel MR, Simpfendorfer CA (2005) Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar Biol 147:1239–1249. https://doi.org/10.1007/s00227-005-0004-7
Heyman WD, Graham RT, Kjerfve B, Johannes RE (2001) Whale sharks Rhincodon typus aggregate to feed on fish spawn in Belize. Mar Ecol Prog Ser 215:275–282. https://doi.org/10.3354/meps215275
Hinde RA (1976) Interactions, Relationships and Social Structure. Man 1-17. https://doi.org/10.2307/2800384
Hoppitt WJ, Farine DR (2018) Association indices for quantifying social relationships: how to deal with missing observations of individuals or groups. Anim Behav 136:227–238. https://doi.org/10.1016/j.anbehav.2017.08.029
Jacoby DM, Busawon DS, Sims DW (2010) Sex and social networking: the influence of male presence on social structure of female shark groups. Behav Ecol 21:808–818. https://doi.org/10.1093/beheco/arq061
Jacoby DM, Croft DP, Sims DW (2012) Social behaviour in sharks and rays: analysis, patterns and implications for conservation. Fish Fish 13:399–417. https://doi.org/10.1111/j.1467-2979.2011.00436.x
Jacoby DM, Fear LN, Sims DW, Croft DP (2014) Shark personalities? Repeatability of social network traits in a widely distributed predatory fish. Behav Ecol Sociobiol 68:1995–2003. https://doi.org/10.1007/s00265-014-1805-9
Jacoby DM, Papastamatiou YP, Freeman R (2016) Inferring animal social networks and leadership: applications for passive monitoring arrays. J R Soc Interface 13:20160676. https://doi.org/10.1098/rsif.2016.0676
Jaine FRA, Couturier LI, Weeks SJ, Townsend KA, Bennett MB, Fiora K, Richardson AJ (2012) When giants turn up: sighting trends, environmental influences and habitat use of the manta ray Manta alfredi at a coral reef. PLoS One 7:e46170. https://doi.org/10.1371/journal.pone.0046170
Jaine FRA, Rohner CA, Weeks SJ, Couturier LI, Bennett MB, Townsend KA, Richardson AJ (2014) Movements and habitat use of reef manta rays off eastern Australia: offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar Ecol Prog Ser 510:73–86. https://doi.org/10.3354/meps10910
Kalbitzer U, Bergstrom ML, Carnegie SD, Wikberg EC, Kawamura S, Campos FA & Fedigan, LM (2017). Female sociality and sexual conflict shape offspring survival in a Neotropical primate. Proc Nat Acad Sci, 114(8), 1892-1897. https://doi.org/10.1073/pnas.1608625114
Karczmarski L, Würsig B, Gailey G, Larson KW, Vanderlip C (2005) Spinner dolphins in a remote Hawaiian atoll: social grouping and population structure. Behav Ecol 16:675–685. https://doi.org/10.1093/beheco/ari028
Kashiwagi T, Marshall AD, Bennett MB, Ovenden JR (2011) Habitat segregation and mosaic sympatry of the two species of manta ray in the Indian and Pacific oceans: Manta alfredi and M. birostris. Mar Biodivers Rec 4:e53. https://doi.org/10.1017/S1755267211000479
King SL, Janik VM (2015) Come dine with me: food-associated social signalling in wild bottlenose dolphins (Tursiops truncatus). Anim Cogn 18:969–974. https://doi.org/10.1007/s10071-015-0851-7
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Krause J, James R, Franks DW, Croft DP (eds) (2014) Animal social networks. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780199679041.001.0001
Kurvers RH, Krause J, Croft DP, Wilson AD, Wolf M (2014) The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol Evol 29:326–335. https://doi.org/10.1016/j.tree.2014.04.002
Lachlan RF, Crooks L, Laland KN (1998) Who follows whom? Shoaling preferences and social learning of foraging information in guppies. Anim Behav 56:181–190. https://doi.org/10.1006/anbe.1998.0760
Lawson JM, Fordham SV, O’Malley MP, Davidson LN, Walls RH, Heupel MR, Ender I (2017) Sympathy for the devil: a conservation strategy for devil and manta rays. PeerJ 5:e3027. https://doi.org/10.7717/peerj.3027
Lewis SA, Setiasih N, Fahmi F, Dharmadi D, O’Malley MP, Campbell SJ, Yusuf M, Sianipar AB (2015) Assessing Indonesian manta and devil ray populations through historical landings and fishing community interviews. PeerJ PrePrints 3:e1642
Lisney TJ, Yopak KE, Montgomery JC, Collin SP (2008) Variation in brain organization and cerebellar foliation in chondrichthyans: batoids. Brain Behav Evol 72:262–282. https://doi.org/10.1159/000171489
