Abstract
Social learning is considered one of the hallmarks of cognition. Observers learn from demonstrators that a particular behavior pattern leads to a specific consequence or outcome, which may be either positive or negative. In the last few years, social learning has been studied in a variety of taxa including birds and bony fish. To date, there are few studies demonstrating learning processes in cartilaginous fish. Our study shows that the cartilaginous fish freshwater stingrays (Potamotrygon falkneri) are capable of social learning and isolates the processes involved. Using a task that required animals to learn to remove a food reward from a tube, we found that observers needed significantly (P < 0.01) fewer trials to learn to extract the reward than demonstrators. Furthermore, observers immediately showed a significantly (P < 0.05) higher frequency of the most efficient “suck and undulation” strategy exhibited by the experienced demonstrators, suggesting imitation. Shedding light on social learning processes in cartilaginous fish advances the systematic comparison of cognition between aquatic and terrestrial vertebrates and helps unravel the evolutionary origins of social cognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social learning enables a naïve animal to acquire information from a knowledgeable one, thus avoiding the costs of learning solely by individual experience (Boyd and Richerson 1998; Byrne 2003; Heyes 2009; Hoppitt and Laland 2008; Huber et al. 2009). An observer utilizes information obtained by watching a demonstrator to achieve a goal, learning about a location, object, reward, or method (Thorpe 1963; Tomasello et al. 1993; Zentall 2006). There are several forms of social learning, many of which have been shown in mammals (Byrne 2003; Heyes 2009; Hoppitt and Laland 2008; Huber et al. 2009), birds (Dorrance and Zentall 2001; Huber et al. 2009; Slagsvold and Wiebe 2011), reptiles (Davis and Burghardt 2011; Wilkinson et al. 2010), bony fish (Brown et al. 2011), sharks (Guttridge et al. 2013), and even in invertebrates (Fiorito and Scotto 1992; Leadbeater and Chittka 2007).
Hoppitt and Laland (2008) outlined the different processes involved in social learning in an effort to describe these behaviors as they occur in a wide variety of animals. One of the simpler forms of social learning is drawing the attention of an observer to a specific object or location. In stimulus enhancement (described by Spence 1937) exposure to a demonstrator draws the attention of the observer to a specific object; in local enhancement (described by Thorpe 1963), attention is drawn to a specific location. Thus, in many social learning studies, where the object of interest is located at a fixed position, stimulus and local enhancement are often indistinguishable.
Observational conditioning further expands on stimulus enhancement, when the observer learns of a relationship between a stimulus and a subsequent reinforcement (Heyes 1994). The observation of a performing demonstrator may not only draw the observer’s attention to the object of manipulation, but also to the rewarding of the demonstrator. This might lead to the establishment of a Pavlovian association.
In social facilitation, the presence of a demonstrator induces the observers to engage in similar activity, making it more likely that the observer will spend time in a certain area or will start interacting with a certain stimulus. This requires concurrent behavior by both observer and demonstrator.
Imitation, a complex form of social learning, is subdivided into contextual imitation and production imitation. In contextual imitation, the observer will become more likely to perform a certain action (which in this case is not a novel action to the observer) when placed in the same context as the demonstrator. In production imitation, the observer becomes more likely to use the same action or sequence of actions as employed by the demonstrator, actions not in the animal’s original behavioral repertoire.
Some researchers argue that a behavior can only be considered true imitation if it can be shown that the observer recognizes the intentional structure of the demonstrator’s action. This makes it difficult to demonstrate imitation in animals (Huber et al. 2009; Tomasello et al. 1993). Although imitation and social learning have been primarily studied in mammals and birds (Huber et al. 2009), a recent study on learning in sharks (Guttridge et al. 2013) showed, for the first time, that social learning is also found in cartilaginous fish. However, while they managed to show that in sharks there is an effect of learning by observation of conspecifics, they could not distinguish between the different mechanisms of social learning that might be involved. The goal of our present study is to show that, following the definitions outlined by Hoppitt and Laland (2008), freshwater stingrays are capable of social learning. Using a recently developed testing apparatus for problem solving and tool use in stingrays (Kuba et al. 2010), we investigate the type of social learning performed by stingrays.
Methods
Experimental animals
Ten naïve freshwater stingrays Potamotrygon falkneri, wild caught in the Nanay River, a tributary river of the Amazon River west of Napo in Peru, were housed at the Vienna Zoological Garden Schönbrunn. Sex was determined by the presence of claspers; there were 4 males and 6 females, ranging from 18 to 36 cm in disk diameter.
