Abstract
Social environments can influence diverse aspects of animal behavior. In reproductive activity, females may adjust their pre-mating preference for males and their post-mating investment in offspring according to the social environment and thus maximize their fitness, and how these two adjustments interact may significantly impact a male’s fitness. But such adjustments may also depend on intrinsic factors, such as age or condition, implying that both should be considered to reliably estimate the strength and direction of sexual selection. We investigated the impact of plasticity in female pre- and post-mating behaviors on male reproductive success at two age points, using a population of the flightless bush cricket Ephippiger diurnus (Orthoptera: Tettigoniidae), a long-lived and multiply-mating chorusing insect. We reared females in different social environments, each represented by an array of male signals with a certain attractiveness, and tested the females’ reproductive behavior over adult life and its repeatability across years. We found that variation in the social environment influenced both mate preference and egg investment, and that these adjustments were repeatable across the two ages tested. Specifically, females reared with attractive mates were more selective and invested more in eggs whereas females reared with unattractive mates laid fewer eggs. That is, plasticity in egg investment reinforced female pre-mating preferences across social environments, resulting in a threefold difference in the reproductive success of unattractive versus attractive males. Our results thus demonstrate how plasticity in female behaviors can play a major role in driving an evolutionary process.
Significance statement
Animals often adjust their behavior to information provided by the social environment in order to maximize their fitness. In reproduction, females may use that information to adjust their pre-mating preference for males and their post-mating investment in offspring. Many studies document these adjustments independently, but the two may interact in a way that can drastically influence a male’s reproductive success. Using the bush cricket Ephippiger diurnus, we show that variation in the social environment influences both female pre- and post-mating traits such that they reinforce one another: females increase oviposition when attractive males are present and decrease oviposition when unattractive males are present in their environment. These adjustments resulted in up to a threefold difference in the total reproductive success of attractive and unattractive males. Our results thus highlight how plasticity in female behaviors can dictate evolutionary processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social environments can influence various aspects of an animal’s behavior, and they often do so throughout development and aging (e.g., Bretman et al. 2011; Kasumovic and Brooks 2011; Rodríguez et al. 2013b; Han and Brooks 2014; Wey et al. 2015). The influence of the social environment on reproductive behavior, both prior to and after mating, may be particularly strong. For example, males may adjust the rate and length of their advertisement signals during courtship (e.g., Höbel 2015; Rebar and Rodríguez 2016) or the extent of their post-copulatory mate guarding (e.g., Barbosa 2012; Bretman et al. 2012) in response to rivals in the environment. Plasticity in reproductive behaviors may play a significant role in evolutionary processes because changes in mating behaviors can have a profound effect on an individual’s fitness (West-Eberhard 1983, 2014; Kokko et al. 2006; Taborsky and Oliveira 2012).
In females, pre-mating and post-mating behaviors seem particularly sensitive to the social environment. For examples, females may control their level of selectivity for potential mates (e.g., Bailey and Zuk 2008; Karlsson et al. 2010; Fowler-Finn and Rodríguez 2012a, b) and their investment in offspring based on traits of their mate (e.g., Sheldon 2000; Arnold et al. 2016), making both adjustments in response to the number and “quality” of males in the local population. These two female adjustments may influence a male’s fitness synergistically if the second, post-mating adjustment reinforces the advantage that the male obtained from having been chosen in the first place (e.g., Rebar et al. 2011; Albo et al. 2013), but such alignment is not a priori the case. A complex interplay between a female’s adjustments to pre- and post-mating behaviors is possible, and this interplay could, in turn, drastically impact a given male’s fitness either upward or downward. Consequently, measuring both pre- and post-mating female behavior—and making the assessments across social environments—is necessary for reliably estimating the strength and direction of sexual selection (Evans and Garcia-Gonzalez 2016).
The potential interplay between pre- and post-mating behavioral adjustments across social environments only accounts for one aspect of plasticity. Often, behavioral adjustments depend on intrinsic factors, i.e., physiological age and condition (Widemo and Sæther 1999; Cotton et al. 2006), as well as the social environment. Extrinsic and intrinsic factors may interact, where adjustments in response to the social environment are more pronounced in younger than aging animals, an effect best explained as terminal investment when senescence approaches and extrinsic factors are ignored (reviewed in Duffield et al. 2017). In general, and notably in species where females mate multiple times, reproductive behaviors remain flexible throughout much of adulthood (e.g., Rodríguez et al. 2013a; Swanger and Zuk 2015), and the combined effect of extrinsic and intrinsic factors may significantly impact an individual’s lifetime reproductive success. A corollary of this expectation is that male lifetime reproductive success may differ significantly from estimates made on the strength of sexual selection at a given point in time (Qvarnström 2001; Chaine and Lyon 2008; Hebets and Sullivan-Beckers 2010; Verzijden et al. 2012).
Here, we investigate the impact of plasticity in female pre- and post-mating behaviors, as expressed over the course of aging, on male reproductive success. We first measure how females adjust pre- and post-mating behavior in response to varying social environments shortly after reaching reproductive maturity and again when older. We then ask whether the adjustments are repeatable within individuals as they age, and whether the adjusted behaviors act synergistically such that post-mating investment reinforces pre-mating preference. Finally, we use the information on female pre- and post-mating behaviors to estimate their combined impact on male lifetime reproductive success.
We address these questions in a population of the flightless bush cricket Ephippiger diurnus (Orthoptera: Tettigoniidae), a long-lived and multiply-mating chorusing insect (Hockham et al. 2004). Males produce rhythmically repeated calls comprised of 1–8 “syllables” in daily choruses (Duijm 1990; Ritchie 1991, 1992b). Populations and individuals vary in the number of syllables per call, but syllable number within individuals is highly repeatable (Ritchie 1992b; Barbosa et al. 2016b) and unaffected by rivals’ calls (Rebar et al. 2016). Thus, females can use call syllable number as a reliable trait with which to evaluate male songs. In most E. diurnus populations, including the one studied here, females consistently prefer a call syllable number higher than the mean value (Barbosa et al. 2016a). At mating, males transfer a nuptial gift, a spermatophore with a large, external part, the nutrient-rich spermatophylax. Females only begin to lay eggs after consuming or removing the spermatophylax (Busnel and Dumortier 1954; Wedell 1994a; Gwynne 2001). The high repeatability of male calls and known female preferences make E. diurnus an ideal species for testing how social environments influence female pre- and post-mating behaviors as they age and thus impact male reproductive success.