Lusseau D, Schneider K, Boisseau OJ, Haase P, Slooten E, Dawson SM (2003) The bottlenose dolphin community of Doubtful Sound features a large proportion of long-lasting associations. Behav Ecol Sociobiol 54:396–405. https://doi.org/10.1007/s00265-003-0651-y
Lusseau D, Wilson BEN, Hammond PS, Grellier K, Durban JW, Parsons KM, Thompson PM (2006) Quantifying the influence of sociality on population structure in bottlenose dolphins. J Anim Ecol 75:14–24. https://doi.org/10.1111/j.1365-2656.2005.01013.x
Lusseau D, Whitehead H, Gero S (2008) Incorporating uncertainty into the study of animal social networks. Anim Behav 75:1809–1815. https://doi.org/10.1016/j.anbehav.2007.10.029
Marshall AD (2008) Biology and population ecology of Manta birostris in southern Mozambique. PhD thesis, The University of Queensland
Marshall AD, Bennett MB (2010) Reproductive ecology of the reef manta ray Manta alfredi in southern Mozambique. J Fish Biol 77:169–190. https://doi.org/10.1111/j.1095-8649.2010.02669.x
Marshall AD, Holmberg J (2019) MantaMatcher photo-identification library. Accessed January 21, 2019, from https://www.mantamatcher.org/
Marshall AD, Dudgeon CL, Bennett MB (2011) Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Mar Biol 158:1111–1124. https://doi.org/10.1007/s00227-011-1634-6
Marshall AD, Kashiwagi T, Bennett MB, Deakos M, Stevens G, McGregor F, Clark T, Ishihara H, Sato K (2018a) Mobula alfredi (amended version of 2011 assessment). The IUCN Red List of Threatened Species 2018:e.T195459A126665723, https://doi.org/10.2305/IUCN.UK.2011-2.RLTS.T195459A126665723.en
Marshall AD, Bennett MB, Kodja G, Hinojosa-Alvarez S, Galvan-Magana F, Harding M, Stevens G, Kashiwagi T (2018b) Mobula birostris (amended version of 2011 assessment). The IUCN Red List of Threatened Species 2018:e.T198921A126669349, http://www.iucnredlist.orq/details/198921/0
Martin RA (2007) A review of shark agonistic displays: comparison of display features and implications for shark–human interactions. Mar Freshw Behav Physiol 40:3–34. https://doi.org/10.1080/10236240601154872
Matich P, Heithaus MR (2015) Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas). Oecologia 178:347–359. https://doi.org/10.1007/s00442-015-3253-2
Mourier J, Vercelloni J, Planes S (2012) Evidence of social communities in a spatially structured network of a free-ranging shark species. Anim Behav 83:389–401. https://doi.org/10.1016/j.anbehav.2011.11.008
Mourier J, Brown C, Planes S (2017) Learning and robustness to catch-and-release fishing in a shark social network. Biol Lett 13:20160824. https://doi.org/10.1098/rsbl.2016.0824
Newman MEJ (2006) Modularity and community structure in networks. Proc Nat Acad Sci 103 (23):8577-8582. https://doi.org/10.1073/pnas.0601602103
O’Malley MP, Lee-Brooks K, Medd HB (2013) The global economic impact of manta ray watching tourism. PLoS One 8:e65051. https://doi.org/10.1371/journal.pone.0065051
Opsahl T, Agneessens F, Skvoretz J (2010) Node centrality in weighted networks: generalizing degree and shortest paths. Soc Networks 32:245–251. https://doi.org/10.1016/j.socnet.2010.03.006
Parker GA (2006) Sexual conflict over mating and fertilization: an overview. Phil Trans R Soc B, 361(1466), 235-259. https://doi.org/10.1098/rstb.2005.1785
Pierce SJ, Holmberg J, Kock AA, Marshall AD (2018) Photographic identification of sharks. In: Carrier JC, Heithaus MR, Simpfendorfer CA (eds) Shark research: emerging technologies and applications for the field and laboratory. CRC Press, Boca Raton, pp 219–234. https://doi.org/10.1201/b21842
Rohner CA, Flam AL, Pierce SJ, Marshall AD (2017) Steep declines in sightings of manta rays and devilrays (Mobulidae) in southern Mozambique (no. e3051v1). PeerJ Preprints 5:e3051v1. https://doi.org/10.7287/peerj.preprints.3051v1
Setyawan E, Sianipar AB, Erdmann MV, Fischer AM, Haddy JA, Beale CS, Lewis SA, Mambrasar R (2018) Site fidelity and movement patterns of reef manta rays (Mobula alfredi): Mobulidae using passive acoustic telemetry in northern Raja Ampat, Indonesia. Nat Conserv Res 3:1–15. https://doi.org/10.24189/ncr.2018.043