All animals were kept together in an all glass aquarium (300 cm long × 150 cm wide × 70 cm high) filled with deionised, aerated, filtered water at 27 ± 2 °C with a depth of 62 cm and a gravel sand bedding. The room was kept at a standardized dark/light schedule (14/10 h).
Animals were allowed 3 months to acclimatize to their surroundings. Prior to start of experiments, food preference was determined by providing a variety of food items throughout the day (tubifex, bloodworms, earthworms, snails, pieces of fish, and squids). Within 3 weeks, animals showed a preference for earthworms, which were subsequently used as reward in all experiments. Outside the experimental sessions, animals were fed ad libitum on earthworms, tubifex, and bloodworms. Four animals were randomly designated as demonstrators (2 male and 2 females) and six as observers (2 male and 4 females).
Experimental set-up
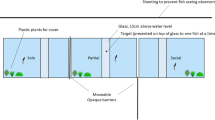
All experiments were conducted in the home tank. The experimental arena was part of the home tank and remained there until the end of experiments. The experimental arena (Fig. 1) was divided into 2 compartments, a starting compartment (85 cm × 90 cm) and a goal compartment (95 cm × 90 cm), separated by an opaque sliding door. For observation sessions, an additional third compartment (85 × 90) was attached opposite to the starting compartment and separated from the experimental compartment by duroplastic mesh, through which an animal could clearly view the experimental compartment, but not enter it. All compartments were adjacent to the glass wall and otherwise opaque. All sessions were filmed (Sony VX2000 video camera) from behind a curtain. The experimental apparatus described in Kuba et al. (2010) consisted of a gray PVC tube (23 cm long and 3 cm in diameter) baited with a food reward. During each trial, the tube was placed in a central position in the last third of the experimental compartment. The experiment was conducted by two experimenters, one of the experimenters operated the compartment doors and experimental apparatus, the other operated the video camera and recorded duration of the trials, strategies used, and the success of the animal.
Demonstrator sessions
At the start of each experimental session, a single demonstrator swam freely in the experimental arena for 30 min, with the sliding door open, allowing it to familiarize itself with all compartments. Each demonstrator session consisted of ten trials in a row. At the beginning of every trial, the demonstrator was placed in the starting compartment and the sliding door was closed. The gray tube, baited with a food reward (a 2 cm long piece of earthworm), was placed in the experimental compartment. The trial began when the sliding door between compartments was opened. To complete the trial, the animal had to enter the experimental compartment, approach the tube, and extract the food reward from it. Trials lasted a maximum of 3 min or until the animal had extracted the food reward. Demonstrator sessions were completed when all individuals reached the learning criterion, the extraction of the food reward within 3 min in 10 out of 10 consecutive trials. Training was then continued until demonstrators showed a consistent strategy for removing the food from the tube. All trials were videotaped for later analysis. For each trial, success, trial time, tube interaction time, and all tube manipulation strategies were recorded. Manipulation strategies were defined as: “touch/test” if the animal engaged in contacts with the pipe using the nose or part of the rim of the fins; “push forward” if the animal came in contact with the tube and used its body to push/move the tube around the arena; “toss” if the animal manipulated the tube aggressively, by biting and throwing the tube around the arena; “suck” if the animal placed its mouth on the tube opening applying only suction pressure; “blow” if the animal placed its mouth on or near the tube and used water jets; and “suck and undulation” if the animal combined the “suck” strategy with an undulating fin movement creating a water current from the tube toward the animal (see Kuba et al. (2010). All behavioral strategies were recorded from video playback.
Observer sessions
For the observer sessions, an observer compartment (opposite the starting compartment) was added to the experimental set-up. The observer was guided into the observation compartment, while the demonstrator was allowed to swim freely between the starting and experimental compartments for 30 min. From this compartment, the observer watched the demonstrator perform five trials in a row on each experimental day. During these trials, the demonstrator animal completed every trial successfully and used a consistent strategy. Analyses of video recordings confirmed that all observers attended to the demonstrator during each of the trials conducted for them. In particular, we noted that the observer followed the demonstrator performance by orientating body and eyes toward the model. Immediately after the observation period, the demonstrator was removed from the experimental arena and the observer was allowed to explore the starting and experimental compartments for 30 min. Observer testing and learning criterion were identical to those for demonstrators.
Data analyses
All statistical tests reported are two tailed. Comparison of the number of trials needed to fulfill the criterion between the two groups was made using a Mann–Whitney test, as these data were nonparametric. Successfulness of strategies was tested for each animal using a χ 2 test comparing percent success of the two most successful strategies. Frequencies of strategy use were compared using t tests, after Kolmogorov–Smirnov tests established that data were approximately normally distributed.