Our previous work on socially mediated plasticity in E. diurnus has revealed complex adjustments by both females and males alike. First, we found some evidence that females adjust their mate preferences and offspring investment (i.e., post-mating oviposition) in response to different social environments that varied in call syllable number (Rebar et al. 2016). We did not, however, address plasticity in female reproductive behaviors as they age (i.e., between matings), how pre- and post-mating behaviors may interact, or whether these responses are even repeatable across years. Furthermore, females were reared in social environments that ranged from two to four syllables per call, which did not fully encompass the natural variation of two to eight syllables per call found in that population. Second, we also found that males adjust their investment in spermatophores according to the social environment when young (Rebar et al. 2016; Rebar and Greenfield 2017), but produce uniformly large spermatophores when old (Rebar and Greenfield 2017). Just as males make differential adjustments according to the social environment and their age, we now assess how the various adjustments that females make before and after mating, taken together, as they age influence the strength and direction of sexual selection.
We reared females from late juvenile stages through adulthood in one of five social (= acoustic) environments that varied in call syllable number: below (Low), equal to (Mid), and above the population mean (High), along with a mixture of those three (Mixed), and a silent environment (Silent). Females remained in those environments throughout their development, receiving feedback about available mates from the daily broadcast of synthetic calls. This approach reflects the chorusing that females would be exposed to in the field naturally. We then tested female mate preferences before each of two mating events, once as a young but sexually mature female and a second time as an older but not senescent female, and allowed females 7 days after each mating event to deposit eggs in the substrate. The two tests of a female’s pre- and post-mating behavior allowed us to evaluate the consistency in her adjustments: behavioral adjustments that are repeatable across female age would have a strong potential to influence male lifetime reproductive success. We muted males during mating events, limiting female information on male calls to their respective social (acoustic) environment. We also collected and tested females during 2 consecutive years to assess whether any behavioral adjustments were consistent across years.
We predicted that (i) female pre- and post-mating behaviors would be repeatable across the range of ages tested, (ii) post-mating investment in eggs would reinforce female mate preference, and (iii) the lifetime reproductive success of males would vary according to their perceived attractiveness. Specifically, when males are rare (Silent environment), we predicted that females would be less selective and increase their investment in eggs because of the reduced likelihood of encountering a future mate. When males are present, females would adjust their behaviors based on average male attractiveness and on variation in male calls. When potential mates are uniformly unattractive (Low treatment), females would express less selectivity, and would invest in fewer eggs. On the other hand, when attractive males are available (Mid and High treatments), females would become more selective and increase their egg investment. However, if those attractive males were mixed among less-attractive ones (Mixed treatment), females would still be selective, but they would invest in fewer eggs because of uncertainty in their specific mate’s attractiveness relative to those of other males in the vicinity as males were muted during mating. The predicted synergistic effects of pre- and post-mating behaviors would thus impact a male’s total reproductive success, with attractive males having greater reproductive success than unattractive males.
Methods
Study species
Ephippiger diurnus is distributed as small, isolated populations across central and southern France and northeastern Spain. The populations cluster into several clades and subclades, each sharing male call and female preference characteristics and found in a specific geographical region (Spooner and Ritchie 2006; Party et al. 2015). We studied a population collected from Vias, France (43° 18′ N, 3° 22′ E.; elevation 27 m). Males from Vias produced an average of 2.1 syllables per call in 2013, and individuals varied from 1 to 3 syllables per call (Greenfield et al. 2016).
We collected young nymphs up to the penultimate instar from Vias in May 2014 and May 2015 (N = 55 and 50 males and females in 2014 and 2015, respectively). Males and females were housed individually in plastic containers (107 W × 119 H mm) with acoustically transparent plastic mesh sides. We reared individuals in the same climate-controlled chamber maintained at 25 °C and a photoperiod of L:D 16:8 h during both years. We fed them cabbage, pollen, and fish flakes ad libitum, and misted individuals daily with water.
Acoustic environments
The equipment and its setup used in this experiment are given in detail elsewhere (Rebar et al. 2016). Briefly, we used five identical 100 L × 100 W × 80 H cm boxes lined with acoustic isolation foam (Flexolan, Dierdorf, Germany) for the five social environments, described below. One side of each box was left open, and on that side we placed a sun-simulating bulb (Solar Glo PT2193; Rolf C. Hagen Inc., Mansfield, MA, USA) and a loudspeaker (FT17H; Fostex Corp., Tokyo, Japan) pointing into the box. The loudspeaker, with a frequency response range from 5 to 50 kHz, covered the energy contained in E. diurnus male calls (10 to 50 kHz). We compensated for the spread of sound energy by rotating individual containers within each box daily. We verified that the boxes were acoustically isolated from one another and that the abiotic conditions at the position of the individual containers were similar among boxes. We also rotated the position of the acoustic environments within the climate-controlled chamber between years.
We randomly assigned individuals to one of five social (= acoustic) environments: Silent, Low, Mid, High, and Mixed. These experimental environments were designed to test our predictions on plasticity in female reproductive behavior. We introduced individuals to their respective environment upon reaching the penultimate instar to standardize acoustic experience (N = 10–11 males and females per environment per year). Individuals remained in their respective acoustic environment throughout the experiment, only being removed to assay mate preferences and during mating (see below). We always positioned the individual cages such that the mesh sidewall faced the loudspeaker and rotated individual cages daily within the environment box to homogenize abiotic and biotic experience. To limit acoustic experience to the broadcast stimuli only, we rendered all males mute by waxing their elytra together, thereby preventing stridulation. We broadcast acoustic stimuli for 6 h per day through a multi-channel playback device (Tascam DR-680; TEAC Corp., Tokyo, Japan) connected to a custom-built multi-channel, high-frequency amplifier to drive the loudspeakers. We calibrated the peak amplitude of stimuli presented in each environment to 90 dB SPL, equal to that of a male calling at 1-m distance (Greenfield et al. 2004; Party et al. 2014).
In the Silent environment, individuals were never exposed to any acoustic stimuli. The other four environments varied in the number of syllables of the synthetic male calls broadcast. We centered variation in syllables per call around the population mean and mode for Vias (2 syllables). The Low environment presented calls that were one syllable less than the mean (1 syllable), the Mid environment presented the population mean (2 syllables), and the High environment presented calls one syllable more than the mean (3 syllables). The Mixed environment presented Low, Mid, and High calls that were broadcast in equal amounts (1, 2, and 3 syllables). Females from Vias prefer calls with more syllables than the population mean (Barbosa et al. 2016a), and we use this known preference to predict how females would respond to available males: the Low environment thus consisted of unattractive males whereas the High environment consisted of attractive males.
We constructed the stimuli from a single, standard syllable that had average acoustic features for the population. We repeated that syllable to create polysyllabic calls and adjusted the amplitude to create four calls of varying amplitude (relative dB: 0 dB, − 4 dB, − 8 dB, − 12 dB) for the Low, Mid, and High environments. The amplitude adjustments simulated distance between calls and thus the perception of a chorus, accounting for the fact that the synthetic calls were broadcast from a single location. We presented calls using randomization without replacement within blocks of four calls, one of each amplitude. Calls were presented every 2 s, which falls within the natural range of alternating calls between males (Brunel 2012; Party et al. 2014). We constructed 20 different blocks of four calls for each acoustic environment, and randomized those blocks to create each acoustic environment stimulus, which was looped to broadcast for 6 h per day, the daily chorus duration of males in the field (Duijm 1990; Ritchie 1991, 1992b).