Shizuka D, Farine DR (2016) Measuring the robustness of network community structure using assortativity. Anim Behav 112:237–246. https://doi.org/10.1016/j.anbehav.2015.12.007
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088. https://doi.org/10.1016/j.anbehav.2013.02.017
Sih A, Hanser SF, McHugh KA (2009) Social network theory: new insights and issues for behavioral ecologists. Behav Ecol Sociobiol 63:975–988. https://doi.org/10.1007/s00265-009-0725-6
Simcox H, Colegrave N, Heenan A, Howard C, Braithwaite VA (2005) Context-dependent male mating preferences for unfamiliar females. Anim Behav 70:1429–1437. https://doi.org/10.1016/j.anbehav.2005.04.003
Snijders L, Blumstein DT, Stanley CR, Franks DW (2017) Animal social network theory can help wildlife conservation. Trends Ecol Evol 32:567–577. https://doi.org/10.1016/j.tree.2017.05.005
Sperone E, Micarelli P, Andreotti S, Brandmayr P, Bernabò I, Brunelli E, Tripepi S (2012) Surface behaviour of bait-attracted white sharks at Dyer Island (South Africa). Mar Biol Res 8:982–991. https://doi.org/10.1080/17451000.2012.708043
Stevens G (2016) Conservation and population ecology of manta rays in the Maldives. PhD thesis, University of York
Stevens G, Hawkins JP, Roberts CM (2018) Courtship and mating behaviour of manta rays Mobula alfredi and M. birostris in the Maldives. J Fish Biol 93:344–359. https://doi.org/10.1111/jfb.13768
Stewart JD, Beale CS, Fernando D, Sianipar AB, Burton RS, Semmens BX, Aburto-Oropeza O (2016a) Spatial ecology and conservation of Manta birostris in the Indo-Pacific. Biol Conserv 200:178–183. https://doi.org/10.1016/j.biocon.2016.05.016
Stewart JD, Stevens GM, Marshall GJ, Abernathy K (2016b) Are mantas self aware or simply social? A response to Ari and D’Agostino 2016. J Ethol 35:145–147. https://doi.org/10.1007/s10164-016-0491-7
Stewart JD, Jaine FR, Armstrong AJ et al (2018) Research priorities to support effective manta and devil ray conservation. Front Mar Sci 5:314. https://doi.org/10.3389/fmars.2018.00314
Sumpter DJ (2006) The principles of collective animal behaviour. Philos Trans R Soc B 361:5–22. https://doi.org/10.1098/rstb.2005.1733
Sutherland WJ (1998) The importance of behavioural studies in conservation biology. Anim Behav 56:801–809. https://doi.org/10.1006/anbe.1998.0896
Swaney W, Kendal J, Capon H, Brown C, Laland KN (2001) Familiarity facilitates learning of foraging behaviour in the guppy. Anim Behav 62:591–598. https://doi.org/10.1006/anbe.2001.1788
Team RC (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Thonhauser KE, Gutnick T, Byrne RA, Kral K, Burghardt GM, Kuba MJ (2013) Social learning in cartilaginous fish (stingrays Potamotrygon falkneri). Anim Cogn 16:927–932. https://doi.org/10.1007/s10071-013-0625-z
Trivers RL (1971) The Evolution of Reciprocal Altruism. Quart Rev Biol 46 (1):35-57. https://doi.org/10.1086/406755
Venables S, McGregor F, Brain L, van Keulen M (2016a) Manta ray tourism management, precautionary strategies for a growing industry: a case study from the Ningaloo Marine Park, Western Australia. Pac Conserv Biol 22:295–300. https://doi.org/10.1071/PC16003
Venables S, Winstanley G, Bowles L, Marshall AD (2016b) A giant opportunity: the economic impact of manta rays on the Mozambican tourism industry - an incentive for increased management and protection. Tour Mar Environ 12:51–68. https://doi.org/10.3727/154427316X693225
Ward AJ, Herbert-Read JE, Sumpter DJ, Krause J (2011) Fast and accurate decisions through collective vigilance in fish shoals. Proc Natl Acad Sci U S A 108:2312–2315. https://doi.org/10.1073/pnas.1007102108
Wearmouth VJ, Sims DW (2008) Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv Mar Biol 54:107–170
Wearmouth VJ, Southall EJ, Morritt D, Thompson RC, Cuthill IC, Partridge JC, Sims DW (2012) Year-round sexual harassment as a behavioral mediator of vertebrate population dynamics. Ecol Monogr 82:351–366. https://doi.org/10.1890/11-2052.1
Wetherbee BM, Cortés E, Bizzarro JJ (2004) Food consumption and feeding habits. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Boca Raton, pp 225–246
Whitehead H (1995) Investigating structure and temporal scale in social organizations using identified individuals. Behav Ecol 6:199–208. https://doi.org/10.1093/beheco/6.2.199