Video analysis was done watching the tapes at a random order in order to minimize bias. Observer reliability was calculated between the initial recordings during the experiment and the later video analysis (for success of trials κ = 0.908 ± 0.064, P < 0.0001 and for the strategies employed κ = 0.707 ± 0.073, P < 0.0001). In addition, extra observer reliability testing was conducted by having one experimenter who was not involved in filming or previous analysis watch 5 % of the experiments; observer reliability was between 94 and 96 %.
Results
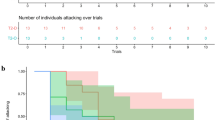
All 10 individuals learned to extract the reward. Observers reached criterion after 10–30 trials, whereas the demonstrators required 47–61 trials with no overlap (Table 1). Observers needed significantly fewer trials to reach criterion than did demonstrators (Mann–Whitney N = 4, 6, U = 0, P = 0.01). Furthermore, there was a trend of faster decline in trial durations over days of observers compared to those of demonstrators (Fig. 2a, Movie S1).
a Relationship between training day and mean trial latency to solving the problem by demonstrator and observer stingrays. Each data point represents the daily mean and standard error for each animal. Gray squares refer to observers, black squares to original training of demonstrators. b Mean frequency of the use of suck and undulation (t = 2.60; df = 8; P < 0.05) and push forward strategies (t = 4.28, df = 8; P < 0.005) by demonstrator and observer groups within the first 10 trials
Demonstrators attempted a variety of strategies to extract the food reward. However, strategies such as pushing forward, biting and then tossing the tube, or just applying suction to one end generally proved less successful than suck and undulation. In fact, when looking at the individual performances of animals, in 9 of the 10 animals, suck and undulation was significantly more successful than any other strategy (53.3–100 % success rate, for individual performance values see Table 2). One animal (observer Animal 6) had 47 % success rate using suck and undulation (within a total of 17 interactions) and 50 % success rate using suck (within a total of 6 interactions). The successful strategy of suck and undulation entailed the creation of a counter current toward the animal by a combination of using the disk-shaped body like a suction cup and undulating fin-movements. Experienced demonstrators correctly performed the extraction task in front of the observers, consistently using only the suck and undulation strategy. In contrast to the early stages of demonstrator training, observers quickly adopted the demonstrated strategy (suck and undulation) and manipulated the tube with a significantly higher frequency of suck and undulation (t = 2.60; df = 8; P < 0.05) and a significantly lower frequency of push forward behavior (t = 4.28, df = 8; P < 0.005; Fig. 2b) than did the demonstrators in their first 10 trials. Observers also made fewer failed attempts at extracting the reward, using any strategy, than demonstrators in the first 10 trials completed correctly (t = 3.83; df = 18; P = 0.001, Fig. 3). Demonstrators and observers showed no significant difference in the more exploratory touch and test behavior (t = 1.45, df = 8, P = ns).
Discussion
Our results clearly show that freshwater stingrays are capable of social learning. Using the pipe shape testing apparatus (Kuba et al. 2010), we introduced the animals to a problem that requires them to perform a set of behaviors/movements in order to retrieve a food reward. Observers required significantly fewer trials to reach the criterion of successfully completing the task. In the present study, observers immediately showed a significantly higher frequency of the most efficient “suck and undulation” strategy, as presented by the experienced demonstrators. The more frequent use of the most efficient strategy, as well as fewer failed reward extraction attempts, contributed to the trend of shorter trial durations for observers.
To test whether animals used imitation as the social learning mechanism, we chose the deferred imitation procedure, in which a delay was introduced between presenting the demonstration and testing the observer. This insures that the observer’s response is true imitation, and not simply a reflexive response or a less complex form of social interaction such as local enhancement or social facilitation (Hoppitt and Laland 2008; Zentall 2006). This method was used to show imitation in Japanese quail using a 30-min duration interval between demonstrations and testing the observer (Dorrance and Zentall 2001). In our study, observational conditioning is very likely to have been part of the social learning process, as observers saw demonstrators consuming their reward. Yet, it is not the only factor involved, as it would not lead to the imitation of the method or speed of acquisition.
Therefore, we suggest that the observer’s response might be imitation, and not simply a reflexive response or a less complex form of social interaction such as local enhancement, social facilitation, or observational conditioning (Bandura 1969; Zentall 2006). Following the definitions by Hoppitt and Laland (2008), there are two types of imitation: contextual and production. In this initial experiment, the distinction between contextual and production imitation is difficult. While contextual imitation might be the more likely form found here, the combination of behaviors seen here might be entirely novel and thus be production imitation. In order to clearly prove imitation, a bidirectional control procedure would have been desirable (Hoppitt and Laland 2008). Due to the constraints of the testing apparatus, this may not be possible as the fish, not the apparatus, dictated the solution employed. While this might be the first evidence for imitation learning in fish, further work is needed.