Female pre-mating reproductive behavior
We focused on female preference for the number of syllables per call because of its diversity between males and among populations, and the strong preferences females exhibit for this call feature (Ritchie 1996; Party et al. 2014; Barbosa et al. 2016a). Before each preference test, we placed each female in acoustic isolation for 24 h to increase the likelihood of female receptivity during testing while also shifting the focus towards long-term effects of the acoustic environment on female mate preferences. We tested each female’s preference twice, at 14 days and 22 days. The first test at 14 days is the youngest age at which nearly all females are sexually mature. The second test 8 days later reflected our best attempt to balance a female’s post-mating investment (see below) with her physiological age due to mating and oviposition. Whereas unmated females may live and be receptive for 60 days (Greenfield et al. 2004), young mated females rarely remain receptive and/or mate after 30 days (Rebar, Barbosa, and Greenfield pers. obs.). That is, the two female ages marked a significant physiological difference yet a small enough time lapse such that the insects were alive and responsive to the preference bioassay in the second test. One limitation of this timeline was that we could not mate all females before their first preference test at 14 days to standardize the mating status between the two preference tests. We justified this approach in two ways. First, there are a restricted number of animals available in any one population of E. diurnus. Second, in nature, most females will be unmated when young (i.e., 14 days) and mated about 8 days later, and will likely mate more than once during adulthood (Hockham et al. 2004). Thus, the actual effect of age on female preferences at these two time points includes both age and mating status, but this is akin to merely age in natural populations.
We tested female preference for syllable number with a locomotion compensation sphere (TrackSphere LC-300; Syntech Equipment and Research, Kirchzarte, Germany). An overhead camera tracks female movement and continuously transmits her position to motors mounted orthogonally to the sphere that then compensates for her movement, always keeping her on top of the sphere. The movement information is then recorded by TrackSphere software (version 2.2; Syntech Equipment and Research, Kirchzarte, Germany).
We placed each female on top of the sphere and allowed her to settle. We first broadcast a recording of a calling Vias male for 10 s to ensure that each female was receptive. We then presented her with 10 acoustic stimuli that ranged from 1 to 10 syllables per call. This range extends beyond the number of syllables produced by males across all populations of E. diurnus. We broadcast stimuli through a loudspeaker (Scanspeak; Avisoft Bioacoustics, Glienicke, Germany), and calibrated each stimulus to an amplitude of 85 dB SPL (0 dB = μPa) at the position of the female. Each stimulus presented 10 advertisement calls that were identical in call rate (20 calls/min), syllable length (150 ms for Vias), and length (30 s). The only difference between stimuli was the number of syllables per call. We presented the stimuli in a randomized order to limit any order effects, and females were given a minimum of 1 min between each stimulus to rest and dishabituate. We used the movement information to calculate a female’s net movement, in centimeters, towards each stimulus over the 30 s of testing. In total, all 102 females examined in the first preference test positively responded to all 10 stimuli and were included in our analysis (3 died before testing). Of those 102 females, 12 died before the second preference test. We thus tested 90 females, and only 42 of them successfully responded to all 10 stimuli and were included in our analysis. Ten failed to respond to any stimuli, and the other 38 females were receptive but stopped responding during the sequence of stimuli. While these receptive females were excluded from analysis here, we retained them for a second mating (see below).
Statistical analysis
We used the 10 responses of each female to construct her mate preference function with non-parametric regression by generating cubic splines, which make no assumption about the shape of the function other than it is smooth in nature. We calculated cubic splines with the mgcv package and a custom-written script in R 3.1.3 (R Core Team 2018), allowing the package to choose the smoothing parameter for each individual spline.
We adopted a function-valued approach to test for variation in female mate preference functions among acoustic environments and at two time points (hereafter age) (Meyer and Kirkpatrick 2005; Stinchcombe and Kirkpatrick 2012; Kilmer et al. 2017). This approach uses the entire preference function as the trait of interest such that each female contributes one preference function to the analysis. We used a linear mixed model with female response to male stimuli as the dependent variable. The final model included acoustic environment and age as fixed effects, along with their interaction, and year nested within acoustic environment and female identity nested within acoustic environment and year as random effects. The model also included linear and quadratic stimulus terms, their interactions with acoustic environment and age separately, and a three-way stimulus × acoustic environment × age interaction term. The acoustic environment and age terms describe differences in the overall responsiveness of females across acoustic environments and age, respectively. The acoustic environment × age interaction term tests for differences in the overall responsiveness of females across environments as they aged. Stimulus interaction terms test for variation in the shape of preference functions. Linear stimulus interaction terms describe female response as a function of increasing or decreasing syllables per call (e.g., open preference). Quadratic stimulus terms describe female response as a curvilinear function of syllables per call (e.g., closed preference). We were particularly interested in the stimulus interaction terms, and by examining the significance of these terms we evaluated how preference functions varied across acoustic environments, age, and years.
Variability in female mate preference functions
We described variation in female preference functions in terms of two traits, i.e., peak preference and selectivity. Peak preference is the stimulus value that elicited the greatest response from a female while selectivity describes how females discriminate against males as they (males) deviate from her peak preference (Bailey 2008; Fowler-Finn and Rodríguez 2012b; Rebar and Rodríguez 2013, 2014; Rodríguez et al. 2013a, b). Selectivity was derived from a principal component analysis (PCA) on three correlated traits: responsiveness, tolerance, and strength (Fowler-Finn and Rodríguez 2012b; Rebar and Rodríguez 2013, 2014; Rodríguez et al. 2013b). Responsiveness is the mean response across all stimuli, tolerance is the range of stimuli accepted at 2/3 the height of the peak preference, and strength describes the steepness of the descent from the peak preference (calculated as the coefficient of variation squared). We performed PCA on these three traits for the first and second matings separately. We also performed them separately for each year. The first principal component created the composite trait termed selectivity, and in all instances had a minimum eigenvalue of 2.21 that explained at least 73.7% of the variance across all PCAs. Responsiveness, tolerance, and strength loaded similarly and quite heavily on this axis for all PCAs performed (minimum of 0.50, 0.59, and − 0.55, respectively).