Whitehead H (2007) Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Commun Stat Simulat 36:1233–1246. https://doi.org/10.1080/03610910701569531
Whitehead H (2008a) Analyzing animal societies: quantitative methods for vertebrate social analysis. University of Chicago Press, Chicago. https://doi.org/10.7208/chicago/9780226895246.001.0001
Whitehead H (2008b) Precision and power in the analysis of social structure using associations. Anim Behav 75:1093–1099. https://doi.org/10.1016/j.anbehav.2007.08.022
Whitehead H (2009) SOCPROG programs: analysing animal social structures. Behav Ecol Sociobiol 63:765–778. https://doi.org/10.1007/s00265-008-0697-y
Whitehead H, Dufault S (1999) Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations. Adv Study Behav 28:33–74. https://doi.org/10.1016/S0065-3454(08)60215-6
Whitehead H, James R (2015) Generalized affiliation indices extract affiliations from social network data. Methods Ecol Evol 6:836–844. https://doi.org/10.1111/2041-210X.12383
Whitehead H, Rendell L (2004) Movements, habitat use and feeding success of cultural clans of South Pacific sperm whales. J Anim Ecol 73:190–196. https://doi.org/10.1111/j.1365-2656.2004.00798.x
Williams R, Lusseau D (2006) A killer whale social network is vulnerable to targeted removals. Biol Lett 2:497–500. https://doi.org/10.1098/rsbl.2006.0510
Wilson AD, Brownscombe JW, Krause J, Krause S, Gutowsky LF, Brooks EJ, Cooke SJ (2015) Integrating network analysis, sensor tags, and observation to understand shark ecology and behavior. Behav Ecol 26:1577–1586. https://doi.org/10.1093/beheco/arv115
Wolf JB, Mawdsley D, Trillmich F, James R (2007) Social structure in a colonial mammal: unravelling hidden structural layers and their foundations by network analysis. Anim Behav 74:1293–1302. https://doi.org/10.1016/j.anbehav.2007.02.024
Yano K, Sato F & Takahashi T (1999) Observations of mating behavior of the manta ray, Manta birostris, at the Ogasawara Islands, Japan. Ich Res, 46(3), 289-296. https://doi.org/10.1007/BF02678515
Zanardo N, Parra GJ, Diaz-Aguirre F, Pratt EA, Möller LM (2018) Social cohesion and intra-population community structure in southern Australian bottlenose dolphins (Tursiops sp.). Behav Ecol Sociobiol 72:156. https://doi.org/10.1007/s00265-018-2557-8
Acknowledgments
We would like to thank Papua Explorers Dive Resort, Raja Ampat SEA Centre, University of Papua, Barefoot Conservation, and KemenRisTek-Dikti for their invaluable support in enabling our fieldwork in Raja Ampat. We are grateful to all citizen scientists, recreational divers, and photographers who have submitted photographs and videos to MantaMatcher.org. Thanks to E. Germanov, E. Sinderson, G. Winstanley, and J. Holmberg for support with MantaUtil and MantaMatcher and E. Germanov for help with illustrations. We would also like to thank D. Jacoby and one anonymous reviewer for their helpful comments.
Funding
No external funding was received for this research. Research was funded by the authors with some funds sourced via donations to Marine Megafauna Foundation and through RJYP student research budget at Macquarie University (during 2017–2018).
Author information
Authors and Affiliations
Contributions
RJYP was the primary author of the manuscript. RJYP and DWF conceived the central idea of the manuscript. RJYP, SKV, ADM, and RFT collected and input data. RJYP conducted all statistical analyses with input from DWF and CB, ADM, and SKV contributed to editing and manuscript revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for conducting research on animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the corresponding author is based.
Additional information
Communicated by I. Hamilton
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1380 kb)
Rights and permissions
About this article
Cite this article
Perryman, R.J.Y., Venables, S.K., Tapilatu, R.F. et al. Social preferences and network structure in a population of reef manta rays. Behav Ecol Sociobiol 73, 114 (2019). https://doi.org/10.1007/s00265-019-2720-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2720-x