Our study differs from other studies in bony fish (Brown et al. 2011) and cartilaginous fish (Guttridge et al. 2013) as the animals had to perform a manipulation task that resulted in the development of specific apparatus manipulation strategies. While other forms of social learning have been shown in many taxa (fish: Brown et al. 2011, Guttridge et al. 2013; reptiles: Davis and Burghardt 2011; Wilkinson et al. 2010), many fish, reptiles, birds, and mammals do not show evidence of social learning (Huber et al. 2009, Heyes 2009). This raises the question about the evolutionary origin of social learning in vertebrates. Either social learning developed much earlier on the phylogenetic history of vertebrates than heretofore assumed or it has a polyphyletic origin, perhaps as a consequence of ecological, perceptual, and social factors.
References
Bandura A (1969) Social-learning theory of identificatory processes. In: Goslin DA (ed) Handbook of socialization theory and research. Rand-McNally, Chicago, pp 213–262
Boyd R, Richerson PJ (1998) An evolutionary model of social learning: the effect of spatial and temporal variation. In: Zentall TR, Galef BG (eds) Social learning: psychological and biological perspectives. Erlbaum, New Jersey, pp 29–48
Brown C, Laland K, Krause J (2011) Fish cognition and behaviour. Wiley-Blackwell, Oxford. doi:10.1002/9781444342536
Byrne RW (2003) Imitation as behaviour parsing. Philos Trans R Soc Lond B Biol Sci 358:529–536
Davis KM, Burghardt GM (2011) Turtles (Pseudemys nelsoni) learn about visual cues indicating food from experienced turtles. J Comp Psych 125:404–410
Dorrance BR, Zentall TR (2001) Imitative learning in Japanese quail (Coturnix japonica) depends on the motivational state of the observer quail at the time of observation. J Comp Psych 115:62–67
Fiorito G, Scotto P (1992) Observational learning in Octopus vulgaris. Science 256:545–547
Guttridge TL, Dijk S, Stamhuis EJ, Krause J, Gruber SH, Brown C (2013) Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim Cogn 16:55–64
Heyes CM (1994) Social learning in animals: categories and mechanisms. Biol Rev 69:207–231
Heyes CM (2009) Evolution, development and intentional control of imitation. Philos Trans R Soc Lond B Biol Sci 364:2293–2298
Hoppitt W, Laland N (2008) Social processes influencing learning in animals: a review of the evidence. Adv Stud Behav 38:105–165
Huber L, Range F, Voelkl B, Szucsich ZV, Miklosi A (2009) The evolution of imitation: what do the capacities of non-human animals tell us about the mechanisms of imitation? Philos Trans R Soc Lond B Biol Sci 364:2299–2309
Kuba MJ, Byrne RA, Burghardt GM (2010) A new method for studying problem solving and tool use in stingrays (Potamotrygon castexi). Anim Cogn 13:507–513
Leadbeater E, Chittka L (2007) Social learning in insects—from miniature brains to consensus building. Curr Biol 17:703–713
Slagsvold T, Wiebe KL (2011) Social learning in birds and its role in shaping a foraging niche. Philos Trans R Soc Lond B Biol Sci 366:969–977
Spence KW (1937) Experimental studies of learning and higher mental processes in infra-human primates. Psych Bul 34:806–850
Thorpe WH (1963) Learning and instinct in animals, 2nd edn. Methuen, London
Tomasello M, Kruger AC, Ratner HH (1993) Cultural learning. Behav Brain Sci 16:495–552
Wilkinson A, Kuenstner K, Mueller J, Huber L (2010) Social learning in a non- social reptile (Geochelone carbonaria). Biol Lett 6:614–616
Zentall TR (2006) Imitation: definitions, evidence, mechanisms. Anim Cogn 9:335–353
Acknowledgments
We gratefully acknowledge the Vienna Zoo for the experimental framework requirements and their support. Furthermore, we want to thank the animal keeper team in the Aquarium house who supported and enriched this project with their help and ideas. We especially would like to thank Prof. M. J. Gutnick for his critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerstin E. Thonhauser and Tamar Gutnick: Equal contribution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 25428 kb)
Rights and permissions
About this article
Cite this article
Thonhauser, K.E., Gutnick, T., Byrne, R.A. et al. Social learning in Cartilaginous fish (stingrays Potamotrygon falkneri). Anim Cogn 16, 927–932 (2013). https://doi.org/10.1007/s10071-013-0625-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-013-0625-z