We used peak preference and selectivity as response variables in linear mixed models to assess variation in these traits arising from variation in acoustic environments and age. Each model included acoustic environment and age as fixed effects, plus their interaction, female identity nested within acoustic environment as a random effect, and year nested within acoustic environment as a random effect. The environment and age terms identified differences in peak or selectivity among acoustic environments or age, respectively, while the interaction term identified whether those responses changed as females aged within each acoustic environment. All of these terms were of interest to us, and we performed post hoc tests using the false discovery rate (FDR) method (Benjamini and Hochberg 1995) to identify significant differences in female responses.
Repeatability of female mate preference functions
We quantified repeatability as the female identity percentage variance component of the linear mixed models on peak preference and selectivity using the REML method (Lessells and Boag 1987). We cross-validated the repeatability estimates by pooling across acoustic environments to calculate Pearson product-moment correlations, which include variation from genetic and environmental inputs, for peak preference and selectivity between the first and second measures of female pre-mating behavior (i.e., age).
Female post-mating reproductive investment
We randomly paired each female with a male of equivalent age and mating experience at least 10 min after testing a female’s preference. We used males that were reared in the same acoustic environment as the females, and the same pool of males was used for each mating event to standardize mating experience, but we randomized males with respect to the female such that each female mated with a novel male each time. Ideally, we would have paired females with males from a stock colony to limit any potential influence of the acoustic environment on male phenotype, but natural populations of Ephippiger diurnus are sufficiently small, restricting that possibility. Further, developmental times slightly, albeit non-significantly, differed between acoustic environments. As female age was controlled for in both matings and we were concurrently interested in male spermatophore investment (Rebar and Greenfield 2017), pairing individuals within environments was most practical. Nonetheless, each male’s elytra were waxed together throughout the experiment, rendering them mute, and females therefore received limited information about male “quality” prior to mating. Second, while males adjust spermatophore size to the acoustic environment (Rebar et al. 2016; Rebar and Greenfield 2017) and our design does not restrict the possibility that males adjusted accessory gland proteins or other traits that influence female oviposition, we found no relationship between spermatophore size and female oviposition within or across acoustic environments (see “Results”). In addition, we account for as many male aspects as possible in our statistical analysis (see below).
We weighed males and females just before pairing, and then placed them in a new, clean plastic container to mate. We mated pairs in a given corner of a room also maintained at the standard 25 °C (range from 24 to 25.5 °C). The opposite side of this room contained an active chorus of at least 30 males from various Ephippiger populations that formed a continuous, background din of conspecific song, which stimulated pairs to mate. In this high-density chorus, members masked one another’s song features, and mating pairs would not have perceived the syllable number of individual males in the chorus. Moreover, the chorus, being > 5 m distant from the mating pair, was at least 15 dB SPL lower than the calls broadcast in the acoustic environments. These factors minimized the possibility that a mating pair would have been influenced by the background chorus in the manner we predicted it was by the acoustic environments, where songs of several males were broadcast in alternation. Once mating was completed, we separated the pair and again weighed both individuals. The average change in weight of the male and female was used to estimate spermatophore size. We returned the female to her respective individual container that had been supplied with fresh sand in which to lay eggs. We then placed each female back into her respective acoustic environment. We allowed females 7 days to lay eggs after each mating, at which time we collected the sand to count and weigh eggs. In total, 75 females successfully mated twice and survived long enough to lay eggs and were thus retained for analysis.
While the potential for the chorus to influence female behavior existed, we note two important aspects of our design. First, all individuals experienced a similar chorus environment during mating. Second, we returned females to their respective acoustic environment immediately after mating, and they remained there throughout our determination of post-mating investment. Thus, any difference in egg investment across acoustic environments is best explained by adjustments to the acoustic environments themselves rather than to any potential effects induced during mating.
Statistical analysis
We initially performed two linear mixed models, with the number of eggs laid and egg weight as dependent variables, to test for adjustments in egg investment due to acoustic environments and age. We included male identity, male size, and male spermatophore size in the model to account for any male effects, but they were subsequently removed as they did not contribute to explaining any variation in female investment (all p > 0.30). Given no qualitative difference in the two models, we opted to use the number of eggs laid for reporting purposes. The final model included terms for acoustic environment and age as fixed effects, plus their interaction, and female size as a covariate. It also included year nested within acoustic environment, and female identity nested within acoustic environment and year as random effects. We reran this model, excluding the six females that failed to oviposit after the first mating (4 Low, 1 Mid, 1 Mixed), but found no qualitative differences in the model results and thus retained these females for analysis.
The acoustic environment and age terms identified differences in reproductive investment among acoustic environments or age, respectively, while the acoustic environment × age interaction term identified whether reproductive investment changed across matings within each acoustic environment. A significant acoustic environment × age interaction term (see “Results”) prompted us to compare female investment between acoustic environments at each age tested. We performed a total of 20 post hoc pairwise comparisons: 10 pairwise comparisons between acoustic environments at each age. We controlled for the FDR of all 20 comparisons (Benjamini and Hochberg 1995). We carried out all statistical analyses with JMP 10 (SAS Institute Inc., Cary, North Carolina).
Repeatability of egg investment
As with the mate preference functions, we quantified repeatability from the female identity variance component of the linear mixed model using the REML method (Lessells and Boag 1987). We cross-validated the repeatability estimates as above by pooling across acoustic environments to calculate a Pearson product-moment correlation for oviposition after the two matings, controlling for female weight at each time point.
Pre- to post-mating reinforcement and its impact on male reproductive success
To estimate the combined impact of plasticity in female pre- and post-mating behaviors across age on male reproductive success, we devised a “total reproductive success” coefficient that estimated the multiplicative effect of those behaviors. Our index is based on calculations of the total opportunity for sexual selection (ITS) in which relative male reproductive success is a product of relative pre- and post-mating “success” (e.g., Collet et al. 2012; Janicke et al. 2015; Evans and Garcia-Gonzalez 2016). We first assumed that male syllable number is heritable, basing this assumption on its high repeatability (Barbosa et al. 2016a; Rebar et al. 2016) and the intermediate syllable numbers produced by males from hybrid crosses of different populations (Ritchie 1992a, b). Second, we assumed that the population-level female preference function from the Mixed environment reflected the strength of selection that females imposed on males of varying call attractiveness, as natural choruses of Vias males contain calls from 1 to 3 syllables (Greenfield et al. 2016). We used the height of that preference function at 1, 2, and 3 syllables (representative of males in the Low, Mid, and High environments), and divided these values by their mean to create a relative measure of pre-mating selection. We calculated this relative value at each mating and for each year independently to account for differences in female mate preference functions.
We then estimated each male’s relative post-mating success from the oviposition by his mate in relation to the mean oviposition by all females reared in the Low, Mid, and High environments. In our experiment, the only acoustic information a female received about her mate’s attractiveness was the synthetic calls in her environment, which continued until immediately prior to and resumed just after mating. These call stimuli were 15 dB louder than the background chorus present in the separate room where mating took place. We thus assume that a female’s oviposition within her respective acoustic environment (Low, Mid, or High) reflects how she may invest in eggs after mating with a male of a given call quality, either 1, 2, or 3 syllables, in a mixed chorus, and recognize that this assumption may not accurately reflect the true relationship between male call quality and female oviposition. Nonetheless, it provides a reasonable estimate, as female size did not differ among environments, indicating that all females could oviposit similarly.
We estimated each male’s fertilization success from work on the Mireval population of E. diurnus (Hockham et al. 2004), a population geographically close to ours (Vias; 40 km distant) and the most similar population genetically and behaviorally (Greenfield et al. 2016; Esquer-Garrigos et al. 2019). Hockham et al. (2004) found that, in the lab, E. diurnus is characterized by last male sperm precedence. The authors also tested field-caught females, finding that the average paternity success of the two males in females with two sires was 89.5 and 10.5% (Hockham et al. 2004), consistent with the extreme P2 precedence found in the lab. We used these two field estimates of paternity success to assign eggs to a given male. Thus, for the first mating, each male’s success was the number of eggs that his mate laid in the 7-day post-mating plus 10.5% of the eggs laid by that female in the 7 days following her second mating. For the second mating, each male’s success was calculated as 89.5% of the number of eggs that his mate laid in the 7-day post-mating. We calculated the global mean number of eggs laid by pooling across post-mating periods, age, and acoustic environments. We then divided each male’s first and second success by that global mean to determine his relative fertilization success for each mating. By using a global mean, the relative values still include the between-age and between-acoustic environment variation in oviposition (Table 3, Fig. 3).
The total reproductive success of each male was calculated by multiplying the relative pre-mating and post-mating values at the first and second mating separately, and then summing those values together. As all values were relative to one another, a male whose total reproductive success was thoroughly average would have a value of 1 after the first mating, and a value of 1 after the second mating, and thus an overall total reproductive success of 2.
Results
Female mate preference functions: effects of age and acoustic environment
Mate preference functions were significantly influenced by the interaction between age and the acoustic environment (Fig. 1; Table 1), the latter indicating that females differed among acoustic environments in how responsive they were as they aged. Females also differed between years in how responsive they were to male calls, but the adjustments were similar across acoustic environments and age (Fig. 1, Table 1). Importantly, the shape of the preference functions differed significantly among acoustic environments (acoustic environment × stimulus interaction term, acoustic environment × stimulus2 term; Table 1), prompting us to perform a trait-by-trait analysis of the preference functions.
The influence of acoustic environment and age on the mate preference functions of Ephippiger diurnus females for the number of syllables in male calls. Females were reared in one of five acoustic environments, and their preferences were assayed when young but sexually mature and then again 8 days later. The phonotactic responses (in cm) to 10 synthetic male calls that ranged from 1 to 10 syllables per call were used to construct each female’s preference function. The curves display the mean preference function of females reared in each acoustic environment. Black and gray lines denote the mean preference when young and older, respectively. Solid and dashed lines denote the mean preference of females collected in 2014 and 2015, respectively
Variability and repeatability of peak preference
We found that neither the acoustic environment nor age significantly influenced peak preference (Fig. 2a, Table 2). In addition, there was no difference in peak preference between years (Fig. 2a, Table 2). Female peak preference was highly repeatable, evidenced by the significant female identity term and estimate (r = 0.67; Table 2). When pooling across acoustic environments, we found a strong positive relationship between peak preference when young and older, confirming the strength of the repeatability estimate (Pearson’s r; r = 0.71, p < 0.001, N = 42).
The influence of acoustic environment and age on two traits that describe the shape of female mate preference functions. Female preferences were assayed when young but sexually mature and then again 8 days later. a Means ± SE display the peak preferences of females reared in one of five acoustic environments when young and older (filled and unfilled circles, respectively). b Relationship between the first and second peak preference of females pooled across acoustic environments. c Means ± SE display the selectivity of females when young and older (filled and unfilled circles, respectively). d Relationship between the first and second selectivity of females pooled across acoustic environments. Acoustic environments not sharing a letter in a and c are significantly different (post hoc tests using the FDR method)
Variability and repeatability of selectivity
We found that the acoustic environment, but not age, significantly influenced female selectivity (Fig. 2c, Table 2). We did not find a significant difference in selectivity between years (Fig. 2c, Table 2). Post hoc analysis revealed that females were more selective having had the experience of attractive mates alone or in concert with increased variation in available mates, and this pattern of selectivity was repeatable as females aged (High and Mixed; Fig. 2c). However, female selectivity was only moderately repeatable (r = 0.26; Table 2), likely influenced by underlying changes in female responsiveness as they aged across the several acoustic environments (Fig. 1, Table 1). When pooling across acoustic environments, we found a stronger positive relationship between the first and second measures of selectivity than the repeatability estimate (Pearson’s r; r = 0.51, p < 0.001, N = 42).
Female post-mating reproductive investment: effects of age and acoustic environment
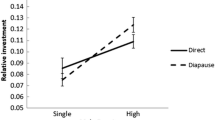
We found that females adjusted their investment in eggs in response to both the acoustic environment and age, and a significant interaction occurred between the two factors (Table 3). We also found that both female identity and size contributed significantly to female investment patterns (Table 3).
Having found significant acoustic environment × age interaction, we performed post hoc pairwise comparisons between acoustic environments at each mating event separately, statistically controlling for all 20 comparisons (FDR method; Benjamini and Hochberg 1995). This analysis revealed similar patterns across mating events. First, females deposited more eggs when experiencing a lack of mates or attractive mates compared to unattractive or average mate types (Silent and High vs. Low and Mid; Fig. 3a, b). Second, when a variety of mates were available, females still invested more than when only unattractive mates were available (Mixed vs. Low; Fig. 3a, b).
The influence of acoustic environment and age on female post-mating reproductive investment. a Means ± SE display the number of eggs that females laid in the 7 days following their first mating when young and second mating when older (filled and unfilled circles, respectively). Eggs laid after the first mating sharing a capital letter are not significantly different whereas eggs laid after the second mating sharing a lowercase letter are not significantly different (post hoc tests using the FDR method from the full model in Table 3). b Relationship between the number of eggs that females laid after their first mating when young and their second mating when older, pooling across acoustic environments
We found that female egg investment was moderately repeatable (r = 0.42; Table 3), despite the significant acoustic environment × age interaction. Pooling across the several acoustic environments, we found a stronger positive relationship in the number of eggs laid across matings by females than the repeatability estimate (partial correlation; r = 0.56, p < 0.001, N = 75; Fig. 3c).
Pre- to post-mating reinforcement and its impact on male reproductive success
Females reinforced their consistent preference for higher syllable numbers through adjustments in post-mating egg investment. Specifically, females deposited more eggs when attractive mates were present in their environment and deposited fewer eggs when less attractive mates were present (Fig. 3). This pattern of investment resulted in up to a threefold difference in the total reproductive success of High vs. Low “quality” males (one-way ANOVA: F2,42 = 62.61, p < 0.001; Fig. 4).
A relative index of the total reproductive success of males reared in the Low, Mid, and High acoustic environments. The index is the multiplication of estimates of a male’s pre-mating attractiveness and post-mating reproductive success (see “Methods”). Individual values are jittered for clarity (white circles) around the mean ± SE (black circles). Acoustic environments not sharing a letter are significantly different (post hoc tests using the FDR method)
Discussion
By rearing E. diurnus females in different acoustic environments and testing their responses over adult life and across 2 years, we reveal a pattern of pre- and post-mating behaviors that support our predictions: consistent adjustments in both behaviors reinforce one another in a way that impacts male reproductive success. These findings expand on previous studies in several ways. First, our results bolster work on how females adjust their pre-mating responsiveness and selectivity to acoustic experience (e.g., Bailey and Zuk 2008; Fowler-Finn and Rodríguez 2012b). Second, we provide some of the first repeatability estimates of pre- and post-mating behaviors across social environments. Finally, we reveal that female post-mating adjustments in egg investment reinforce their pre-mating preferences such that the total opportunity for sexual selection to act on male signals is greatly strengthened.
Given that males developed in the same acoustic environment as females, how likely is it that oviposition was influenced by male spermatophores rather than or in addition to female behavior? First, we found no evidence that spermatophore size or male size contributed to variation in oviposition. Second, we recently documented a pattern of terminal investment in the size of spermatophores produced by aging E. diurnus males reared in varying acoustic environments (i.e., second mating; Rebar and Greenfield 2017). If the contents of spermatophores were influential, then one would predict no difference in oviposition after the second mating as males produced uniformly large spermatophores. This prediction stems from the fact that the two components of spermatophores, the sperm-filled ampulla and the spermatophylax, positively and strongly covary (Wedell 1994b; Jarrige et al. 2015), suggesting that larger spermatophores likely contain more accessory gland proteins to influence oviposition. This is not to say that the contents of spermatophores do not influence oviposition rates, but that the moderately repeatable pattern of oviposition in aging females indicates that plasticity in oviposition is more strongly under female control. Consistent with this conclusion, Hockham et al. (2004) found that relative spermatophore size did not predict P2 paternity in doubly mated E. diurnus females.
Unlike the pattern of age-related investment in spermatophores by males, a female’s age had a much weaker influence on post-mating investment in oviposition than her social environment did. Whereas life history theory predicts individuals to bias investment towards current reproduction when future reproduction is unlikely (Clutton-Brock 1984; Roff 1992; Stearns 1992), females continued to reinforce their pre-mating preference by strategically investing more in eggs when male calls more closely matched their peak preference. This combined effect across matings resulted in a threefold difference in the relative reproductive success between attractive and unattractive males. One possibility is that the range in ages that we tested was insufficient for observing a terminal effect in the population studied here. Whereas we detected significant interaction terms between the acoustic environment and age on preference functions and oviposition, the repeatability estimates of peak preference, selectivity, and oviposition support the notion that females had not yet begun to terminally invest. Relaxed female selectivity is consistent with terminal investment, but this effect had not manifested in post-copulatory decisions. But other ecological factors that are relatively independent of age may also be at play. First, animals may estimate environmental parameters, including local habitat quality, via information perceived from conspecifics (Danchin et al. 2004; Valone 2007). Thus, males may be attracted to the calls of competitors when searching for a suitable habitat (Muller 1998; Pfennig et al. 2000; Bee 2007), and in our present study attractive songs in the environment may inform females that they are in a good habitat where increased oviposition after each mating would be rewarding. If there is uncertainty in a given male’s attractiveness, as would occur with satellite males intercepting searching females or the muted males with whom females mated in this experiment (particularly in the Mixed environment), females may reduce their investment or express more variability in oviposition than if only attractive songs were present in the environment. Conversely, the presence of unattractive songs may prompt females to reduce their egg investment and search for a more suitable habitat.
Another possibility is that females may reabsorb oocytes that are not oviposited as an adaptive mechanism to reallocate resources to survival. Oosorption is widespread in insects (Bell and Bohm 1975), and it has been shown to occur in response to poor environmental quality, notably low resource availability (Moore and Attisano 2011; Moore 2014). Here we suggest that social cues may function similarly to resource cues, and plasticity in reproductive physiology may allow females to increase their life span and thus their chances of finding a more attractive mate. For example, short-winged, non-migratory milkweed bug females increased their levels of oosorption when reared in a poor environment (Attisano et al. 2013). As E. diurnus are flightless and distributed in geographically isolated populations, oosorption may represent an adaptive response to perceived poor mate quality. While our study did not measure residual fitness via female life span, this hypothesis predicts that females in an unattractive acoustic environment would live longer albeit with a reduced lifetime reproductive output relative to females in an attractive environment.
In some cases, variability in the relationship between pre- and post-mating behaviors may reflect artifacts of experimental design. For example, our work on another population of E. diurnus (Font Romeu) found that young females laid fewer eggs when reared in an acoustic environment containing calls with more, as opposed to fewer, syllables (Rebar et al. 2016), which contrasts with our findings reported here. We suggest that these earlier findings result from a mismatch between the range of call syllable numbers used in our test stimuli and the actual range of call syllable numbers broadcast by males in the population. Our test stimuli ranged from 2 to 4 syllables per call, similar to the 3-syllable range used in the current study, but males from Font Romeu averaged more syllables per call than in previous years (4.5 syllables per call), and individuals ranged from 3 to 8 syllables. This variation is in line with the previously documented pattern of between-male variation in syllable number increasing as the population mean increases (Ritchie 1996). Consequently, the range of calls we used may not have reflected the range to which females had been selected to increase their post-mating investment. Furthermore, the acoustic environments broadcast calls that were less than the population mean for Font Romeu, and female oviposition may have reflected the acoustic information that there were not any attractive males present. Had we tested calls with more syllables than the mean, it might have revealed a pattern of post-mating reinforcement similar to that reported here (Vias population), as females from that population (Font Romeu) also prefer males’ songs with more syllables than the population mean (Barbosa et al. 2016a; Rebar et al. 2016). Alternatively, females may use additional cues to evaluate mate attractiveness, e.g., cuticular hydrocarbons (CHCs), which are known to play a role in female mate choice in field crickets (Thomas and Simmons 2009; Simmons et al. 2013). Such cues may have offered conflicting or reinforcing information to the acoustic environment, altering female perception of mate attractiveness, and thus her post-mating investment.
Behavioral consistency, arising from genetic and environmental inputs (Boake 1989; Dohm 2002), indicates how females may act as an agent of selection on males. The medium to large effect sizes that we found for the repeatability of peak preference, selectivity, and egg investment in the Vias population of E. diurnus are consistent with the general finding that female preference functions have driven male signal evolution in this species (Barbosa et al. 2016a). Coupled with the high repeatability of male syllable number, found in all E. diurnus populations examined thus far (Ritchie 1992b; Barbosa et al. 2016b; Rebar et al. 2016), selection imposed by female mate choice would favor the same males over the breeding season. Moreover, in the Vias population, female pre- and post-mating reproductive traits remained stable across years. Females exert strong, consistent, directional selection on males, indicated by a consistent mismatch between female peak preference and male syllable number. The pre-mating female preference is then reinforced through post-mating investment, leading to the large variation in male reproductive success and thus driving male call evolution in the population.
In conclusion, our results highlight how plasticity in female behaviors can dictate evolutionary processes. Consistent plasticity in female pre- and post-mating behaviors across social environments and mating events indicates the ability of females to not only respond to selection, but to impose selection on male traits and influence their subsequent evolution. This finding is most evident in its impact on the total reproductive success of males of varying attractiveness. Against the background pattern of call and preference diversity between populations of E. diurnus (Barbosa et al. 2016a; Greenfield et al. 2016), adaptive plasticity in female behaviors to mate availability has been favored via selection and, in turn, likely contributes to ongoing divergence between the isolated populations in this species.
Data availability
All supporting data will be deposited in an appropriate repository (e.g., Dryad), pending manuscript acceptance.
References
Albo MJ, Bilde T, Uhl G (2013) Sperm storage mediated by cryptic female choice for nuptial gifts. Proc R Soc B 280
Arnold KE, Gilbert L, Gorman HE, Griffiths KJ, Adam A, Nager RG (2016) Paternal attractiveness and the effects of differential allocation of parental investment. Anim Behav 113:69–78
Attisano A, Tregenza T, Moore AJ, Moore PJ (2013) Oosorption and migratory strategy of the milkweed bug, Oncopeltus fasciatus. Anim Behav 86:651–657
Bailey NW (2008) Love will tear you apart: different components of female choice exert contrasting selection pressures on male field crickets. Behav Ecol 19:960–966
Bailey NW, Zuk M (2008) Acoustic experience shapes female mate choice in field crickets. Proc R Soc B 275:2645–2650
Barbosa F (2012) Males responding to sperm competition cues have higher fertilization success in a soldier fly. Behav Ecol 23:815–819
Barbosa F, Rebar D, GreenfieldMD (2016a) Female preference functions drive inter-population divergence in male signaling: call diversity in the bushcricket Ephippiger diurnus. J Evol Biol 1–10
Barbosa F, Rebar D, Greenfield MD (2016b) Reproduction and immunity trade-offs constrain mating signals and nuptial gift size in a bushcricket. Behav Ecol 27:109–117
Bee MA (2007) Selective phonotaxis by male wood frogs (Rana sylvatica) to the sound of a chorus. Behav Ecol Sociobiol 61:955–966
Bell WJ, Bohm MK (1975) Oosorption in insects. Biol Rev 50:373–396
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Boake CRB (1989) Repeatability: its role in evolutionary studies of mating behavior. Evol Ecol 3:173–182
Bretman A, Gage MJG, Chapman T (2011) Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol 26:467–473
Bretman A, Westmancoat JD, Gage MJG, Chapman T (2012) Individual plastic responses by males to rivals reveal mismatches between behaviour and fitness outcomes. Proc R Soc B 279:2868–2876
Brunel O (2012) De la communication acoustique au sein du groupe: contraintes et mécanismes. Ph. D. thesis, Université François-Rabelais, Tours
Busnel RG, Dumortier B (1954) Observations sur le comportement acousticosexuel de la ♀ d’Ephippiger bitterensis. C R Seances Soc Biol Fil 148:1589–1592
Chaine AS, Lyon BE (2008) Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319:459–462
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
Collet J, Richardson DS, Worley K, Pizzari T (2012) Sexual selection and the differential effect of polyandry. Proc Natl Acad Sci 109:8641–8645
Cotton S, Small J, Pomiankowski A (2006) Sexual selection and condition-dependent mate preferences. Curr Biol 16:R755–R765
Danchin E, Giraldeau LA, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
Dohm MR (2002) Repeatability estimates do not always set an upper limit to heritability. Funct Ecol 16:273–280
Duffield KR, Bowers EK, Sakaluk SK, Sadd BM (2017) A dynamic threshold model for terminal investment. Behav Ecol Sociobiol 71:185
Duijm N (1990) On some song characteristics in Ephippiger (Orthoptera: Tettigonioidae) and their geographic variation. Neth J Zool 40:428–453
Esquer-Garrigos YS, Streiff R, Party V, Nidelet S, Navascués M, Greenfield MD (2019) Pleistocene origins of chorusing diversity in Mediterranean bush-cricket populations (Epippiger diurnus). Biol J Linn Soc bly195
Evans JP, Garcia-Gonzalez F (2016) The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J Evol Biol 1–24
Fowler-Finn KD, Rodríguez RL (2012a) Experience-mediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66:459–468
Fowler-Finn KD, Rodríguez RL (2012b) The evolution of experience-mediated plasticity in mate preferences. J Evol Biol 25:1855–1863
Greenfield MD, Siegfreid E, Snedden WA (2004) Variation and repeatability of female choice in a chorusing katydid, Ephippiger ephippiger: an experimental exploration of the precedence effect. Ethology 110:287–299
Greenfield MD, Esquer-Garrigos Y, Streiff R, Party V (2016) Animal choruses emerge from receiver psychology. Sci Rep 6:34369
Gwynne DT (2001) Katydids and bush-crickets: reproductive behavior and evolution of the Tettigoniidae. Cornell University Press, Ithaca
Han CS, Brooks RC (2014) Long-term effect of social interactions on behavioral plasticity and lifetime mating success. Am Nat 183:431–444
Hebets E, Sullivan-Beckers L (2010) Mate choice and learning. In: Breed MD, Moore J (eds) Encyclopedia of animal behavior. Elsevier B V, Amsterdam, pp 389–393
Höbel G (2015) Socially mediated plasticity of chorusing behavior in the gladiator frog Hypsiboas rosenbergi. Acta Ethol 18:145–152
Hockham LR, Graves JA, Ritchie MG (2004) Sperm competition and the level of polyandry in a bushcricket with large nuptial gifts. Behav Ecol Sociobiol 57:149–154
Janicke T, David P, Chapuis E (2015) Environment-dependent sexual selection: Bateman’s parameters under varying levels of food availability. Am Nat 185:756–768
Jarrige A, Body M, Giron D, Greenfield MD, Goubault M (2015) Amino acid composition of the bushcricket spermatophore and the function of courtship feeding: variable composition suggests a dynamic role of the nuptial gift. Physiol Behav 151:463–468
Karlsson K, Eroukhmanoff F, Svensson EI (2010) Phenotypic plasticity in response to the social environment: effects of density and sex ratio on mating behaviour following ecotype divergence. PLoS One 5:1–6
Kasumovic MM, Brooks RC (2011) It’s all who you know: the evolution of socially cued anticipatory plasticity as a mating strategy. Q Rev Biol 86:181–197
Kilmer JT, Fowler-Finn KD, Gray DA, Höbel G, Rebar D, Reichert MS, Rodríguez RL (2017) Describing mate preference functions and other function-valued traits. J Evol Biol 30:1658–1673
Kokko H, Jennions MD, Brooks R (2006) Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst 37:43–66
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities : a common mistake. Auk 104:116–121
Meyer K, Kirkpatrick M (2005) Up hill, down dale: quantitative genetics of curvaceous traits. Philos Trans R Soc B 360:1443–1455
Moore PJ (2014) Reproductive physiology and behaviour. In: Shuker DM, Simmons LW (eds) The evolution of insect mating systems. Oxford University Press, Oxford, pp 78–91
Moore PJ, Attisano A (2011) Oosorption in response to poor food: complexity in the trade-offbetween reproduction and survival. Ecol Evol 1:37–45
Muller KL (1998) The role of conspecifics in habitat settlement in a territorial grasshopper. Anim Behav 56:479–485
Party V, Brunel-Pons O, Greenfield MD (2014) Priority of precedence: receiver psychology, female preference for leading calls and sexual selection in insect choruses. Anim Behav 87:175–185
Party V, Streiff R, Marin-Cudraz T, Greenfield MD (2015) Group synchrony and alternation as an emergent property: elaborate chorus structure in a bushcricket is an incidental by-product of female preference for leading calls. Behav Ecol Sociobiol 69:1957–1973
Pfennig KS, Rapa K, McNatt R (2000) Evolution of male mating behavior: male spadefoot toads preferentially\rassociate with conspecific males. Behav Ecol Sociobiol 48:69–74
Qvarnström A (2001) Context-dependent genetic benefits from mate choice. Trends Ecol Evol 16:5–7
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rebar D, Greenfield MD (2017) When do acoustic cues matter? Perceived competition and reproductive plasticity over lifespan in a bushcricket. Anim Behav 128:41–49
Rebar D, Rodríguez RL (2013) Genetic variation in social influence on mate preferences. Proc R Soc B 280:20130803
Rebar D, Rodríguez RL (2014) Genetic variation in host plants influences the mate preferences of a plant-feeding insect. Am Nat 184:489–499
Rebar D, Rodríguez RL (2016) Males adjust their signalling behaviour according to experience of male signals and male-female signal duets. J Evol Biol 29:766–776
Rebar D, Zuk M, Bailey NW (2011) Mating experience in field crickets modifies pre- and postcopulatory female choice in parallel. Behav Ecol 22:303–309
Rebar D, Barbosa F, Greenfield MD (2016) Acoustic experience influences male and female pre- and postcopulatory behaviors in a bushcricket. Behav Ecol 27:434–443
Ritchie MG (1991) Female preference for “song races” of Ephippiger ephippiger (Orthoptera: Tettigoniidae). Anim Behav 42:518–520
Ritchie MG (1992a) Behavioral coupling in tettigoniid hybrids (Orthoptera). Behav Genet 22:369–379
Ritchie MG (1992b) Variation in male song and female preference within a population of Ephippiger ephippiger (Orthoptera: Tettigoniidae). Anim Behav 43:845–855
Ritchie MG (1996) The shape of female mating preferences. Proc Natl Acad Sci 93:14628–14631
Rodríguez RL, Boughman JW, Gray DA, Hebets EA, Höbel G, Symes LB (2013a) Diversification under sexual selection: the relative roles of mate preference strength and the degree of divergence in mate preferences. Ecol Lett 16:964–974
Rodríguez RL, Rebar D, Fowler-Finn KD (2013b) The evolution and evolutionary consequences of social plasticity in mate preferences. Anim Behav 85:1041–1047
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman & Hall, New York
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Simmons LW, Thomas ML, Simmons FW, Zuk M (2013) Female preferences for acoustic and olfactory signals during courtship: male crickets send multiple messages. Behav Ecol 24:1099–1107
Spooner LJ, Ritchie MG (2006) An unusual phylogeography in the bushcricket Ephippiger ephippiger from Southern France. Heredity 97:398–408
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stinchcombe JR, Kirkpatrick M (2012) Genetics and evolution of function-valued traits: understanding environmentally responsive phenotypes. Trends Ecol Evol 27:637–647
Swanger E, Zuk M (2015) Cricket responses to sexual signals are influenced more by adult than juvenile experiences. J Insect Behav 28:328–337
Taborsky B, Oliveira RF (2012) Social competence: an evolutionary approach. Trends Ecol Evol 27:679–688
Thomas ML, Simmons LW (2009) Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol Biol 9:162
Valone TJ (2007) From eavesdropping on performance to copying the behavior of others: a review of public information use. Behav Ecol Sociobiol 62:1–14
Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI (2012) The impact of learning on sexual selection and speciation. Trends Ecol Evol 27:511–519
Wedell N (1994a) Dual function of the bushcricket spermatophore 258:181–185
Wedell N (1994b) Variation in nuptial gift quality in bush crickets (Orthoptera: Tettigoniidae). Behav Ecol 5:418–425
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
West-Eberhard MJ (2014) Darwin’s forgotten idea: the social essence of sexual selection. Neurosci Biobehav Rev 46(Part 4):501–508
Wey TW, Spiegel O, Montiglio P-O, Mabry KE (2015) Natal dispersal in a social landscape: considering individual behavioral phenotypes and social environment in dispersal ecology. Curr Zool 61:543–556
Widemo F, Sæther SA (1999) Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol Evol 14:26–31
Acknowledgements
We thank C. Maurin for the assistance in animal collection and husbandry, M. Deluen and C. Hebert for their help with animal husbandry and data collection, and K. Ratzlaff for the construction of the amplifier. This work was funded by a Fondation Fyssen Postdoctoral Fellowship to DR, a National Science Foundation Biology Postdoctoral Fellowship FY 2012 (Award ID 1202761) to FB, and the Agence Nationale de la Recherche de France (contrat ANR-11-BSV7-025-01) to MDG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Shaw
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rebar, D., Barbosa, F. & Greenfield, M.D. Female reproductive plasticity to the social environment and its impact on male reproductive success. Behav Ecol Sociobiol 73, 48 (2019). https://doi.org/10.1007/s00265-019-2661-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2661-4